Abstract
A direct competitive fluorescence immunoassay (dc-FIA) and a direct competitive enzyme-linked immunosorbent assay (dc-ELISA) for the screening of dimethyl phthalate (DMP) in water samples were developed. The immunoassays utilise polyclonal antibodies against DMP raised in rabbits. The anti-DMP antibodies were linked to horseradish peroxidase (HRP) and fluorescein isothiocyanate (FITC). Under the optimal experimental conditions, the dc-ELISA has a linear working range of 0.1–2000 ng/ml (R 2=0.993) with a limit of detection of 0.09 ng/ml. In the dc-FIA, the linear working range was 0.05–30 ng/ml (R 2=0.996), and the limit of detection was 0.02 ng/ml, which is approximately four-fold more sensitive than the dc-ELISA using the same antibody and coating antigen. The results show low cross-reactivity with other structurally related compounds. The proposed methods are successfully applied to determine the DMP contaminants with a simple extraction procedure, and good recoveries were obtained.
Introduction
Phthalate esters (PAEs) are synthetic compounds which have been used primarily as plasticizers in order to gain the desired flexibility and durability, such as in building materials, home furnishings, transportation, clothing, food and medical products (Wang, Fan, & Gu, Citation2003; Zhang et al., Citation2009). Since PAEs are not chemically but physically bound to the polymer chains, they may leach into the environment. The contamination has been widely reported in the soil, water and sediment in the middle and lower reaches of the Yellow River in China, lake water and drinking water (Li, Zhong, Xu, & Sun, 2006; Liu, Shen, Zhang, & Zhang, Citation2009; Prapatpong & Kanchanamayoon, Citation2010; Sha, Xia, Yang, & Huang, Citation2007). As a result, phthalate esters are ubiquitous pollutants in the environment. What is worse, many works have shown that certain phthalate esters have an adverse effect on the male reproductive system (Foster, Citation2006; Howdeshell, Rider, Wilson, & Earl Gray Jr., Citation2008; Martino-Andrade & Chahoud, Citation2010; Wilson et al., Citation2004). Because PAEs can leach out from plastics, they typically are unintentionally added to food during processing and packaging. Therefore, PAEs are toxic to humans through diet. Dimethyl phthalate (DMP) belongs to the phthalate esters family, and has been considered as a priority environmental pollutant by the US Environmental Protection Agency (USEPA), European Union and the China National Environmental Monitoring Center (Xu et al., Citation2009).
Therefore, there is a strong need to look for an effective detection method for such pollutants. In recent years, numerous methods had been developed for dimethyl phthalate analysis and monitor, including gas chromatography (Li et al., Citation2006), gas chromatography with mass spectroscopy detection (Peñalver, Pocurull, Borrull, & Marcé, Citation2000), and reversed-phase liquid chromatography (De Orsi et al., Citation2006). In general, time consuming, high instrumentation costs, skilled personnel and complex sample treatments are involved in these instrumental methods. However, immunoassays have been proved to be a new convenient and economical analytical tool to detect phthalate esters in various environmental matrices (Kuang, Xu, Cui, Ma, & Xu, 2010; Zhang, Cong, Sheng, & Liu, 2010; Zhang & Sheng, Citation2010; Zhang, Wang, & Zhuang, 2006, 2007). As for the detection of DMP, immunoassay was first applied by Ius, Bacigalupo, Meroni, Pistillo, and Roda (1993). However, this assay was only appropriate for determining PAEs as a group because of the high cross-reactivity with components structurally related to DMP.
The aim of this study was to develop two simple and sensitive methods for detection of dimethyl phthalate in water samples, and compare the sensitivity and accuracy of two methods using the same antibody and coating antigen.
Materials and methods
Reagents
Phthalate esters standards dimethyl phthalate (DMP), diethyl phthalate (DEP), dipropyl phthalate (DPrP), dibutyl o-phthalate (DBP), di-n-pentyl phthalate (DPP), diethylhexyl phthalate (DEHP), dicyclohexyl phthalate (DCHP) and o-phenylenediamine (OPD), were purchased from Shanghai Chemical Reagent Co. (Shanghai, China). Bovine serum albumin (BSA, MW = 67,000), ovalbumin (OVA, MW = 45,000), goat anti rabbit IgG, sephadex G-50, horseradish peroxidase (HRP) and fluorescein isothiocyanate (FITC) were obtained from Sigma (St. Louis, MO, USA). Twenty-five per cent of glutaraldehyde was purchased from Hefei BoMei Biotechnology Co. (Hefei, China). Freund's complete adjuvant (lanoline:mineral oil 1:2, with heat-killed mycobacterium tuberculosis) and Freund's incomplete adjuvant (lanoline:mineral oil 1:2) were prepared in our laboratory. All reagents were of analytical grade unless specified otherwise.
Solutions
(1) Coating buffer: 50 mM pH 9.6 carbonate buffer; (2) assay buffer (PBS): 10 mM pH 7.5 phosphate-buffered saline, containing 137 mM NaCl and 2.7 mM KCl; (3) washing buffer (PBST): assay buffer with 0.05% (v/v) of Tween-20; (4) blocking solution: 1% of OVA in assay buffer; (5) substrate solution (OPD + H2O2): 4 mg o-phenylenediamine dissolved in 10 ml pH 5.0 citric acid-phosphate, and 15 µl 5% H2O2 was added before using; (6) stop solution: 2 M sulphuric acid; (7) DMP standard solution was dissolved in ethanol, and diluted in PBS.
Apparatus
Absorbance and fluorescence intensity were obtained from the Microplate Reader (Synergy™ HT; Bio-Tek instruments, Inc., USA). The pH values of all buffer solutions were measured using a pHS-3C pH meter (Shanghai Precision & Scientific Instrument Co., Shanghai, China). Ultraviolet-visible (UV-vis) spectra were recorded on a spectrophotometer (UV-3010; Hitachi, Japan). Polystyrene microtiter plates (96 well) were purchased from Shanghai Sangon Biotechnology Technology Co. (Shanghai, China).
Hapten synthesis
As is generally known, the immune system does not recognise relatively small molecules. 4-Nitrophthalic acid was selected to synthesise dimethyl 4-aminophthalate (DMAP) as the hapten according to previous synthetic method of dibutyl phthalate (Zhang et al., Citation2006). Briefly, concentrated sulphuric acid (1.0 ml) was added slowly to the stirred solution of 4-nitrophthalic acid (20 g, 0.0947 mol) in 25 ml of methanol at room temperature (RT), and it was heated to reflux (120°C) for 7 h. After that, the solvent was evaporated under reduced pressure. The oily residue was added into cold ice water, then the mixture was washed three times with 10% aqueous Na2CO3. The crude product was washed again, adjusted to pH 6.5–7.0 by using distilled water, and recrystallised from ethanol. The produced pale yellow crystals were dried in a vacuum desiccator overnight to obtain dimethyl 4-nitrophthalate.
Synthesis of hapten (dimethyl 4-aminophthalate): dimethyl 4-nitrophthalate (4.5 g, 18.8 mmol) was added slowly to the stirred solution of 300 ml benzene and 10 ml concentrated hydrochloric acid. Then, 5.0 g zinc dust was added. After reacting for 15–20 min, other 5.0 g zinc dust was added and the reactive mixture was stirred for 15 h at RT. Five hundred millilitres of distilled water was added and the pH of reaction mixture was set at 7.0 by adding solid sodium hydroxide. Then the mixture was transferred to a separatory funnel and the benzene layer was removed. The combined benzene extracts were distilled under reduced pressure to get the pale yellow crude product. The crude product was recrystallised from ethanol; 2.8 g dimethyl 4-aminophthalate was obtained. The products were characterised by IR (KBr) and 1H NMR.
Synthesis of hapten–protein conjugates
Hapten–protein conjugates were synthesised by a diazotising method. The hapten was covalently attached to BSA to be used as immunogen or OVA to be used as coating antigen. Briefly, 0.0871 g dimethyl 4-aminophthalate was dissolved in 0.1 ml concentrated hydrochloric acid and 6 ml H2O was added. Then 5 ml 0.1 mol/L NaNO2 was added dropwise. The mixture was stirred in an ice bath for 30 min, and urea was added to react with remaining NaNO2. BSA solution (500 mg dissolved in 100 ml pH 9.20 sodium borate) was added dropwise. The colour of the reaction mixture changed from colourless to intense copper brown. The mixture was stirred in an ice bath for 3 h. Finally, the conjugates were dialyzed against doubly distilled water (pH 7.0) that was changed with fresh water twice a day for five days at 4°C. The conjugation of DMAP with OVA was prepared in the same way as above.
Production of polyclonal antibody
Three male New Zealand white rabbits weighing 2–3 kg were immunised with DMAP-BSA (rabbits DMP-1#, DMP-2#, and DMP-3#) according to the immunisation protocol already described (Zhang & Sheng, Citation2010). The antibody titre was assessed after each boost. After an acceptable antibody titre was observed, blood was obtained from the rabbit's heart; the antisera were separated by centrifugation. The crude antisera were purified using the saturated ammonium sulphate precipitation method (Gill, Forouzandeh, Rahbarizadeh, Ramezani, & Rasaee, Citation2006). The purified antibody was stored at –20°C until use after lyophilising.
Preparation and characterisation of antibody-HRP conjugate
Antibody-HRP conjugates were prepared by a modified glutaraldehyde method. Briefly, 0.1 ml 25% glutaraldehyde was added to 0.4 ml HRP solution (10 mg, dissolved in 50 mM pH 9.6 carbonate buffers). After incubation for 2 h at 37°C, 2 ml cold anhydrous ethanol was added. The mixture was centrifuged for 10–15 min at 2500 rpm. After washing two times using ethanol (80%, v/v), the precipitate was dissolved in the carbonate buffer. Then, 10 mg anti-DMP antibody was added to the solution. The reaction mixture was kept at 4°C overnight. The second day, NaH2PO4 was added to adjust the pH to 7.0. For removing unconjugated enzyme, the conjugates were purified by semi-saturated ammonium sulphate precipitation method and gel filtration. The purified antibody-HRP conjugates were testified by UV spectral absorption value at 280 and 403 nm. The concentration of labelled HRP and IgG was 1.01 and 2.22 mg/ml by the equations (Equation1), (Equation2). The mole ratio of HRP with antibody was 1.82 according to equation (Equation3). The further assay indicated that the conjugation of antibody with HRP was successful.
Where [Labelled HRP], [IgG] are the concentration of labelled HRP and anti-DMP antibodies, respectively; A280, A403 are the absorption values of conjugates at 280 and 403 nm, respectively. In the formula (2), 0.94 was a correction factor because the absorbance of HRP-labelled IgG by glutaric dialdehyde method increased 6% at approximately 280 nm; 0.62 was a conversion factor because IgG concentration was 0.62 mg when the absorbance was 1.0 at 280 nm.
Preparation and characterisation of antibody-FITC conjugate
The conjugation of FITC to anti-DMP antibody was synthesised according to the described procedure by Zhang and Sheng (Citation2010). The conjugates were dialysed against pH 7.1 phosphate-buffered saline for 4 h and treated with Sephadex G-50 for filtration of the remaining free FITC. The characterisation of final conjugates was done by UV-vis spectral data. The absorbance of conjugates and a known amount of standard FITC solution were determined at λ = 495, 280 and 260 nm, respectively. The standard curves in different wavelength for FITC were established; the concentrations of FITC and antibody in the conjugates were calculated. Thus, mole ratio of FITC with antibody was approximately 5.0 according to formula: Mole ratio of FITC with antibody = 0.41 [IgG]/[FITC]. Here, [FITC], [IgG] are the concentration of FITC and antibody in conjugates, respectively. Further assay indicated that the conjugation of antibody with FITC was successful.
Direct competitive immunoassay procedure
The immunoassay was performed in 96-well polystyrene microtiter plates. Antigen (DMAP-OVA diluted in coating buffer: 5.0 µg/ml for ELISA, 40 µg/ml for FIA, 100 µl/well) was coated onto the plate and incubated for 2 h at 37°C. The plate was washed three times (every time for 3 min) with PBST to remove any excess antigen (Stage I). The non-specific binding sites were blocked using 1% OVA solution (1 g OVA diluted in 100 ml PBS, 150 µl/well), incubated for 1 h at 37°C (Stage II). The plate was washed three times again, 50 µl either sample solution or standard solution and 50 µl HRP-labelled anti-DMP antibody (or FITC-labelled anti-DMP antibody in FIA) were added to each well, incubated for 4 h (or 3 h in FIA) at 37°C (Stage III). After incubation, an important washing step was used to remove unbound antibodies (Stage IV). One hundred microlitres of the substrate solution (4 mg o-phenylenediamine dissolved in 10 ml pH 5.0 citric acid-phosphate, and 15 µl H2O2 was added before using) was added (Stage V), incubated for 30 min at 37°C, the reaction was stopped by adding 50 µl 2 M H2SO4 (Stage VI). Finally, the absorbance was read at 490 nm (630 nm as reference). Standard curves were obtained by plotting the absorbance values against the logarithm of DMP concentration or sample. For the dc-FIA, The procedure was the same as dc-ELISA except that Stage V and Stage VI. After Stage IV, The fluorescence intensity was measured in the microplate reader at λex = 485 nm, λem = 528 nm. Standard curves were obtained by plotting the fluorescence intensity against the logarithm of the DMP concentration. Curves were simulated by means of Origin 6.0 software.
Cross-reactivity
The specificity of immunoassay was investigated by cross-reactivity (CR). Eight structurally related substances were selected for testing CR. Standard solution of each compound was dissolved in ethanol, diluted in PBS with the concentration range of 0.1–10,000 ng/ml and applied to the ELISA and FIA procedure. The cross-reactivity (CR) values were calculated according to the follow equation:
Sample preparation
Direct contact with phthalate containing surfaces of processing equipment and packaging material is a major source of phthalates in the diet, especially in food items of high lipid contents (Chou & Wright, Citation2006). Thus, we chose the following samples. Domestic water (potable) out of plastic pipe was from student dormitory and plastic bag for liquid food packaging and plastic water bottles (PET bottle) for beverage packaging were obtained from supermarket in Wuhu, China. Domestic water was collected in the glass bottles, filtered, and adjusted pH to 7.0 using 1 mol/L HCl or 1 mol/L NaOH, stored at 4°C until required. Plastic bag for liquid food packaging and plastic water bottles were cut into small pieces (about 0.5 cm2). An accurately weighed sample was then transferred into a 100 ml flask filled with double distilled water. The flask was kept in the water bath at 50°C for 12 h (Zhang et al., Citation2006). Each sample, approximately 100 ml, was extracted using hexane in a 125 ml separatory funnel for two times. The extracts were transferred to the distilling flask and evaporated to dryness on a heating block at 40°C, then redissolved in 1 ml ethanol for immunoassay.
Results and discussion
Synthesis of hapten–protein conjugates
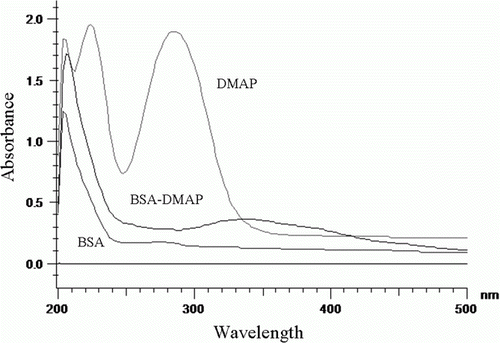
The key step in developing an immunoassay for small molecules is the design and preparation of optimum hapten as immunogen (Galve, Camps, Sanchez-Baeza, & Marco, Citation2000). First, we synthesised hapten (dimethyl 4-aminophthalate, DMAP) that provided the desired amino group for protein conjugation. DMAP was covalently attached to BSA to be used as immunogen or OVA to be used as coating antigen with a diazotation reaction. The structures of the final conjugates were supported by the UV-vis spectra data (). BSA had two characteristic absorption peaks at 278 and 205 nm. DMAP had two characteristic absorption peaks at 235 and 286 nm. DMAP-BSA conjugate had two peaks at 205 and 331 nm, it remained the peak at 205 nm, and that the absorption peak strength of DMAP-BSA was stronger than that of an equal concentration of BSA in same wavelength. This result suggested that DMAP would be likely to conjugate to BSA successfully. The hapten density (the number of hapten molecules per molecule of protein) of conjugates was estimated directly according to the following formula (Le et al., Citation2009; Wu, Chang, Ding, & He, Citation2008):
Characterisation of polyclonal antibody
The antibody titre was assessed by checkerboard titration based on non-competitive indirect ELISA (Cao, Lu, Long, Hong, & Sheng, Citation2005). The binding of serial dilutions (1/1000 to 1/64,000 in PBS) of each antiserum to microtiter plate coated with series of coating antigen (1–10 µg/ml in coating buffer, 100 µl/well) was measured. In the absence of DMP, it was found that the antiserum of rabbit (DMP-1#) displayed a high level of affinity to coating antigen. The highest dilution of antiserum was noted as the end point of the titre when Aantiserum/Awithout antiserum=2.0. In this paper, the 1/64,000 dilution was suitable for the subsequent immunoassay.
Optimisation of immunoassay procedure
Choice of coating antigen concentration and antibody dilution
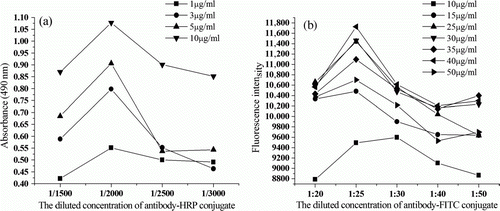
We determined the optimal antigen concentration and antibody dilution in each method by the direct competitive protocol described above. The binding of each labelled anti-DMP antibody at serial dilutions (1/1500–1/3000 for ELISA or 1/20–1/50 for FIA) to microtiter plates coated with different concentrations of coating antigen (1–10 µg/ml for ELISA or 10–50 µg/ml for FIA) was measured. The corresponding concentration was considered to be suitable in the following test, when the absorbance value was approximately 0.8–1.0 units in the absence of analytes by the ELISA. As shown in a, the results indicated that the best concentration of coating antigen was 5 µg/ml and the best dilution of antibody-HRP conjugate was 1:2000. b showed that the best concentration of coating antigen was 40 µg/ml and the best dilution of antibody-FITC conjugate was 1:25 by the FIA.
Choice of the blocking solution and the blocking time
The plates were blocked using the different proteins (such as OVA, skimmed milk powder) with different time. As a result, 1% OVA in PBS showed the lowest background value, and the suitable blocking time was 1 h (data not shown).
Concentration of Tween-20
To reduce nonspecific binding and improve sensitivity, Tween-20 at the concentration ranging from 0% to 0.5% was investigated. A concentration around 0.05% was found to reduce completely the nonspecific interactions while providing an assay with good immunoassay features (data not shown).
Effect of pH
The immunoassays reported in this paper are stable under a neutral or slightly alkaline condition. Both under acidic condition and alkaline condition gave lower absorbance (or fluorescence intensity) and higher IC50. This behaviour is similar to the immunoassays of other phthalate esters previously reported (Kuang et al., Citation2010; Zhang et al., Citation2006, Citation2007; Zhang et al., Citation2010). Based on the sensitivity and the maximal absorbance of these assays, a pH value around 7.5 was considered to be optimum condition for the dc-ELISA and dc-FIA.
Influence of ionic strength
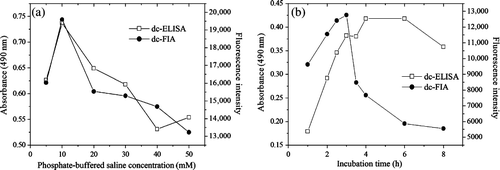
To evaluate the influence of the ionic strength on the immunoassay, assays were carried out at increasing phosphate-buffered saline concentrations varying from 5 to 50 mM. a showed absorbance and fluorescence intensity were significantly affected by increasing the salt content of the buffers, with a maximum value at the concentration of 10 mM. Therefore, 10 mM was selected as the optimal concentration due to its high sensitivity and acceptable signal.
Effect of incubation time
The incubation time of antigen and antibody was investigated from 1 to 8 h at 37°C. As shown in b, the absorbance and fluorescence intensity increased continuously by increasing the incubation time. The curves reached the maximum at 4 and 3 h, respectively. Therefore, 4 and 3 h were selected as the optimal time for dc-ELISA and dc-FIA, respectively, because more reaction time resulted in more antibody adsorbed on the microplate (Kuang et al., Citation2010). In general, most incubation for stationary assays involving the reaction of antigen and antibody are 1–3 h at 37°C (Crowther, Citation2009). Based on the maximum values and sensitivity, the assays were carried out at 4 and 3 h, respectively.
Sensitivity of immunoassay
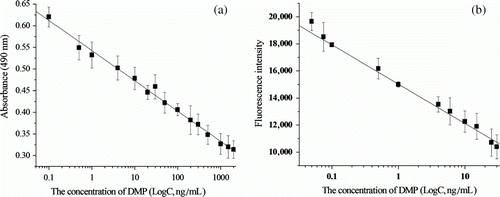
Under the optimal conditions, we developed two sensitive immunoassays for DMP as above procedure. The typical standard curves for DMP in the present assay with DMAP-OVA as the solid-phase antigen are shown in , while a shows the standard curve of DMP by direct competitive ELISA. The assay showed a good sensitivity with limit of detection (LOD) of 0.09 ng/ml and wide dynamic range (0.1–2000 ng/ml), the linear correlation coefficient was R 2=0.993. b shows the standard curve of DMP by direct competitive FIA, gave a good response for DMP at concentrations of 0.05–30 ng/ml, the linear correlation coefficient was R 2=0.996, a good sensitivity with LOD was 0.02 ng/ml. Here, LOD is defined as the concentration corresponding to three standard deviations of A0 (or F0); A0, F0 are the absorbance and fluorescence intensity, respectively, without DMP solution. Compared with time-resolved fluoroimmunoassay as described by Ius et al. (Citation1993), the DMP polyclonal antibody developed here has greater affinity and these methods present lower limit of detection. In case of results obtained from chromatography methods (Gimeno, Marcé, & Borrull, Citation2003; Li et al., Citation2006; Prapatpong & Kanchanamayoon, Citation2010), they are less sensitive than these developed methods and also require a complex sample clean-up step. The newly developed simple and rapid dc-ELISA and dc-FIA methods are superior to earlier assays in terms of limit of detection, dynamic range and recovery.
Specificity of immunoassay
The specificity of immunoassay was evaluated by cross-reactivity (CR) of the anti-DMP antibody with eight structurally related substances. The IC50 and CR values for each substance were listed in . The assay showed high cross-reactivity with DMNP and DMAP, this effect is similar to the previous reported immunoassays of phthalate esters (Kuang et al., Citation2010; Zhang et al., Citation2006, Citation2007; Zhang et al., Citation2010). The high level of CR might be due to the same aromatic structure of these two compounds. Thus, the cross-reactivity with DMP is understandable. Similarly, other PAEs have the same aromatic structure and similar alkyl groups with DMP, the assay also showed low cross-reactivity with DMP. Such as, DEP was 8.82% cross-reactive, DPrP and DBP were 2.1%, 2.4% cross-reactive, respectively. DPP, DEHP, DCHP did not react with the anti-DMP antibody at the concentrations assayed. However, DMAP and DMNP were not present in the real samples; they were only used as hapten in this work. The interference observed was negligible. Therefore, it can be concluded that the developed methods are highly specific for DMP.
Table 1. Cross-reactivity of structure related compounds to DMP determined by competitive direct immunoassays.
Analysis and recovery of spiked samples
The reliability of the proposed methods was examined using three spiked samples. The extracted domestic water (potable) out of plastic piping, leachate from a plastic bag for food packaging and plastic water bottles were analysed by dc-ELISA and dc-FIA. To study the recovery, the samples were spiked with DMP standard solution at three designated concentrations (10, 30, 100 ng/ml for ELISA, or 0.5, 4, 10 ng/ml for FIA). Results listed in . The recoveries of DMP were very good, ranged from 81.9% to 109.2% by dc-ELISA, and ranged from 96% to 107.5% by dc-FIA, respectively.
Table 2. Recovery of DMP from spiking water samples.
Conclusions
This work provides two simple methods for the rapid screening of DMP contaminations with a simple pretreatment. Three spiked water samples were analysed by the proposed methods. The results indicated that the methods are specific, sensitive and reliable for quantitative analysis of DMP in water samples. Compared with chromatographic methods, the methods offer a simple and low-cost alternative for DMP measurement. Therefore, they have great potential for determination of DMP at trace level in food and in water analysis.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 20875004), the Natural Science Foundation of Anhui Province, China (Grant No. 2006KJ149B), the Anhui Postdoctoral Sustentation Fund, China and Program for Innovative Research Team in Anhui Normal University. We express our deep thanks for financial support.
References
- Cao , Y.S. , Lu , Y.T. , Long , S.Y. , Hong , J.B. and Sheng , G.Q. 2005 . Development of an ELISA for the detection of bromoxynil in water . Environment International , 31 : 33 – 42 .
- Chou , K. and Wright , R.O. 2006 . Phthalates in food and medical devices . Journal of Medical Toxicology , 2 : 126 – 135 .
- Crowther , J.R. 2009 . Stages in ELISA . In Walker , J.M. (), The ELISA guide book , 2nd ed. (p. 70 ). Humana Press New York NY .
- De Orsi , D. , Gagliardi , L. , Porrà , R. , Berri , S. , Chimenti , P. Granese , A. 2006 . An environmentally friendly reversed-phase liquid chromatography method for phthalates determination in nail cosmetics . Analytica Chimica Acta , 555 : 238 – 241 .
- Foster , P.M. 2006 . Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters . International Journal of Andrology , 29 : 140 – 147 .
- Galve , R. , Camps , F. , Sanchez-Baeza , F. and Marco , M.-P. 2000 . Development of an immunochemical technique for the analysis of trichlorophenols using theoretical models . Analytical Chemistry , 72 : 2237 – 2246 .
- Gill , P. , Forouzandeh , M. , Rahbarizadeh , F. , Ramezani , R. and Rasaee , M.J. 2006 . Production of anti-digoxigenin antibody HRP conjugate for PCR-ELISA DIG detection system . Journal of Immunoassay & Immunochemistry , 27 : 303 – 318 .
- Gimeno , R.A. , Marcé , R.M. and Borrull , F. 2003 . Determination of plasticizers by high-performance liquid chromatography and atmospheric pressure chemical ionization mass spectrometry in water and sediment samples . Chromatographia , 58 : 37 – 41 .
- Howdeshell , K.L. , Rider , C.V. , Wilson , V.S. and Earl Gray , Jr L. 2008 . Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats . Environmental Research , 108 : 168 – 176 .
- Ius , A. , Bacigalupo , M.A. , Meroni , G. , Pistillo , A. and Roda , A. 1993 . Development of a time-resolved fluoroimmunoassay for phthalate esters in water . Fresenius,' Journal of Analytical Chemistry , 345 : 589 – 591 .
- Kuang , H. , Xu , L.G. , Cui , G. , Ma , W. and Xu , C.L. 2010 . Development of determination of di-n-octyl phthalate (DOP) residue by an indirect enzyme-linked immunosorbent assay . Food and Agricultural Immunology , 21 : 265 – 277 .
- Le , T. , Yu , H. , Guo , Y.C. , Ngom , B. , Shen , Y.A. and Bi , D.R. 2009 . Development of an indirect competitive ELISA for the detection of doxycycline residue in animal edible tissues . Food and Agricultural Immunology , 20 : 111 – 124 .
- Li , X. , Zhong , M. , Xu , S.F. and Sun , C. 2006 . Determination of phthalates in water samples using polyaniline-based solid-phase microextraction coupled with gas chromatography . Journal of Chromatography A , 1135 : 101 – 108 .
- Liu , W.L. , Shen , C.F. , Zhang , Z. and Zhang , C.B. 2009 . Distribution of phthalate esters in soil of e-waste recycling sites from Taizhou City in China . Bulletin of Environmental Contamination and Toxicology , 82 : 665 – 667 .
- Martino-Andrade , A.J. and Chahoud , I. 2010 . Reproductive toxicity of phthalate esters . Molecular Nutrition & Food Research , 54 : 148 – 157 .
- Peñalver , A. , Pocurull , E. , Borrull , F. and Marcé , R.M. 2000 . Determination of phthalate esters in water samples by solid-phase microextraction and gas chromatography with mass spectrometric detection . Journal of Chromatography A , 872 : 191 – 201 .
- Prapatpong , P. and Kanchanamayoon , W. 2010 . Determination of phthalate esters in drinking water using solid-phase extraction and gas chromatography . Journal of Applied Sciences , 10 : 1987 – 1990 .
- Sha , Y.J. , Xia , X.H. , Yang , Z.F. and Huang , G.H. 2007 . Distribution of PAEs in the middle and lower reaches of the Yellow River, China . Environmental Monitoring and Assessment , 124 : 277 – 287 .
- Wang , Y. , Fan , Y. and Gu , J.D. 2003 . Microbial degradation of the endocrine-disrupting chemicals phthalic acid and dimethyl phthalate ester under aerobic conditions . Bulletin of Environmental Contamination and Toxicology , 71 : 810 – 818 .
- Wilson , V.S. , Lambright , C. , Furr , J. , Ostby , J. , Wood , C. Held , G. 2004 . Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis . Toxicology Letters , 146 : 207 – 215 .
- Wu , J.E. , Chang , C. , Ding , W.P. and He , D.P. 2008 . Determination of florfenicol amine residues in animal edible tissues by an indirect competitive ELISA . Journal of Agricultural and Food Chemistry , 56 : 8261 – 8267 .
- Xu , B. , Gao , N.Y. , Cheng , H.F. , Xia , S.J. , Rui , M. and Zhao , D.D. 2009 . Oxidative degradation of dimethyl phthalate (DMP) by UV/H2O2 process . Journal of Hazardous Materials , 162 : 954 – 959 .
- Zhang , D. , Liu , H. , Liang , Y. , Wang , C. , Liang , H.C. and Cai , H.S. 2009 . Distribution of phthalate esters in the groundwater of Jianghan plain, Hubei, China . Frontiers of Earth Science in China , 3 : 73 – 79 .
- Zhang , M.C. , Wang , Q.E. and Zhuang , H.S. 2006 . A novel competitive fluorescence immunoassay for the determination of dibutyl phthalate . Analytical and Bioanalytical Chemistry , 386 : 1401 – 1406 .
- Zhang , M.C. , Wang , Q.E. and Zhuang , H.S. 2007 . Determination of dibutyl o-phthalate by antigen-coated competitive fluorescence immunoassay . Analytical Letters , 40 : 127 – 137 .
- Zhang , M.C. , Cong , Y. , Sheng , Y.L. and Liu , B.L. 2010 . A direct competitive enzyme-linked immunosorbent assay by antibody coated for diethyl phthalate analysis . Analytical Biochemistry , 406 : 24 – 28 .
- Zhang , M.C. and Sheng , Y.L. 2010 . An indirect competitive fluorescence immunoassay for determination of dicyclohexyl phthalate in water Samples . Journal of Fluorescence , 20 : 1167 – 1173 .