Abstract
Immunoblotting analysis of wheat allergens can be a two-step procedure: an incubation with a human serum and one with an anti-IgE-horseradish peroxidase (HRP) conjugate, visualised by chemiluminescence. Different procedures produced side reactions that appeared as non-specific additional bands which hampered the identification of specific IgE-reacting proteins. We then showed that the rabbit anti-human IgE-HRP conjugate most likely contains anti-wheat antibodies that bind with the proteins. We investigated another procedure to accurately identify wheat specific IgE-reacting proteins. The use of polyvinylpyrrolidone-40 throughout the procedure and 3% cow milk for the conjugate incubation provided signals clear-cut enough to detect proteins up to the allelic level. Tested against patients, the procedure identified IgE-reacting proteins. It also provided new data in terms of gliadins for patients suffering from hypersensitivity to hydrolysed wheat proteins. Lastly, we investigated why dried cow milk blocks. Using rabbit serum containing anti-wheat antibodies, we detected wheat cross-reacting polypeptides in it.
Introduction
Wheat allergies can be triggered simply by inhaling wheat flour (baker's asthma; Baur & Posch, Citation1998), by coming into contact with wheat-derived products (Lauriere et al., Citation2006; Leduc, Moneret-Vautrin, Guerin, Morisset, & Kanny, Citation2003; Pecquet, Lauriere, Huet, & Leynadier, Citation2002) and by ingesting wheat-containing products, such as wheat-dependant exercise induced anaphylaxis (WDEIA) (Varjonen, Vainio, & Kalimo, Citation1997). In each case, different allergens are involved (Mittag et al., Citation2004). These allergens are wheat proteins found in the grain that are divided into two groups: albumins and globulins form one group, and they are soluble in water or salt containing solutions, respectively. Prolamins form the second group, and they are insoluble under the former conditions but are soluble in concentrated alcoholic solutions. Prolamins are further divided into gliadins and glutenins which are themselves divided into low-molecular weight glutenin sub-units (LMW-GS) and high-molecular weight glutenin sub-units (HMW-GS). Gliadins are monomeric proteins divided into α/β-, γ- and ω-gliadins according to their decreasing acid-polyacrylamide gel electrophoresis (PAGE) mobility, ω1–ω5 referring to the acid-PAGE mobility of individual bands (Kasarda, Autran, Lew, Nimmo, & Shewry, Citation1983). Albumins/globulins, gliadins and LMW-GS have been shown to be major IgE-binding proteins in various wheat allergies (Battais et al., Citation2003). Most of the allergens involved in baker's asthma are either albumins or globulins (Amano et al., Citation1998). Omega-5 gliadins have been identified as major allergens in WDEIA (Palosuo et al., Citation1999) and some LMW-GS have been identified as strongly IgE reacting for this same allergy (Mittag et al., Citation2004). Some IgE-reacting proteins have been reported (Lauriere et al., 2006, 2007; Snegaroff et al., Citation2007) in the case of immediate hypersensitivity to hydrolysed wheat proteins (IHHWP) by either skin contact (c-IHHWP) or by skin contact and food ingestion (c/f-IHHWP). For this latter allergy, ω1,2 gliadins have been shown to be the main IgE-reacting proteins (Lauriere et al., Citation2007).
Immunoblotting analysis is used for the detection of IgE-reacting proteins in the case of wheat allergies. It can be a two-step procedure. The first step involves the incubation of a membrane with the human serum diluted in a blocking reagent. The second step involves the incubation of the given membrane with an anti-IgE antibody linked with an enzyme horseradish peroxidase (HRP) diluted in a blocking reagent. The incubation with the substrate of HRP induces a reaction that reveals the proteins that bind to IgE. For washing throughout both steps of the procedure, blocking reagents are also used. These reagents are usually polyvinylpyrrolidone-40 (PVP-40), dried cow milk, or other proteins such as BSA or gelatine.
When confronted with very low levels of IgE, such as in the case of allergies, very sensitive detection methods like radioactivity or chemiluminescence are used to “see” the proteins that bind. Bird, Gearing, and Thorpe (Citation1988), Lasne (Citation2003) and Maruyama et al. (Citation1998) have all encountered problems with immunoblotting analysis. To investigate similar problems in our case, we first compared three protocols based on three commercially available immunoblotting analysis kits. We report here that in the case of these three kits, the immunoblotting procedures produce side reactions that appear as non-specific additional bands which hamper the identification of specific IgE-reacting proteins.
Barnes, Johnson, Blears, Harvey, and Finn (Citation1988) and Rumbo, Chirdo, Anon, and Fossati (Citation1998) have found that antibodies specific to food antigens are present in human serum. Following their lead, we asked whether or not the problems arising with the immunoblotting-chemiluminescence procedure were due to antibodies in the conjugate used in the second step as they are commercialised thanks to animals. More specifically, we asked if rabbit anti-IgE-HRP conjugate could contain anti-wheat antibodies that would bind with the proteins on the membrane, causing the side reactions which appear as non-specific additional bands. Once the animal conjugate was found to be the culprit (in our case the rabbit) and given the cost or the complexity of other methods now available to overcome this problem, we set out to design and verify an easy and cost-efficient procedure that would produce signals clear-cut enough to allow an accurate identification of specific IgE-reacting proteins. We investigated the use of different reagents in the two steps of the procedure as well as different percentages of dried cow milk in the second step to provide the fine-tuned procedure presented here.
We then asked if the proposed procedure would hold up under testing with hospital patients. We thus verified the procedure using sera of hospital patients suffering from a wide range of allergies. In addition, this procedure also provided new data about patients suffering from c-IHHW. Lastly, we investigated why cow milk blocks and more specifically, what compounds were preventing the side reactions that were veiling the identification of specific IgE-reacting proteins.
Materials and methods
Materials
All reagents were of analytical grade. Rabbit anti-human IgE-horseradish peroxidase (HRP) conjugated antibodies were supplied by Dako SA (Trappes, France). Goat anti-human IgE-alkaline phosphatase (AP) conjugated antibodies and PVP-40 was supplied by Sigma (St Louis, MO). Goat anti-rabbit IgG-HRP conjugated antibodies were supplied by Bio-Rad laboratories (Hercules, CA). SuperSignal West Dura Extended Duration® substrate was from Pierce Biotechnology (Rockford, IL). Aurora™ western blot chemiluminescent detection kit was from MP Biomedicals (Irvine, CA). Opti-4 CN substrate kit and western blot amplification module were from Bio-Rad Laboratories (Hercules, CA). Dried defatted milk was from Régilait (St Martin Belle Roche, France). Blocking reagent from Roche Diagnostics (Bâle, CH) was also tested. Black Indian drawing ink was from Pelikan AG (Hanover, Germany). Immobilon-P™, a polyvinylidene difluoride (PVDF) membrane, was purchased from Millipore Corporation (Bedford, MA) and NuPAGE precast gels and buffers were purchased from Invitrogen (Carlsbad, CA). X-OMAT AR-5 films were purchased from GE Healthcare (Buckinghamshire, GB).
Human sera
A high-wheat specific IgE titre serum (Pharmacia CAP, Class 6) was used for the research and development of the procedure. It came from a patient (male, 45 years old) with allergic rhinitis. It was purchased from PlasmaLab International (Everett, WA).
To confirm whether or not the procedure would hold up under testing, 34 adult patients were selected: 10 patients (No. 1–10), identified at Cochin Hospital (Paris, France) and Tenon Hospital (Paris, France), were suffering from baker's asthma; 13 patients (No. 11–23), identified at Tenon Hospital and Delafontaine Hospital (St Denis, France), were suffering from characterised WDEIA triggered by unmodified wheat products; and five patients (No. 24–28), identified at Tenon Hospital, Saint-Jacques Hospital (Besançon, France) and in Martigues (France) were suffering from c-IHHWP. Six patients (No. 29–34), identified at Tenon Hospital, St Eloi Hospital (Montpellier, France) and Saint-Jacques Hospital, were suffering from c/f-IHHWP. The present study received the consent of the patients and the approval of the Cochin Hospital Ethical Committee.
Animal serum
Rabbit anti-prolamins polyclonal serum was raised against purified ω-gliadins from hexaploid wheat (Triticum aestivum) cultivar (cv. Capelle) and was characterised as specifically reacting with all prolamins from this cereal, except HMW-GS.
Protein extracts
Wheat or milk total proteins were extracted, respectively, from 40 mg of a blend of four flours from hexaploid wheat (Triticum aestivum) or from 5.5 mg of dried defatted milk by gentle mixing with 1 ml of Laemmli's sample buffer (Laemmli, Citation1970), 63 mM tris-HCl pH 6.8, 10% (w/v) glycerol, 2% (w/v) SDS containing 5% (v/v) 2-mercaptoethanol, for 1 hour, then boiled for 5 min. After centrifugation (5 min at 11,000 g), the supernatant was recovered.
Wheat albumins and globulins were extracted according to Marion, Nicolas, Popineau, Branlard, and Landry (Citation1994) and gliadins and glutenins sub-fractions according to Singh, Shepherd, and Cornish (Citation1991).
Ethanol-soluble proteins from milk were first extracted for 1 hour by adding 44 ml of Laemmli's sample buffer containing 5% (v/v) 2-mercaptoethanol to 5 g of dried milk. Then 118 ml of ethanol 96% (v/v) was added to the slurry and further mixed for 1 hour. After centrifugation at 12,000 g for 30 min, supernatant was collected. An aliquot containing 20 µg of proteins was evaporated and solubilised in Laemmli's sample buffer containing 5% (v/v) 2-mercaptoethanol for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotting
For the analysis of the reactivity of wheat allergic patient sera on wheat protein extracts, SDS-PAGE was carried out according to Singh et al. (Citation1991). For the other analyses, SDS-PAGE was performed using NuPAGE precast 4–12% polyacrylamide gradient gels with MOPS buffer according to the recommendations of the manufacturer. Analyses were performed on 20 µg of proteins.
Electroblottings were carried out under semi-dry conditions using the protocol of Lauriere (Citation1993) on PVDF membranes. This protocol allowed an almost complete transfer of the proteins from the gel onto the membrane.
Immuno-probing for human IgE
In preliminary experiments, three protocols, which combined two types of blocking reagents, two anti-IgE conjugates and three detection systems, were compared. They were used according to the manufacturer's instructions and are given in the legends of the figures.
Standard procedure: PVDF membranes were first saturated for 2 hours in 50 mM phosphate buffer, pH 7.4, 150 mM NaCl, 0.1% (w/v) Tween 20 (PBST) and 2% (w/v) PVP-40 (PBST-PVP40). They were then incubated overnight at 4°C with human serum diluted 10 times in PBST-PVP40. After three washing steps, the rabbit anti-human IgE-HRP conjugate was added, at 1/25,000 in PBST, 3% (w/v) dried cow milk, for 2 hours. After seven washing steps, the membranes were incubated for 5 min with the substrate of the conjugated enzyme. All washing steps lasted 10 min each in PBST-PVP40, except the last two, which were in PBS. All steps were under gentle rocking, except staining, and performed at room temperature, except the incubation with the patient serum. The fine-tuning of the proposed procedure, involving the blocking buffers, conjugate dilutions and incubation times, is reported in the corresponding legends of the figures.
After immuno-detection, localisation of proteins on the membrane was performed, using Indian ink staining according to the protocol established by Eynard and Lauriere (Citation1998) and Hancock and Tsang (Citation1983).
Immuno-probing for rabbit IgG
The standard procedure was used except that membranes were incubated 1 hour at 4°C with rabbit anti-prolamin serum diluted 1000 times in PBST-PVP40 and the goat anti-rabbit IgG-HRP conjugate was incubated for 2 hours, at 1/125,000 in PBST, 5% (w/v) dried cow milk.
Chemiluminescence detection
For human IgE detection, chemiluminescence was recorded on X-OMAT AR-5 film as for autoradiography according to Eynard and Lauriere (Citation1998). For rabbit IgG detection, chemiluminescent signals were recorded using the luminescent image analyser LAS-3000 supplied by Fujifilm (USA).
Results
Evidence of non-specific reactions of anti-IgE conjugate with wheat proteins
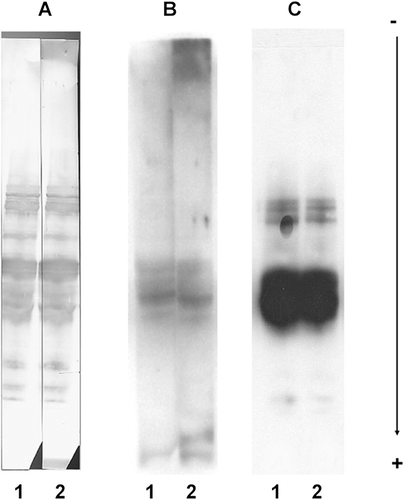
In preliminary experiments set up to identify wheat allergens, three different protocols adapted to the very low level of IgE in human serum were compared. They were based on SDS-PAGE of wheat proteins, blotted on PVDF membranes and immuno-probed with patient's sera (, lane 1) or without patient's sera for the control (, lane 2). The protocols differed mainly by the final reporting reaction, namely a colorimetric method, using the Opti-4CN detection kit (panel A) and two chemiluminescence methods based on, respectively, the Aurora detection kit (panel B) and the SuperSignal West Dura Extended Duration® substrate (panel C). They also differed by the given blocking reagents, either from Roche Diagnostics or from the Aurora detection kit. For all these protocols, bands were elicited but their patterns differed, depending on the given anti-IgE conjugate and the detection method used. Surprisingly, the pattern did not differ between the assay (lane 1) and the control (lane 2). This is evidence of the presence of non-specific reactions due to the anti-IgE conjugated reagents causing side effects. In our case, due to their high intensity (compare lanes 1 and 2, panels A, B and C in ), these unspecific signals were veiling the specific ones, and they could not be subtracted from the whole signal (as suggested by Maruyama et al., Citation1998).
Figure 1. Common side reactions due to secondary antibodies. Direct immuno-probing of blotted total wheat proteins, with human sera (lane 1) or without human sera (for control, lane 2) along with different secondary antibody preparations and quenching reagents. Reactions were detected with, respectively: (A) Bio-Rad Opti-4CN detection kit (rabbit anti-human IgE-HRP at 1/1000, blocking reagent from Roche Diagnostics, washings in PBST); (B) Aurora detection kit (goat anti-human IgE-AP at 1/10,000, blocking reagent from the kit, washings in blocking reagent); (C) Pierce SuperSignal West Dura Extended Duration® 8 substrate (rabbit anti-human IgE-HRP at 1/10,000, blocking reagent from the Aurora detection kit, washings in PBST). B and C are chemiluminescence methods, whereas A is a colorimetric method.
Attempts to overcome the unspecific reactions from the secondary antibody preparations
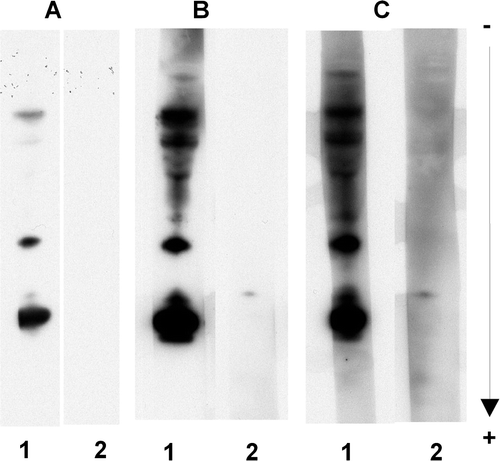
To test a new procedure to overcome the unspecific reactions, we investigated the use of different reagents in the two steps of the procedure as well as different percentages of dried cow milk in the second step. Dilution to the limit of detection of the anti-IgE conjugate reagents, up to the dilution 1/100,000, had no effect. The use of Triton X100 instead of Tween 20 in PBST, at the concentration 0.1% (w/v) and 0.3% (w/v) Triton X100, led to no improvements (results not shown). The blocking reagent PVP-40 recommended by Battais et al. (Citation2003), when used alone at 2% (w/v) PVP-40 in PBST for all incubation and washing steps, also had no effect on the non-specific reactions (A, lane 2). On the contrary, the non-specific reactions of the anti-IgE conjugate reagent disappeared when PVP-40 was replaced by 5% (w/v) dried cow milk, a well known blocking reagent (B, lane 2). However, the chemiluminescent signal became weaker. As the anti-IgE conjugate reagents were thought to be triggering the non-specific reaction, a combination of the two last assays was tested (C). These conditions were 5% (w/v) dried cow milk in PBST added only in the anti-IgE conjugate incubation step and 2% (w/v) PVP-40 in PBST in all other steps. Under these latter conditions, the specific signal increased (B and C, lanes 1) and no non-specific reactions occurred in the control performed with no patient serum (B and C, lanes 2). As dried cow milk showed effects on both specific and non-specific reactions, to fine-tune the procedure, various concentrations were tested (). A weak concentration of dried cow milk (1%) increased the background noise (C), whereas a high one (5%) decreased the specific response (A, lane 1). We found that 3% (w/v) of dried cow milk at the anti-IgE conjugate incubation step (B) was the optimum concentration to provide the most clear-cut chemiluminescent signal without any non-specific reactions due to the anti-IgE conjugate reagent, and without background staining.
Figure 2. Effects of PVP-40 and dried cow milk as blocking reagents. Immunoblotting analyses of total wheat proteins were carried out by adding to PBST, at all steps, the following blocking reagents: panel A, 2% (w/v) PVP-40; panel B, 5% (w/v) dried milk; panel C, as for A, except that 5% (w/v) dried milk, instead of PVP-40, was added at only the incubation step with the secondary antibodies, to adsorb interfering antibodies. Lane 1, immuno-probing successively with wheat-allergic patient serum (1/10) and anti-human IgE-HRP conjugate (1/100,000); Lane 2, control, without incubation with patient serum. Reactions were recorded using the SuperSignal West Dura Extended Duration® 19 substrate.
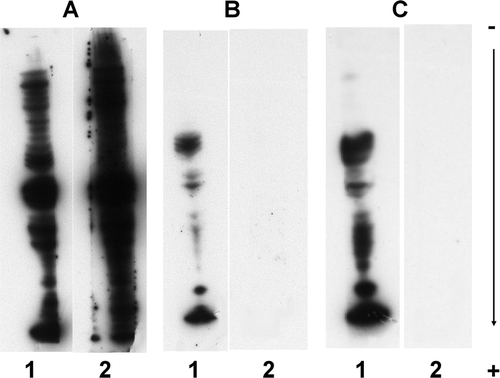
Figure 3. Effects of dried cow milk concentrations on both specific and side reactions. The conditions of the analyses were the same as panel C in , except that the concentration of dried cow milk added to PBST for the incubation with the secondary antibody preparation was: (A) 5% (w/v); (B) 3% (w/v); and (C) 1% (w/v). Reactions were recorded using the SuperSignal West Dura Extended Duration® 25 substrate.
Testing the procedure to detect IgE-reacting wheat proteins in hospital patient sera
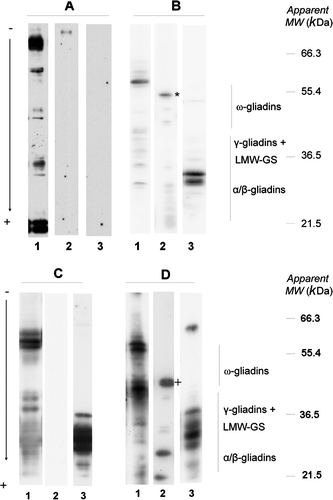
Three elements of wheat proteins (albumins–globulins, gliadins and glutenins) were processed using the standard procedure with sera from patients suffering from Baker's asthma, WDEIA, c-IHHWP and c/f-IHHWP. Differences of IgE specificity were observed between patients but constant features were also observed. They are summarised in . Almost all patients tested displayed IgE reacting with components of the albumin–globulin fraction and to a lesser extent with components of the LMW-GS fraction. Few of them reacted with HMW-GS components. None of the patients suffering from Baker's asthma or c-IHHWP reacted with gliadins. To the contrary, most of the patients (five out of six) suffering from c/f-IHHWP, and all patients with WDEIA, reacted with gliadins. Representative results from selected patients are shown in . The patterns of IgE reactivity with albumins and globulins (, lanes 1) displayed a high variability among all patients. For all these patients, HMW-GS was not IgE reacting (results not shown). However, typical IgE reactivity with gliadins and LMW-GS was observed in patients with WDEIA in which ω5-gliadins and two typical bands of LMW-GS were IgE reacting (B, lanes 2 and 3, respectively). IgE from c/f-IHHWP patients reacted with ω1.2-gliadins and with various LMW-GS (D, lanes 2 and 3, respectively).
Table 1. Frequency of sera reacting with the three main wheat protein groups given different wheat allergies.
Antibodies in the patient serum and in the rabbit anti-human IgE conjugate reacted specifically with dried cow milk proteins
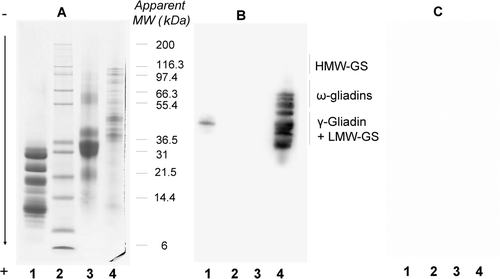
To investigate why dried cow milk blocks, we separated total proteins from this reagent using SDS-PAGE and then blotted them on PVDF membranes. Firstly, they were directly immuno-probed with only rabbit anti-human IgE-HRP, either in the presence of 2% (w/v) PVP-40 in PBST at all steps (A) or under the same conditions but with 3% (w/v) dried cow milk instead of PVP-40 in the case of the antibody incubation medium (B). Two major milk protein bands were elicited in the first experiment. These reactions were inhibited when milk proteins were present in the incubation medium (B). This suggested the presence of antibodies in the rabbit anti-human IgE-HRP reacting with peptides or proteins present in the dried cow milk.
Figure 4. Reactivity of rabbit anti-human IgE-HRP conjugate and wheat-allergic patient serum on a milk total protein extract. Milk proteins, separated using SDS-PAGE and blotted on PVDF, were immuno-probed as follows. Lane A, immuno-probing with only the secondary antibody preparation using PBST with 2% (w/v) PVP-40 at all steps. Lane B, idem A except that reactive immunoglobulins of the secondary antibody preparation were adsorbed with 3% (w/v) dried milk. Lane C, immnuno-probing with the patient serum using the standard procedure. Reactions were recorded using the SuperSignal West Dura Extended Duration® 34 substrate. Lane D, Indian ink staining of proteins present in dried cow milk.
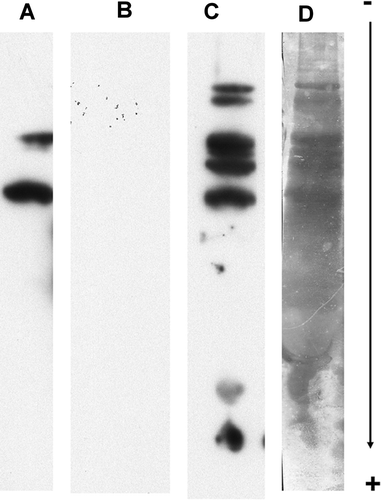
Secondly, the same milk total proteins were immuno-probed with the patient serum and processed according to the new procedure (C). Several bands were elicited which also demonstrated that patient IgE reacted with peptides or proteins present in the dried cow milk. Among all the proteins revealed by Indian ink staining (D), a few had reacted.
Detection of wheat-derived polypeptides in dried cow milk
To further understand why dried cow milk blocks, a total milk protein extract and a sub-fraction of it, soluble in ethanol, were compared to a total wheat protein extract using SDS-PAGE and either immunoblotting or staining with Coomassie blue after SDS-PAGE ().
Milk proteins from the ethanol extract (A, lane 1) or from the total extract (A, lane 3) stained with Coomassie blue revealed a complex pattern. The ethanol-soluble proteins from the dried cow milk (A, lane 1) corresponded to polypeptides below 35 KDa, thus demonstrating the elimination, in this extract, of most of the milk proteins by ethanol precipitation. Immunoblotting experiments were performed using rabbit anti-prolamin antibodies. As expected, the antibodies reacted with all prolamins of the wheat protein extract except HMW-GS (B, lane 4). They also reacted with a minor component with an apparent molecular weight of 45 kDa in the ethanol milk extract (B, lane 1). However, no reaction occurred with proteins of the total milk extract, probably due to the overly high amount of soluble milk proteins which could hide wheat polypeptides on the blot (B, lane 3). As expected, no reaction was observed on a control blot processed under the same conditions but without rabbit anti-prolamin antibodies (C). Unspecific linkages, like hydrophobic bonds between prolamins and rabbit IgG, could theoretically be involved. This possibility was not thought to be so in our case for two reasons: first of all, unspecific linkages could also occur between other proteins from the wheat or the milk and rabbit IgG. However, this was not the case as no response was obtained with HMW-GS (which has an amino-acid composition close to other prolamins) and proteins from the total milk extract. Moreover, the absence of background showed that the washings and blocking steps were efficient. These experiments demonstrated that, in the dried cow milk, we detected at least one polypeptide soluble in alcohol and antigenically related with prolamins.
Figure 5. Reactivity of rabbit anti-prolamin serum on wheat and milk protein extracts. Ethanol-soluble milk proteins (lane 1), molecular weight marker (lane 2), total milk proteins (lane 3) and total wheat proteins (lane 4) were separated using SDS-PAGE and stained with Coomassie brilliant blue (panel A) or blotted on PVDF and immuno-probed with a rabbit anti42 prolamin serum (panel B) or without a control (panel C) as described in the material and methods. Reactions were recorded using the SuperSignal West Dura Extended Duration® 43 substrate.
Discussion
Anti-human IgE conjugate may contain interfering anti-wheat protein antibodies
Side reactions are a common problem in immunoblotting (Lasne, Citation2003; Maruyama et al., Citation1998). The use of very sensitive detection methods such as chemiluminescence to detect very low levels of IgE – for example, in the case of wheat allergies – produce side reactions that appear as non-specific additional bands which hamper the identification of specific IgE-reacting proteins. Those described in this paper only occurred when anti-IgE conjugates were used, and they depended on the given methods of detection (). These methods differed according to the type of conjugate, the type of blocking reagent and the degree of detection sensitivity. Changing the dilution of the sera and the secondary antibody, increasing concentrations of the non-ionic detergents Tween 20 and Triton X100, or using various blocking reagents, except milk, did not eliminate side reactions. BSA, a well known blocking reagent, was not used due to such drawbacks as its cost as well as the specific requirements that must be met for its elimination. The same side reactions (results not shown) were found when a goat anti-IgG-PA conjugate was experimented with. All these observations suggested that compounds present in the sera of the animal used for the conjugate production (the rabbit in our case) may be responsible for the side reactions. The proteins under analysis on the blots were wheat proteins. They can be found in the feed of numerous animals, including rabbit. Rumbo et al. (Citation1998) have found that antibodies against dietary proteins are present in human blood serum and milk. Following their lead, it is reasonable to assume that small amounts of anti-wheat protein antibodies were present alongside the anti-IgE antibodies in the rabbit serum and were conjugated to the reporter enzyme during the preparation of the conjugate. The presence of such anti-wheat protein antibodies would explain the side reactions observed when blots were incubated with only the anti-IgE conjugate in the control. The anti-human IgE used was commercially purified using either a solid-phase absorption with human plasma protein for the anti-human IgE/HRP or an immunospecific purification that removes essentially all goat serum proteins for the anti-human IgE/PA. We have thus shown that this level of purification is inadequate when studying wheat allergens.
Development of an easy and cost-efficient procedure to specifically detect IgE reacting wheat proteins
To overcome the unspecific reactions, we investigated the use of different reagents in the two steps of the procedure. The use of PVP-40 in the first step to prevent interferences in the fixation of IgE on wheat proteins; and 3% dried cow milk in the second step to adsorb interfering antibodies present in the conjugate, provided the most clear-cut signal without any non-specific reactions. It is an easy procedure to specifically detect IgE-reacting wheat proteins and it is much more cost-efficient than a monoclonal HRP-conjugated anti-human IgE antibody.
The procedure was then tested against the sera of hospital patients suffering from a wide range of well-characterised wheat allergies. It allowed us to confirm that patients suffering from Baker's asthma displayed IgE reactivity with albumins/globulins (Amano et al., Citation1998). Those suffering from WDEIA displayed IgE reactivity with ω5-gliadins, some specific allelic forms of ω-gliadins, and with LMW-GS as already described (respectively, Palosuo et al., Citation1999 and Mittag et al., Citation2004). Thus, when associated with high-resolutive electrophoresis, the procedure differentiates IgE-reacting proteins up to the allelic level (). By this mean, Lauriere et al. (2007) observed genetic differences in ω-gliadins involved in two different immediate food hypersensitivities to wheat. In addition, the procedure provided new data for patients suffering from c/f-IHHWP and c-IHHWP. In the case of c/f-IHHWP, Lauriere et al. (2007) have already shown that IgE anti-ω1,2 gliadins are present in the patient sera. In our study, in the case of both allergies, numerous LMW-GS and albumins–globulins were also found to be IgE reacting; however, they displayed different patterns. Moreover, patients suffering from c-IHHWP had no IgE directed against gliadins, strongly differentiating these two allergies.
Figure 6. Reactivity of wheat-allergic patient sera on wheat protein extracts. Albumins/globulins from wheat (lane 1), gliadins (lane 2) and glutenins (lane 3) were separated using SDS-PAGE, blotted on PVDF, and immuno-probed with the serum from patients No. 1 suffering from baker's asthma (panel A), No. 11 from WDEIA (panel B), No. 24 from c-IHHWP (panel C) and No. 29 from c/f-IHHWP (panel D). Reactions 50 were recorded using the SuperSignal West Dura Extended Duration® 51 substrate.*ω5-gliadins; +ω1,2-gliadins.
Evidence for the presence of wheat-derived cross-reacting polypeptides in dried cow milk
During the first step of the procedure, specific compounds in the dried cow milk could compete with wheat proteins fixed on the blotted membrane, for specific IgE interactions, thus explaining the weakness of the specific signals in our case as shown in A. Similarly, during the second step, these compounds could adsorb the anti-wheat protein antibodies present in the anti-human IgE conjugate as shown in B, 2C, 3 and 4B. We set out to identify these compounds. Although a competition of milk IgA and IgG with IgE for binding of wheat proteins cannot be fully excluded, the simplest explanation compatible with the results in B and 3 could be the presence of cross-reacting polypeptides in the dried milk that would interact with both the anti-wheat protein IgE present in the patient serum during the first step and the anti-dietary wheat protein antibodies present in the rabbit anti-human IgE conjugate during the second step. The presence of antibodies reacting with polypeptides present in the dried cow milk was effectively evidenced in both the patient serum and the conjugate ().
To confirm that these polypeptides were antigenically related to wheat proteins, we looked for polypeptides from prolamins in the dried cow milk. Prolamins are the most abundant proteins in the wheat grain. They are specifically soluble in concentrated alcoholic solutions, like 75% v/v ethanol. Rabbit anti-prolamin antibodies were used directly on total milk proteins or after selective extraction using 75% v/v ethanol (). One minor component of the alcohol soluble fraction of milk proteins reacted with the antibodies. It corresponded to 0.016% of the total proteins from the dry milk, when evaluated with a semi-quantitative method. The solubility and the antigenicity of this component present in the dried cow milk strongly suggested it originated from wheat prolamins. It displayed a relative molecular weight similar to that of γ-gliadins or LMW-GS. To our knowledge, this is the first demonstration of the natural presence of a polypeptide cross-reacting with wheat proteins in dried cow milk, most likely of dietary origin. Wheat-derived polypeptides, originating from the human diet, have already been reported in human blood or breast milk (Chirdo, Rumbo, Anon, & Fossati, Citation1998; Kilshaw & Cant, Citation1984; Linna, Citation1996; Troncone et al., Citation1987). Animal feed appears to trigger the same phenomenon, thus explaining why, in our procedure, dried cow milk blocks.
Conclusion
In immunoblotting, in the course of wheat allergen detection, when using very sensitive detection methods like chemiluminescence, side reactions may hamper the identification of specific IgE-reacting proteins. In our case, they appeared as non-specific additional bands. Like in human sera, our results suggest that antibodies against dietary wheat proteins may be present in the serum from the animal (rabbit in our case) used to produce anti-human IgE conjugate and they may cause these side reactions. Indeed, wheat proteins are food proteins which can diffuse throughout the body of people and animals. To overcome side reactions, we have designed an easy and cost-efficient procedure to provide signals clear-cut enough to allow identification of IgE-reacting proteins up to the allelic level. It is based on two different blocking reagents: PVP-40 in the first step used to prevent interferences in the fixation of IgE on wheat proteins; and dried cow milk in the second step used to adsorb interfering antibodies present in the conjugate. The procedure was verified by using sera from hospital patients suffering from well-characterised wheat allergies. IgE-reacting wheat proteins known to be involved in these different allergies were accurately detected. In addition, the procedure provided new data for patients suffering from c-IHHWP. Contrary to those suffering from c/f-IHHWP, they had no IgE directed against gliadins. The absence of detectable interferences simplified the comparison of the reactivity patterns for patients with similar allergies and allowed the detection of common trends or differences up to the allelic level for potential allergenic proteins. Lastly, we sought out what compounds in the dried cow milk could adsorb anti-wheat protein antibodies present in the conjugate. Dried cow milk, like human breast milk, most likely contains dietary wheat cross-reacting polypeptides which bind these antibodies thereby preventing side reactions. More generally speaking, it may be fruitful to further investigate the risk of non-specific reactivity when using reagents originating from animal fluids.
Acknowledgements
Part of this work was supported by the French Ministère de la Recherche, contract AQS No. 01P0622.
References
- Amano , M. , Ogawa , H. , Kojima , K. , Kamidaira , T. , Suetsugu , S. , Yoshihama , M. , Satoh , T. , Samejima , T. and Matsumoto , K. 1998 . Identification of the major allergens in wheat flour responsible for baker's asthma . Biochemical Journal , 330 ( Pt 3 ) : 1229 – 1234 .
- Barnes , R.M.R. , Johnson , P.M. , Blears , J. , Harvey , M.M. and Finn , R. 1988 . Human-serum antibodies reactive with dietary proteins-antigenic specificity . International Archives of Allergy and Applied Immunology , 87 : 189 – 193 .
- Battais , F. , Pineau , F. , Popineau , Y. , Aparicio , C. , Kanny , G. , Guerin , L. , Moneret-Vautrin , D.A. and Denery Papini , S. 2003 . Food allergy to wheat: Identification of immunoglobulin E and immunoglobulin G-binding proteins with sequential extracts and purified proteins from wheat flour . Clinical & Experimental Allergy , 33 ( 7 ) : 962 – 970 .
- Baur , X. and Posch , A. 1998 . Characterized allergens causing bakers’ asthma . Allergy , 53 : 562 – 566 .
- Bird , C.R. , Gearing , A.J. and Thorpe , R. 1988 . The use of Tween 20 alone as a blocking agent for immunoblotting can cause artefactual results . Journal of Immunological Methods , 106 : 175 – 179 .
- Chirdo , F.G. , Rumbo , M. , Anon , M.C. and Fossati , C.A. 1998 . Presence of high levels of non-degraded gliadin in breast milk from healthy mothers . Scandinavian Journal of Gastroenterology , 33 : 1186 – 1192 .
- Eynard , L. and Lauriere , M. 1998 . The combination of Indian ink staining with immunochemiluminescence detection allows precise identification of antigens on blots: Application to the study of glycosylated barley storage proteins . Electrophoresis , 19 : 1394 – 1396 .
- Hancock , K. and Tsang , V.C.W. 1983 . India ink staining of proteins on nitrocellulose paper . Analytical Biochemistry , 133 : 157 – 162 .
- Kasarda , D.D. , Autran , J.C. , Lew , E.J.L. , Nimmo , C. and Shewry , P.R. 1983 . N-terminal amino acid sequences of omega-gliadins and omega-secalins. Implications for the evolution of prolamin genes . Biochimica et Biophysica Acta , 747 : 138 – 150 .
- Kilshaw , P.J. and Cant , A.J. 1984 . The passage of maternal dietary proteins into human breast milk . International Archives of Allergy and Applied Immunology , 75 : 8 – 15 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 ( 5259 ) : 680 – 685 .
- Lasne , F. 2003 . Double-blotting: A solution to the problem of nonspecific binding of secondary antibodies in immunoblotting procedures . Journal of Immunological Methods , 276 : 223 – 226 .
- Lauriere , M. 1993 . A semidry electroblotting system efficiently transfers both high- and low-molecular-weight proteins separated by SDS-PAGE . Analytical Biochemistry , 212 : 206 – 211 .
- Laurière , M. , Pecquet , C. , Bouchez-Mahiout , I. , Snégaroff , J. , Bayrou , O. , Raison-Peyron , N. and Vigan , M. 2006 . Hydrolysed wheat proteins present in cosmetics can induce immediate hypersensitivities . Contact Dermatitis , 54 ( 5 ) : 283 – 289 .
- Laurière , M. , Pecquet , C. , Boulenc , É. , Bouchez-Mahiout , I. , Snegaroff , J. , Choudat , D. , Raison-Peyron , N. , Vigan , M. and Branlard , G. 2007 . Genetic differences in omega-gliadins involved in two different immediate food hypersensitivities to wheat . Allergy , 62 : 890 – 896 .
- Leduc , V. , Moneret-Vautrin , D.A. , Guerin , L. , Morisset , M. and Kanny , G. 2003 . Anaphylaxis to wheat isolates: Immunochemical study of a case proved by means of double-blind, placebo-controlled food challenge . Journal of Allergy and Clinical Immunology , 111 : 897 – 899 .
- Linna , O. 1996 . Specific IgE antibodies to uningested cereals . Allergy , 51 : 849 – 850 .
- Marion , D. , Nicolas , Y. , Popineau , Y. , Branlard , G. and Landry , J. 1994 . Wheat kernel proteins molecular and functional aspects , Viterbo, , Italy : Universita Degli Studi della Tuscia Consiglio Nationale della Ricerche .
- Maruyama , N. , Ichise , K. , Katsube , T. , Kishimoto , T. , Kawase , S. , Matsumura , Y. , Takeuchi , Y. , Sawada , T. and Utsumi , S. 1998 . Identification of major wheat allergens by means of the Escherichia coli expression system . European Journal of Biochemistry , 255 : 739 – 745 .
- Mittag , D. , Niggemann , B. , Sander , I. , Reese , I. , Fiedler , E.M. , Worm , M. , Vieths , S. and Reese , G. 2004 . Immunoglobulin E-reactivity of wheat-allergic subjects (baker's asthma, food allergy, wheat-dependent, exercise-induced anaphylaxis) to wheat protein fractions with different solubility and digestibility . Molecular Nutrition & Food Research , 48 : 380 – 389 .
- Palosuo , K. , Alenius , H. , Varjonen , E. , Koivuluhta , M. , Mikkola , J. , Keskinen , H. , Kalkkinen , N. and Reunala , T. 1999 . A novel wheat gliadin as a cause of exercise-induced anaphylaxis . Journal of Allergy and Clinical Immunology , 103 ( 5 pt 1 ) : 912 – 917 .
- Pecquet , C. , Lauriere , M. , Huet , S. and Leynadier , F. 2002 . Is the application of cosmetics containing protein-derived products safe? . Contact Dermatitis , 46 : 123
- Rumbo , M. , Chirdo , F.G. , Anon , M.C. and Fossati , C.A. 1998 . Detection and characterization of antibodies specific to food antigens (gliadin, ovalbumin and beta-lactoglobulin) in human serum, saliva, colostrum and milk . Clinical & Experimental Allergy , 112 : 453 – 458 .
- Singh , N.K. , Shepherd , K.W. and Cornish , G.B. 1991 . A simplified SDS-PAGE procedure for separating LMW subunits of glutenin . Journal of Cereal Science , 14 : 203 – 208 .
- Snégaroff , J. , Branlard , G. , Bouchez-Mahiout , I. , Laudet , B. , Tylichova , M. , Choudat , T. , Raison-Peyron , N. , Vigan , M. , Kerre , S. and Laurière , M. 2007 . Recombinant proteins and peptides as tools for studying IgE reactivity with low-molecular-weight glutenin subunits in some wheat allergies . Journal of Agricultural and Food Chemistry , 55 : 9837 – 9845 .
- Troncone , R. , Scarcella , A. , Donatiello , A. , Cannataro , P. , Tarabuso , A. and Auricchio , S. 1987 . Passage of gliadin into human breast milk . Acta Paediatrica Scandinavica , 76 : 453 – 456 .
- Varjonen , E. , Vainio , E. and Kalimo , K. 1997 . Life threatening, recurrent anaphylaxis caused by allergy to gliadin and exercise . Clinical & Experimental Allergy , 27 : 162 – 166 .