Abstract
By the titrations of the antibody response to the vaccinations of avian influenza (AI) and Newcastle disease (ND), the present study was conducted to evaluate the effects of Marek's disease (MD)-resistant breeding and vaccination against the immunosuppression induced by virulent Marek's disease virus (MDV). The results showed that haemagglutination inhibition (HI) titres to AI and ND in MDV-unchallenged groups were significantly higher than those of MDV-challenged groups (P<0.05), those of the resistant birds were significantly higher than those of the common birds (P<0.05) and those of the herpes virus of turkeys (HVT)-vaccinated birds were significantly higher than those of the HVT-unvaccinated birds (P<0.05). The results demonstrated that antibody responses to the vaccinations of AI and ND were significantly suppressed by MDV infection, and MD-resistant breeding and HVT vaccination could significantly increase resistance to the suppression.
Introduction
Marek's disease (MD) is a lymphoproliferative disease caused by the Marek's disease virus (MDV), which is ubiquitous in poultry houses. MD is characterised by a mononuclear infiltration of one or more of the following: peripheral nerves, gonad, iris, various viscera, muscle and skin (Calnek & Witter, Citation1997). Three serotypes of MDV strains are recognised. All oncogenic strains are classified as serotype 1 (MDV-1) while the naturally non-oncogenic chicken strains and herpesvirus of turkeys (HVT) belong to serotypes 2 (MDV-2) and 3, respectively. MD incidence has largely been controlled by vaccination with MDV serotypes 1, 2 and 3, sometime in bi- and multivalent combinations since the 1970s (Witter, Citation2001). However, new MDV-1 isolates of increased virulence, such as vvMDV and vv + MDV strains (Witter, Citation1998) have emerged with greater immunosuppressive potential over the past decade (Calnek, Harris, Buscaglia, Schat, & Lucio, Citation1998). MDV-1 infection could cause immunosuppression other than visible tumours in the later phase of life that suppress immunoresponses to vaccination and results in concurrent or secondary infections by other pathogens, which may be one of the major problems found in the field that cause big economic losses. Avian influenza (AI) and Newcastle disease (ND) are the most important diseases in the poultry industry in China and the antibody response of the vaccinations of AI and ND can be detected easily by haemaggulutination (HA) and haemagglutination inhibition (HI) tests with the serum of vaccinated birds.
Xiayan chickens, one of the well known indigenous breeds in China, originate from Xiayan Village of Rong County in Guangxi, and have been selectively bred for MD resistance by a combination of challenge/survival breeding, unvaccinated/survival breeding (through natural infection) and resistant parent's breeding (through the challenge of progeny) (Li et al., Citation1996). These approaches have provided a significant genetic resistance against MD (Li et al., Citation1996). The inbred MD-resistant line of Xiayan chicken has being successfully used to produce commercial broilers and to crossbreed with other lines in the poultry industry in south China, especially in Guangxi and Guangdong provinces for more than 10 years (Liang, Wei, & Li, Citation2007).
In the present study, the influence of the challenge of a MDV-1 field isolate YL040920, which is associated with acute oncogenicity and can overcome the protection of vaccination with HVT (Zuo et al., Citation2007), on antibody responses to the vaccinations of AI and ND vaccines was evaluated with the Xiayan chickens of resistant line and common line, and with and without the vaccination of HVT for the purpose of evaluation the effects of MD-resistant breeding and vaccination of HVT against the virulent MDV induced immunosuppression. This isolate has the molecular characters of vvMDV (Renz, Cheetham, & Walkden-Brown, Citation2008; Zuo et al., Citation2007).
Material studied, area descriptions, methods and techniques
Chicken groups and vaccination procedure
Xiayan chicks of resistant line and common line were used in the experiment. Chicks were hatched at the isolation unit of the University of Guangxi. A total of 74 birds of the common line were divided into groups F (24 birds), G (30 birds) and E (20 birds), while 77 birds of the resistant line were divided into groups T (30 birds), R (27 birds) and S (20 birds). All the birds were reared and maintained under disease-free conditions. Treatments of these groups are described in . Birds of groups G and R were vaccinated with a commercial HVT vaccine at hatching. All the birds were vaccinated with a commercial ND live vaccine strain LaSota at 9 days of age and then twice with commercial inactivated oil-emulsion vaccines of AIV subtype H5 and NDV at 15 and 44 days of age, respectively, and kept in separated isolators (). Spleen, liver, heart, kidney and proventriculus tissue were collected from all birds on day 64 post-infection for further analysis. Birds were euthanased by CO2 inhalation.
Table 1. Trial groups.
Table 2. Vaccination protocols for all three vaccines (HVT, ND and AI).
Marek's disease virus (MDV) challenged
Birds of groups F, G, T and R were injected intra-abdominally with 2500 plaque forming unit (PFU) of the virulent MDV-1 isolate YL040920 grown in duck embryo fibroblasts (DEFs) at 7 days of age, while birds of group E and S were used as unchallenged control, respectively ().
Multiplex PCR detection Marek's disease virus (MDV)
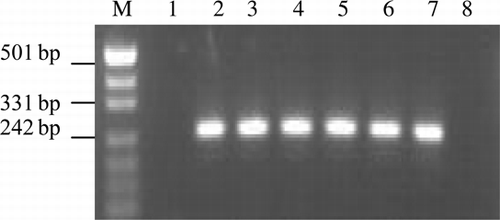
A multiplex PCR () was performed on DNA extracted from virus-infected DEFs and tissue samples of all challenged and unchallenged birds to confirm the infection of vvMDV. Genomic DNA was extracted from PBL samples using a standard phenol–chloroform method. Methods of PCR analysis as followed the description of Wei et al. (Citation2002). The primers () were designed to amplify specific fragment (). Each 50-µl reaction mix contained approximately 100 ng DNA sample, PCR buffer, 25 mM of MgCL2, 0.2 mM of each dNTP, 0.1 mM of each primer, 1U of Taq DNA polymerase. The amplification process consisted of a 1 min initial denaturation at 95°C, followed by 35 cycles (each consisting of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, elongation at 72°C for 1 min) with a final elongation at 72°C for 8 min.
Table 3. PCR primer sequences and amplified segments.
Sera preparation and haemaggulutination (HA)/haemagglutination (HI) test
Blood samples were collected at days 8, 23, 30, 38, 50, 57 and 64 post-infection (dpi). The sera samples were separated and stored at −80°C until used. Antibodies (Ab) titres to AIV–H5 and NDV in sera were detected by HA and HI tests followed the methods described by Brown, King, and Seal (Citation1999). The antiserum was prepared by inoculation of specified pathogen free chickens and had an end point dilution of 1:512. The HI testing of serum samples from inoculated and control chickens were completed as follows. Serial twofold serum dilutions were made in phosphate buffered saline (pH 7.2), and then four HA units of NDV and AIV strain test antigen was added to each dilution and incubated at 37°C for 30 min. An equal volume of 0.5% chicken erythrocytes in PBS was added as a test indicator. The HI end point was determined as the last dilution with complete inhibition of HA activity.
The differences between pairs were analysed by Student's t-tests. For all analyses, differences were considered significantly when P<0.05 unless otherwise designated. Correlation (r) values were determined by linear regression analysis.
Results
Confirmation of Marek's disease virus-1 (MDV-1) infection
The characteristic patterns of electrophoresis of virulent MDV-1 (a band of 317 bp, two copies of 132-bpr) were observed from all the samples detected. DNA bands associated with reticuloendotheliosis virus (REV) and avian leukosis virus (ALV) were not detected ().
Figure 1. Multiplex PCR detection for DNA extracted from infected DEFs and samples of challenged and unchallenged birds. Lane 1: spleen of unchallenged birds; Lane 2: DEFs infected with YL040920; Lanes 3–7: viscera organs (liver, spleen, heart, kidney and proventriculus, respectively) of challenged birds; Lane 8: negative control.
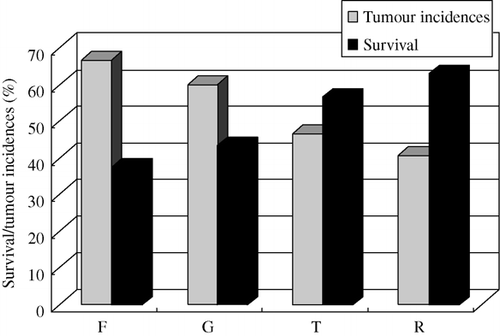
Survival and the tumour occurrences of Marek's disease (MD)
The results () showed that the survival of MD for groups F, G, T and R were 37.50% and 43.33%, 56.67% and 62.96%, respectively. The results () also showed that the tumour incidences of MD for groups F, G, T and R were 66.67% and 60.00%, 46.67% and 40.74%, respectively.
AIV titres and statistical analysis
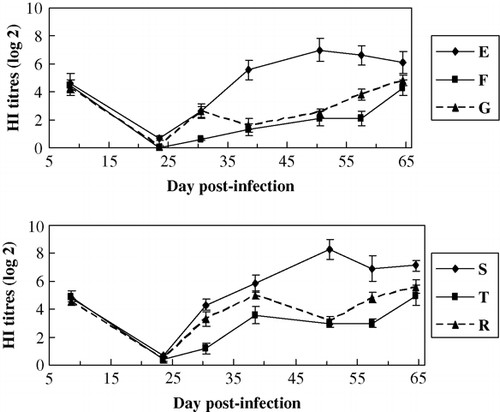
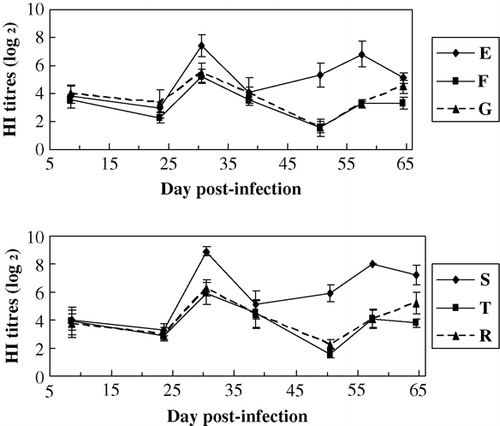
The results () showed that for the HI Ab titres against AIV, that of the MDV-unchallenged birds (group E) were significantly higher than that of the MDV-challenged birds (group F) (P<0.05), HI Ab titres for AIV of the MDV-unchallenged birds (group S) were significantly higher than that of the MDV-challenged birds (group T) (P<0.05) especially at 50 days after the challenge. The AIV antibody titres of the HVT-vaccinated birds (group G) were significantly higher than that of the unvaccinated birds (group F) (P<0.05). HI Ab titres of the HVT-vaccinated birds (group R) were significantly higher than that of the unvaccinated birds (group T) (P<0.05) on the whole. The results showed that Ab responses to the vaccinations of AIV were suppressed by MDV infection and HVT vaccination could significantly decrease the suppression.
Figure 3. HI Ab titres to AIV within groups of different lines. Common line – E (no HVT-vaccinated, no MDV-challenged); F (no HVT-vaccinated, MDV-challenged); G (HVT-vaccinated, MDV-challenged). MD-resistant – S (no HVT-vaccinated, no MDV-challenged); T (no HVT-vaccinated, MDV-challenged); R (HVT-vaccinated, MDV-challenged).
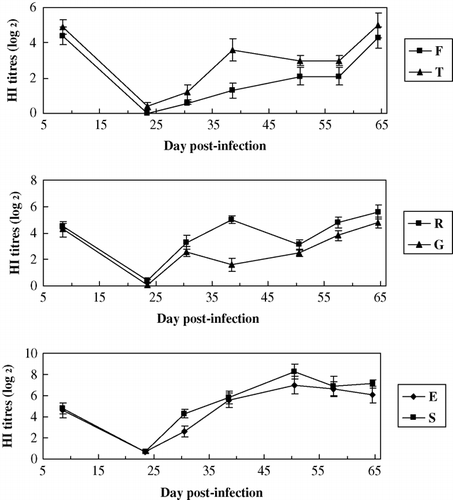
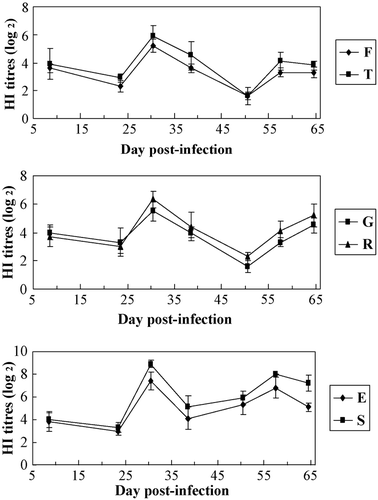
The results () showed that for HI Ab titres to AIV, those of the resistant line (groups T, R and S) were significantly higher than those of the common line (groups F, G and E) (P<0.05), respectively. The results showed that MD-resistant line could significantly increase the resistance to the suppression of MDV-infection on HI Ab response to the vaccination of AI. HI antibody titres of AI were between 4.3 and 4.9 at the age of 8 days indicates a higher maternal antibody level.
Figure 4. HI Ab titres to AIV between groups of different lines. Common line – E (no HVT-vaccinated, no MDV-challenged); F (no HVT-vaccinated, MDV-challenged); G (HVT-vaccinated, MDV-challenged). MD-resistant – S (no HVT-vaccinated, no MDV-challenged); T (no HVT-vaccinated, MDV-challenged); R (HVT-vaccinated, MDV-challenged).
Newcastle disease (ND) titres and statistical analysis
The results () showed that for HI Ab titres to NDV, that of the MDV-unchallenged birds (group E) was significantly higher than that of the MDV-challenged birds (group F) (P<0.05), that of the MDV-unchallenged birds (group S) was significantly higher than that of the MDV-challenged birds (group T) (P<0.05) especially at 57 days after the challenge, and that of the HVT-vaccinated birds (group G) was significantly higher than that of the unvaccinated birds (group F) (P<0.05). Ab titres to NDV of the HVT-vaccinated birds (group R) were significantly higher than that of the unvaccinated birds (group T) (P<0.05). The results showed that Ab responses to the vaccinations of ND were significantly suppressed by MDV infection and HVT vaccination could significantly decrease the suppression.
Figure 5. HI Ab titres to NDV within groups of different lines. Common line – E (no HVT-vaccinated, no MDV-challenged); F (no HVT-vaccinated, MDV-challenged); G (HVT-vaccinated, MDV-challenged). MD-resistant – S (no HVT-vaccinated, no MDV-challenged); T (no HVT-vaccinated, MDV-challenged); R (HVT-vaccinated, MDV-challenged).
The results () showed that for HI Ab titres to NDV, those of the resistant line (groups T, R and S) were significantly higher than those of the common line (groups F, G and E) (P<0.05), respectively. The results showed that MD-resistant line could significantly increase the resistance to the suppression of MDV-infection on HI Ab response to the vaccination of ND. HI antibody titres of ND were between 3.6 and 4.0 at 8 days, indicating a higher maternal antibody level.
Figure 6. HI Ab titres to NDV between groups of different lines. Common line – E (no HVT-vaccinated, no MDV-challenged); F (no HVT-vaccinated, MDV-challenged); G (HVT-vaccinated, MDV-challenged). MD-resistant – S (no HVT-vaccinated, no MDV-challenged); T (no HVT-vaccinated, MDV-challenged); R (HVT-vaccinated, MDV-challenged).
Discussion
One of the consequences of MDV infection is immunosuppression in the host. There is some information (Calnek & Witter, Citation1997; Schat, Chen, Calnek, & Char, Citation1991) on the mechanism of immunosuppression caused by MDV. In the lymphoid organs, MDV primarily causes lytic infection of B cells (Shek, Calnek, Schat, & Chen, Citation1983), which leads to the suppression of humoral immunity (Payne, Franzier, & Powell, Citation1976). During the latent phase, MDV causes suppression of cellular immunity.
Comparable experiments with animals are also necessary to identify the mechanism of immunosuppression. In the present study, birds of MD-resistant and common lines were used and antibody responses in sera to the vaccinations of AIV and NDV by HA test and HI test at various times after infection with MDV were compared with those in uninfected controls.
HI antibody titres of ND and AI were between 3.6 and 4.9 at the age of 8 days and shows that the maternal antibody was at a higher level. Then HI antibody titres of ND and AI reduced and were at very low levels and HI antibody titres of AI hardly detected at of 23 days after vaccination with ND and AI at 9 and 15 days, respectively. This result demonstrate that maternal antibody decreased and can neutralise vaccine highly. Then HI antibody titres of ND rise markedly until 30 days and HI antibody titres of AI rise until 65 days because maternal antibodies disappeared, and HI antibody titres of ND decreased again until age 50 days and increased again to age 65 days.
Significant differences in the levels of antibody titres to the vaccinations of AI and ND between MDV-infected and non-infected birds indicate that MDV infection led to a decrease of antibody responses to the vaccinations.
Antibody titres to AIV and NDV in the resistant line birds were significantly higher than in those of the common line birds during the whole period of experiment from 8 to 64 days of age. The results of the study demonstrate that MD-resistant breeding could significantly increase chicken resistance to the MDV infection and can improve the antibody responses to the vaccinations of ND and AI. Antibody titres to AIV and NDV in the HVT-vaccinated birds were also significantly higher than in those of the unvaccinated birds and indicates that HVT vaccination could improve the resistance of chickens against the immunosuppression caused by MDV infection and then enhance immune response to the vaccinations.
The present study will be helpful in evaluating the significance of MD resistance breeding, and provides fundamental information for screening MD resistance-related genes that enhance the genetic resistance in Xiayan chickens, a complementary avenue to vaccination for controlling MD.
Acknowledgements
This work was supported by the Nature Science Foundation of China (Grant Nos. 30460099, 39860055, 30860206) and the Guangxi University Key Project (2005ZD01).
References
- Brown , C. , King , D.J. and Seal , B.S. 1999 . Pathogenesis of Newcastle disease in chickens experimentally infected with viruses of different virulence . Veterinary Pathology , 36 : 125 – 132 .
- Calnek , B.W. , Harris , R.W. , Buscaglia , C. , Schat , K.A. and Lucio , B. 1998 . Relationship between the immunosuppressive potential and the pathotype of Marek's disease virus isolates . Avian Disease , 42 : 124 – 132 .
- Calnek , B.W. , Witter , R.L. 1997 . Marek's disease . In B.W. Calnek Disease of poultry , 10th ed. 369 413 Ames : Iowa State University Press .
- Li , K.R. , Wei , P. , Liang , M.F. , Zeng , Z.H. , Pan , Q.Z. , He , S.Y. , et al. 1996 . A study on selective breeding of Xiayan chicken for Marek's disease resistance . In : R.F. Silva , H.H. Cheng , P.M. Coussens , L.F. Lee , L.F. Velicer Current research on Marek's disease 75 79 Pennsylvania, PA : AAAP .
- Liang , Y.D. , Wei , P. and Li , K.R. 2007 . Current situation of breeds resources of quality chicken in Guangxi province and their research and exploitation . China Poultry , 29 ( 17 ) : 5 – 7 .
- Payne , L.N. , Franzier , J.A. and Powell , P.C. 1976 . Pathogenesis of Marek's disease . International Review of Experimental Pathology , 16 : 59 – 154 .
- Renz , K.G. , Cheetham , B.F. , Walkden-Brown , S.W. 2008 . Association between meq gene sequence and virulence in Australian and international isolates of Marek's disease virus . In Proceedings of the 8th International Marek's Disease Symposium . James Cook University, Townsville, Australia .
- Schat , K.A. , Chen , C.L , Calnek , B.W. and Char , D. 1991 . Transformation of T-lymphocyte subsets by Marek's disease herpes virus . Journal of Virology , 65 : 1408 – 1413 .
- Shek , W.R. , Calnek , B.W. , Schat , K.A. and Chen , C-L.H. 1983 . Characterization of Marek's disease virus infected lymphocytes: Discrimination between cytolytically and latently infected cells . Journal of the National Cancer Institute , 70 : 485 – 491 .
- Wei , P. , He , X.M. , Wang , G.J. , Li , K.R. , Liu , Y.L. Jiang , L.Y. 2002 . The development of rapid differential diagnosis technique for avian tumors . Journal of Yangzhou University (Agriculture and Life Science Edition) , 23 ( 2 ) : 1 – 5 .
- Witter , R.L. 1998 . The changing landscape of Marek's disease . Avian Pathology , 27 : S46 – S53 .
- Witter , R.L. 2001 . Protective efficacy of Marek's disease vaccines . Current Topics in Microbiology and Immunology , 255 : 57 – 90 .
- Zuo , T.R. , Zhao , Z.Y. , Wei , P. , Wei , X.X. , Li , Y. and Mo , M.L. 2007 . Isolation and identification of a field isolate of Marek's disease virus with acute oncogenicity . Chinese Journal of Virology , 23 : 218 – 223 .