Abstract
Six structurally similar sulfonamide haptens have been linked to ovalbumin by diazo-method used as coating antigen. An enzyme-linked immunosorbent assay (ELISA) was developed to investigate heterologous structure of coating haptens on sensitivity of ELISA for sulfamethazine. The sensitivities of ELISA, expressed as IC50 values, ranged from 94 to 877 ng mL− 1 when six coating antigens were employed. The results suggested that the structural heterology of coating hapten had significant effect on ELISA sensitivity. In order to evaluate the relationship between the degree of hapten heterology and ELISA sensitivity, we used molecular similarity methods to qualitatively represent the degree of coating hapten heterology. The Molecular Access System (MACCS) structural keys and the Tanimoto similarity coefficient were used to calculate and compare the degree of coating hapten's similarity, and the authors found that the sensitivity of ELISA was not in direct proportion with degree of coating hapten heterology in the case of study.
Introduction
Currently, competitive enzyme-linked immunosorbent assay (ELISA) is the mostly used bioanalytical method for the detection of small molecule such as pesticide, toxin, environmental contaminant and veterinary drug (Knopp, Citation2006; Morozova, Levashova, & Eremin, Citation2005; Pastor-Navarro, Morais, Maquieira, & Puchades, Citation2007). The strength of competition between antibody, analyte and coating antigen is of primary importance for sensitivity of ELISA. There are many factors that can influence the sensitivity of ELISA such as temperature, physico-chemical condition, etc. (Grant & Sporns, Citation2005; Kolar, Deng, & Franek, Citation2002); however, the heterology of coating hapten always exerts direct and significant relationship with ELISA sensitivity without considering the characteristics of antibody itself. Coating antigen heterology in competitive ELISA means that the immunising hapten is different from the coating hapten (Kim, Cho, Lee, & Lee, Citation2003a). The heterology of coating hapten often results in weaker decrease of recognition of antibody to the coating hapten compared to homogenous coating hapten, thus allowing the analyte at lower concentrations to compete with the heterogenous coating antigen and higher sensitivity can be obtained (Wang, Zhang, Nesterenko, Eremin, & Shen, Citation2007). The pioneering study made by Harrison stated that the heterology of coating antigen in competitive ELISA is favourable for analytical sensitivity (Harrison, Goodrow, & Gee, Citation1990). In the field of pesticide residue detection, the excellent contribution has been achieved by Hammock, B.D. group. They have reported that heterology of coating hapten structure, bridge length and structure and site attachment heterology, could significantly increase the sensitivity of ELISA (Goodrow & Hammock, Citation1998; Kim et al., Citation2003b; Schneider & Hammock, Citation1992). One excellent example was investigated by using herbicide benzoylphenylurea as molecule model (Siong & Hammock, Citation1984). Kim et al. also investigated several coating hapten of organophosphorus insecticide fenthion with different types of heterology on ELISA sensitivity, finding that the degree of hapten heterology parallels the degree of sensitivity (Kim et al., 2003a; Kim, Kim, Lee, & Lee, Citation2007). However, in these studies, the evaluation of diversity degree of hapten molecules was mostly based on subjective assessment of molecular similarity and not by precise definition and algorithm, thus, sometimes give subjective results. Nowadays, similarity method has been used widely in drug development for a variety of purposes. It can determine whether and how one molecule is similar to another one. The most commonly used similarity method is based on the 2D fingerprint like Molecular Access System (MACCS) structural keys, which consists of a bit string with values of one and zero representing the presence or absence of a particular fragment or fragment counts (Leach & Gillet, Citation2007). The MACCS structural keys were also found to encode information about hydrophobicity, electrostatics, sterics, dispersion interactions and hydrogen bonding that must be considered in antibody–antigen interaction, suggesting that it is appropriate to represent hapten molecule that interacts with antibody molecule (Brown & Martin, Citation1997). The similarity between two molecules represented by 2D fingerprints is frequently quantified using Tanimoto coefficient which gives a measure of the number of fragments in common between the two molecules (Leach & Gillet, Citation2007). Tanimoto coefficient places similarity on scale from 0 (most dissimilar) to 1 (most similar). Krasowski et al. applied similarity analysis to develop computational methods that can predict compound likely to cross-react within immunoassays used for clinical detection of drug of abuse or for therapeutic drug monitoring (Krasowski, Siam, Iyer, & Ekins, Citation2009a; Krasowski et al., Citation2009b).
Although the importance of heterology of coating antigen in competitive ELISA has been generally witnessed, to author's knowledge, there have been few special studies carried out to investigate the effect of hapten heterology on ELISA sensitivity, in particular combining with molecular similarity analysis. In the paper we present here, we used sulfamethazine (SMZ) as a model to investigate influence of heterologous coating haptens on ELISA sensitivity in virtue of molecular similarity analysis.
Experimental
Materials and instruments
SMZ (purity>99%), sulfamerazine (SMR; purity>99%), sulfadimethoxine (SDM; purity>99%), sulfadiazine (SDZ; purity>99%), sulfamethoxazole (SMX; purity>99%), sulfamonomethoxine (SMM; purity>99%) and ovalbumin (OVA; purity >98%) were purchased from Sigma Chemical Co. (St. Louis, MO). All other chemicals and solvents were of analytical grade or better and were obtained from Beijing Chemical Reagent Co. (Beijing, PRC). Deionised water was prepared using a Milli-Q water purification system (Millipore, Bedford, MA). The polyclonal antibody towards SMZ was previously produced by our group (Zhang, Wang, Nesterenko, Eremin, & Shen, Citation2007).
Polystyrene microtitre plates were purchased from Beijing Wanger Bio Tech. Co. (Beijing, PRC). Ultraviolet (UV)-visible spectra were recorded on a Varian Cary spectrophotometer (Thermo, USA). The ELISA plate reader was obtained from TECAN Inc. (Durham, NC, USA).
Standard solutions
Standard stock solutions (5 mg mL−1) of SMZ were prepared by dissolving an appropriate amount of SMZ in methanol. The individual stock solutions were stored at 4°C in amber glass bottles. Working standards (1, 5, 10,…, 1,00,000, 2,00,000 and 5,00,000 ng mL−1) of SMZ were prepared by diluting the stock solution in buffer.
Buffers and solution
The following buffers were used in the ELISA: (1) coating buffer was 0.05 M carbonate buffer, pH 9.6; (2) blocking buffer consisted of 0.02 M phosphate-buffered saline (PBS), pH 7.4, 0.5% casein, 1% BSA, 0.01% NaN3 and 0.05% Tween 20; (3) washing buffer was PBS with 0.05% Tween 20; (4) antibody dilution buffer was PBS, containing 0.05% Tween 20, 0.1% albumin and 0.01% NaN3; (5) enzyme labelled secondary antibody dilution buffer was containing 10% albumin; (6) substrate was 0.1% TMB and H2O2 in 0.05 M citrate buffer, pH 4.5; and (7) 2NH2SO4 was the stopping reagent.
Synthesis of coating antigen
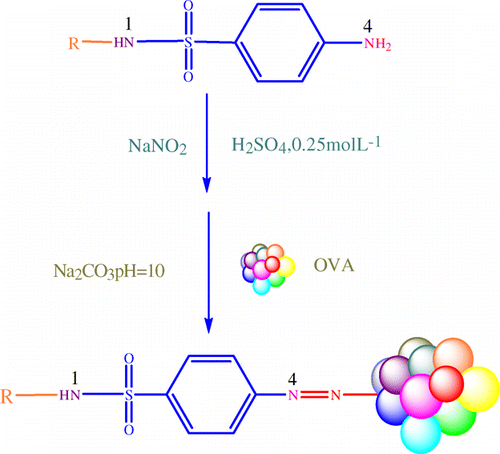
Conjugates of SMZ–OVA were synthesised by diazo-method, which were previously described (; McCaughey, Elliott, & Crooks, Citation1990). An amount of 104 mg SMZ was dissolved in 8 mL 0.25 mol L−1 sulphuric acid and kept in 4°C. Sodium nitrite (38 mg) in 3 mL distilled water was added to the SMZ solution in chopped ice within 15 minutes. After 30 minutes, 8 mL of SMZ solution was added drop-wise with stirring to 200 mg OVA in 8 mL sodium carbonate. The pH value of the reaction mixture was kept at 10 by addition of sodium hydroxide (1 M). The reaction mixture was stirred for six hours at room temperature and then dialysed against 0.9% sodium chloride for three days (each changes for every eight hours). The conjugate of SDZ–OVA, SDM–OVA, SMX–OVA, SMR–OVA and SMM–OVA was simultaneously synthesised by the same procedure. All coating antigens were determined by UV-visible spectra photometer.
Determination of enzyme-linked immunosorbent assay (ELISA) sensitivity
Polystyrene 96-well microtitre plates were coated with coating antigen (100 µl/well) and incubated at 37°C for two hours, and then at 4°C overnight. The plates were washed three times with washing buffer, and then blocked with blocking buffer (300 µL/well) at 37°C for one hour. Antibody (50 µl/well), various concentrations of analytes (50 µl/well) and goat anti-rabbit IgG–HRP (1:3000 in PBS, 100 µl/well) were simultaneously added to the conjugate coated micro-plates and incubated at 37°C for one hour. Following incubation, the plates were washed three times with washing buffer. The substrate solution (100 µl/well) was added and incubated at 37°C for 30 minutes before the enzymatic reaction was stopped by adding 2NH2SO4 (100 µl/well). The absorbance (OD) of each well was measured at 490 nm by the ELISA plate reader.
Standard curves were prepared in assay buffer and each IC50 value was determined in the competitive experiment described above. The four parameter logistic equation as defined below was used to fit the immunoassay data. Calculations were performed using OrginPro7.5 software (OriginLab Corporation, Northampton, MA).
Similarity calculation in molecular operating environment
Six sulfonamide molecules used as coating hapten in the study were constructed in the Molecular Operating Environment (Chemical Computing Group). The 2D fingerprints were calculated by using MACCS structural keys. The Tanimoto similarity coefficients were obtained by ‘similarity searching’ protocol using SMZ as query molecule in molecular operating environment platform.
Results and discussions
Synthesis of coating antigen
In order to investigate the effect of heterologous structure of coating hapten on ELISA sensitivity for SMZ, six sulfonamide haptens were used to prepare coating antigens. The only difference of all coating haptens is the diversity array of R group linked to the nitrogen at position N1 (). The R group structures of these haptens are showed in .
Table 1. Structure of heterologous coating hapten, parameters of ELISA and molecular similarity.
Previous studies reported that the ratio of hapten to carrier protein may have an effect on sensitivity of ELISA (Franek, Diblikova, Cernoch, Vass, & Hruska, Citation2006; Hennion & Barcelo, Citation1998). The methods used to reduce the influence of hapten–protein ratio as low as possible is based on the fact that all coating antigens were synthesised by identical amount of materials and chemical route, which will guarantee to obtain similar ratio of hapten–carrier protein.
Determination of enzyme-linked immunosorbent assay (ELISA) sensitivity
The optimal antibody and coating antigen dilution, ODmax (which is the maximum absorbance value of the inhibition curve) and IC50 values are summarised in . It is well known that competitive ELISA is different from non-competitive one in that an excess of immuno-reagents is used to increase signal response. Limiting concentrations of antibody and coating antigen are required for a competitive ELISA in order to obtain the required analytical sensitivity. The lowest possible coating antigen and antibody concentration is desirable for the highest sensitivity. However, if the dilution of coating antigen and antibody is too low, which will not allow the reliable detection of label and affects the competition between antibody, analyte and coating antigen. Since the objective of the study is to investigate the effect of heterologous coating hapten on the sensitivity of ELISA, the influence of dilution of immuno-reagent on sensitivity should be as low as possible. In the study, the combinations of dilution of coating antigen and antibody were employed in the study when the values of ODmax ranged from 1.2 to 1.5.
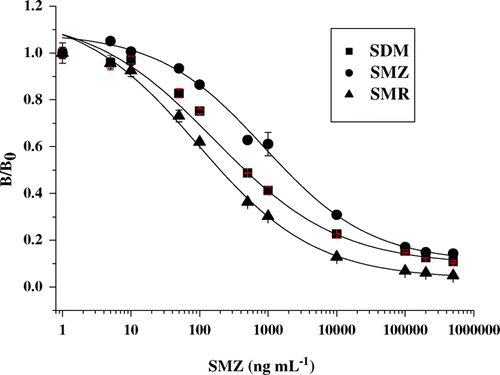
Simple inspection of IC50 values revealed that the dilution of coating antigen and antibody is important for ELISA sensitivity. Significant differences in dilution of antibody and coating antigen were observed. The dilution of antibody optimal to SMZ–OVA is 1/64×104, which was the highest dilution and far more than that of other coating antigens. The highest dilution of coating antigen was obtained by SMR–OVA, while it presented the lowest IC50 value of 94 ng mL−1. Since the antibody was produced to the SMZ, the coating antigen SMZ–OVA exactly was homologous to SMZ–BSA that was used as immunogen. The homologous coating antigen presented the second highest sensitivity with IC50 value of 126 ng mL−1. The IC50 values of other four heterologous coating antigens, SDM–OVA, SDZ–OVA, SMX–OVA and SMM–OVA, were 165, 371, 877 and 610 ng mL−1, respectively. Standard inhibition curves for SMR–OVA, SMX–OVA and SDM–OVA were shown in . It could be observed that the dilution of coating antigen and antibody had some influence on the sensitivity by comparing that of coating antigens from each other, however, exceptions such as SMR–OVA that compared to SMZ–OVA and, SDZ–OVA compared to SDM–OVA do not follow the rule proved that the diversion of structure of coating hapten resulted in the difference of sensitivity for SMZ.
Effect of heterologous structure of coating antigen on enzyme-linked immunosorbent assay (ELISA) sensitivity
Theoretically, a sensitive competitive immunoassay for a small molecule, such as sulfonamides, can be achieved when the antibody affinities for coating hapten and analyte are comparable. However, in practice, the affinity of antibody for the coating hapten is generally higher than for analyte when the structure of coating hapten is identical with that of immunogen hapten (Krasnova, Eremin, Natangelo, Tavazzi, & Benfenati, Citation2001). In the study, due to the immunogen hapten was SMZ, the antibody obtained from SMZ–BSA should have higher affinity to SMZ–OVA than hapten SMZ because of the antibody may recognise the attachment of hapten to carrier protein. Both the structural features of the coating hapten itself and the structure and length of the bridge between hapten molecule and carrier protein, markedly influenced recognition of the coating antigen by the antibody and under conditions of competitive interaction with the analyte, assay sensitivity was modified (Colbert, Eremin, & Landon, Citation1991). In the paper, we pay more attention to the effect of structural heterology of coating hapten on sensitivity of ELISA, thus, all coating antigen were prepared by the same method. The bridge and site heterology of coating antigen theoretically did not exist.
In agreement with previous studies, structural heterology of coating antigen was found to have significant effect on sensitivity of ELISA (Harrison, Goodrow, & Hammock, Citation1991; Wortberg, Goodrow, Gee, & Hammock, Citation1996). It could be seen in , the diversity structure of coating antigen SMR–OVA comparing to SMZ–OVA had positive influence on sensitivity, while, other four coating antigens had negative influence on sensitivity. The subtract of a methyl group on the pyrimidine ring, as seen in SMR, reduced the affinity of antibody to SMR–OVA compared to target SMZ. Thus, the SMZ at lower concentrations could compete with the coating antigen SMR–OVA, which resulted in better assay sensitivity by about 34%. However, in the case of SDZ, SDM, SMM and SMX, the lower sensitivity comparing to SMR–OVA was achieved by these coating antigens that are more structurally diverse from SMZ. The results demonstrated that the structural heterology of coating antigen could increase sensitivity of ELISA in some extent, however, the adverse influence on sensitivity sometimes occurred, and that the degree of heterology of coating antigen may had relationship with sensitivity of ELISA.
To assess the degree of similarity between two molecules is always subjective and mostly relies upon eye-looking comparative judgement in immunoassay for the determination of small molecules. Because of this subjectivity, it is difficult to make the reader, even author, certain of the conclusion obtained from the analysis. Nowadays, molecular similarity analysis is increasingly popular and widely applied in combinatorial chemistry, high-throughput screening and related field. In the paper we presented, we firstly introduced the commonly used molecular similarity analysis, the 2D fingerprint MACCS keys and the Taminoto coefficient, of application in the competitive immunoassay field to better explain the hapten heterology of influence on ELISA sensitivity from a better scientific point of view.
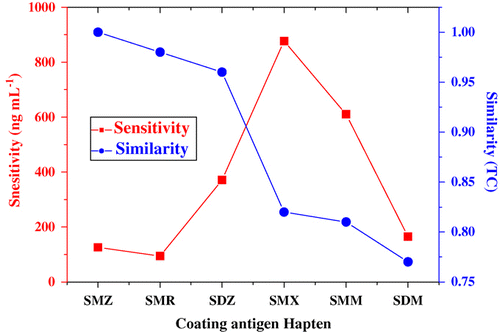
The calculated Tanimoto coefficients of six sulphonamide hapten are listed in . The immunogen hapten SMZ was used as query molecule and thus its Tanimoto coefficient was one. The value of the Tanimoto coefficient was ranged from zero to one. The Tanimoto coefficient of molecule was more close to one, the more similar to the query molecule. The array of degree similarity of all coating haptens was SMZ (1.00), SMR (0.98), SDZ (0.96), SMX (0.82), SMM (0.81) and SDM (0.77) by a decreasing order. The relationship of degree heterology of coating hapten with the ELISA sensitivity was presented in . We found that the degree of heterology of coating antigen was not direct proportion to ELISA sensitivity. The heterology of coating haptens had two-faced influence on ELISA sensitivity.
There are clearly many variables that are difficult to control in the study such as final functional density of antigen on the solid surfaces used, how antigens with different degrees of conjugation lie on the surface and are more or less accessible to binding agents. Another point should be noted is that there are limited numbers of coating antigen molecules in the view of statistics. However, we think this is a useful study to investigate the effect of heterology of coating haptens on sensitivity of ELISA from the aspect of hapten.
Conclusion
In the paper, we have prepared six heterologous sulfonamide coating antigens and investigated the heterology of these coating haptens of influence on sensitivity of ELISA for the determination of SMZ. The results indicated that the heterology of coating antigen had a significant effect on ELISA sensitivity, expressed as IC50, which was ranged from 94 to 877 ng mL−1. The molecular similarity analysis for six coating haptens by using the MACCS structural keys and the Tanimoto coefficient showed that the degree of heterology of coating antigen was not in direct proportion to ELISA sensitivity. The novel study, combining ELISA with molecular similarity analysis, indicates that multidisciplinary research could be used as a tool to investigate heterologous coating hapten effect on competitive immunoassay and provide the information required for the potential design of novel coating hapten and thus, improved immunoassay.
Acknowledgements
This work is supported by the grants from State Key Programme of National Natural Science of China (No. 30830082), National Natural Science Foundation of China (No. 30901086), Beijing Excellent Doctoral Dissertation Fund (YB20081001902) and Research Fund for the Doctoral Programme of Higher Education of China (20090008120004). We thank University of Science and Technology Beijing for molecular operating environment platform.
References
- Brown , R.D. and Martin , Y.C. 1997 . The information content of 2D and 3D structural descriptors relevant to ligand-receptor binding . Journal of Chemical Information and Computer Science , 37 : 1 – 9 .
- Colbert , D.L. , Eremin , S.A. and Landon , J. 1991 . The effect of fluorescein labels on the affinity of antisera to small haptens . Journal of Immunological Methods , 140 : 227 – 233 .
- Franek , M. , Diblikova , I. , Cernoch , I. , Vass , M. and Hruska , K. 2006 . Broad-specificity immunoassays for sulfonamide detection: Immunochemical strategy for generic antibodies and competitors . Analytical Chemistry , 78 : 1559 – 1567 .
- Goodrow , M.H. and Hammock , B.D. 1998 . Hapten design for compound-selective antibodies: ELISAS for environmentally deleterious small molecules . Analytica Chimica Acta , 376 : 83 – 91 .
- Grant , G.A. and Sporns , P. 2005 . Effect of the hapten linking arm on cross-reactivity of sulfonamide-specific antibodies in ELISA . Food and Agricultural Immunology , 16 : 259 – 272 .
- Harrison , R.O. , Goodrow , M.H. , & Gee , S.J. 1990 . Hapten synthesis for pesticide immunoassay development . In M. Vanderlaan L. Stanker B. Watkins & D. Roberts Immunoassays for trace chemical analysis: Monitoring toxic chemicals in humans, food and the environment (pp. 14 – 27 ). Washington, DC : ACS Symposium Series 451, American Chemical Society .
- Harrison , R.O. , Goodrow , M.H. and Hammock , B.D. 1991 . Competitive inhibition ELISA for the s-triazine herbicides: Assay optimization and antibody characterization . Journal of Agricultural and Food Chemistry , 39 : 122 – 128 .
- Hennion , M.C. and Barcelo , D. 1998 . Strengths and limitations of immunoassays for effective and efficient use for pesticide analysis in water samples: A review . Analytica Chimica Acta , 362 : 3 – 34 .
- Kim , Y.J. , Cho , Y.A. , Lee , H.S. and Lee , Y.T. 2003a . Investigation of the effect of hapten heterology on immunoassay sensitivity and development of an enzyme-linked immunosorbent assay for the organophosphorus insecticide fenthion . Analytica Chimica Acta , 494 : 29 – 40 .
- Kim , Y.J. , Cho , Y.A. , Lee , H.S. , Lee , Y.T. , Gee , S.J. and Hammock , B.D. 2003b . Synthesis of haptens for immunoassay of organophosphorus pesticides and effect of heterology in hapten spacer arm length on immunoassay sensitivity . Analytica Chimica Acta , 475 : 85 – 96 .
- Kim , Y.J. , Kim , Y.A. , Lee , Y.T. and Lee , H.S. 2007 . Enzyme-linked immunosorbent assays for the insecticide fenitrothion. Influence of hapten conformation and sample matrix on assay performance . Analytica Chimica Acta , 59 : 183 – 190 .
- Knopp , D. 2006 . Immunoassay development for environmental analysis . Analytical and Bioanalytical Chemistry , 385 : 425 – 427 .
- Kolar , V. , Deng , A. and Franek , M. 2002 . Production and characterization of generic antibodies against s-triazine and sulfonylurea herbicides . Food and Agricultural Immunology , 14 : 91 – 105 .
- Krasnova , A.I. , Eremin , S.A. , Natangelo , M. , Tavazzi , S. and Benfenati , E. 2001 . A polarization fluorescence immunoassay for the herbicide propanil . Analytical Letter , 34 : 2285 – 2301 .
- Krasowski , M.D. , Siam , M.G. , Iyer , M. and Ekins , S. 2009a . Molecular similarity methods for predicting cross-reactivity with therapeutic drug monitoring immunoassays . Therapeutic Drug Monitoring , 31 : 337 – 344 .
- Krasowski , M.D. , Siam , M.G. , Iyer , M. , Pizon , A.F. , Giannoutsos , S. and Ekins , S. 2009b . Chemoinformatic methods for predicting interference in drug of abuse/toxicology immnoassays . Clinical Chemistry , 55 : 1203 – 1213 .
- Leach , A.R. ,& Gillet , V.J. 2007 . An introduction to chemoinformatics . Dordrecht, , The Netherlands : Springer .
- McCaughey , W.J. , Elliott , C.T. and Crooks , S.R. 1990 . Sulphadimidine in the urine of experimentally fed pigs . The Veterinary Record , 126 : 323 – 326 .
- Morozova , V.S. , Levashova , A.I. and Eremin , S.A. 2005 . Determination of pesticides by enzyme immunoassay . Russian Journal of Analytical Chemistry , 60 : 202 – 217 .
- Pastor-Navarro , N. , Morais , S. , Maquieira , A. and Puchades , R. 2007 . Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues: Application to honey samples . Analytica Chimica Acta , 594 : 211 – 218 .
- Schneider , P. and Hammock , B.D. 1992 . Influence of the ELISA format and the hapten-enzyme conjugate on the sensitivity of an immunoassay for s-triazine herbicides using monoclonal antibodies . Journal of Agricultural and Food Chemistry , 40 : 525 – 530 .
- Siong , I.W. and Hammock , B.D. 1984 . Comparison of coating and immunizing antigen structure on the sensitivity and specificity of immunoassays for benzoylphenylurea insecticides . Journal of Agricultural and Food Chemistry , 32 : 1294 – 1301 .
- Wang , Z.H. , Zhang , S.X. , Nesterenko , I.S. , Eremin , S.A. and Shen , J.Z. 2007 . Monoclonal antibody-based fluorescence polarization immunoassay for sulfamethoxypyridazine and sulfachloropyridazine . Journal of Agricultural and Food Chemistry , 55 : 6871 – 6878 .
- Wortberg , M. , Goodrow , M.H. , Gee , S.J. and Hammock , B.D. 1996 . Immunoassay for simazine and atrazine with low cross-reactivity for propazine . Journal of Agricultural and Food Chemistry , 44 : 2210 – 2219 .
- Zhang , S.X. , Wang , Z.H. , Nesterenko , I.S. , Eremin , S.A. and Shen , J.Z. 2007 . Determination of the veterinary drug maduramicin in food by fluorescence polarization immunoassay . International Journal of Food Science and Technology , 42 : 36 – 44 .