Abstract
Based on the common structure of organophosphorus pesticides, O,O-dimethylphosphorothioate, two haptens were synthesised with different spacer arms. The class-specific polyclonal antisera were prepared from the conjugates of haptens and keyhole limpet hemocyanin. Under optimum conditions, a homologous direct competitive enzyme-linked immunosorbent assay (ELISA) method was developed for multi-residue detection of 23 organophosphate pesticides. The most sensitive IC50 values of the multi-residue analysis were estimated as 0.25, 0.65 and 0.80 mg L−1 for methyl parathion (PM), fenitrothion and fenthion, respectively. Furthermore, when the developed ELISA was applied to detection of PM in water samples, adequate spike recoveries in the range from 70.3 to 131% were obtained. It provided a useful multi-residue determination method of organophophorus pesticides.
Introduction
Organophosphorus (OP) pesticides have been widely used to defend insect infestation in agriculture because of its high activity and easy degradation. They act by the acetylcholinesterase enzyme and dispute nerve function, resulting in paralysis and death (Hassal, Citation1990). Although almost OP pesticides degrade relatively fast, some events of food poisoning have taken place. As a result, development of simple and effective detection methods for OP pesticides would be valuable. As we all know, multi-residue determination of organophorsphorus pesticides usually depended on gas chromatography (Schenck et al., Citation2009). These instrumental analytical procedures are expensive, laborious and unsuitable for monitoring a large number of samples. Enzyme-linked immunosorbent assay (ELISA) is accepted and performed due to low cost, high sensitivity and rapid screening. However, reported ELISA of OP pesticides were mostly detecting individual pesticide, example for malathion (Brun et al., Citation2004), methyl parathion (PM) (Kolosova et al., Citation2004), acephate (Lee, Ahn, Stoutamire, Gee, & Hammock, Citation2003), methyl azinphos (Josep & Angel, Citation1999). Although all methods had lower limits of detection, it was difficult to detect multi-residue of OP pesticides simultaneously. So, it is necessary to establish a multi-residue immunoassay method of OP pesticides (Spinks, Citation2000).
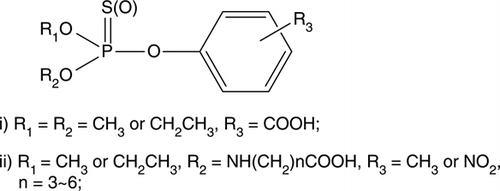
Recently, several studies on indirect competitive ELISA for multi-residue detection of OP pesticide have been reported. Most of the ELISA methods were developed from class-specific antibodies raised against haptens with the functional group common to OP pesticides. Based on the generic structure of O,O-dimethyl or O,O-diethyl phosphorothioate, multi-residue ELISA for OP pesticides were developed (Banks Chaudhry, Haverly, Watkins, & North Way Citation1998; Jang et al., 2002; Piao et al., Citation2009). Based on the generic structure of O,O-dimethyl phosphorodithioate, the class-specific antibodies were obtained and ELISA method for OP pesticides were also developed (Liang Liu, Zhu, Fan, & Liu, Citation2008; Liang, Liu, Liu, Yu, & Fan, Citation2008). Using phosphonic acid as a generic hapten, a broadly specific ELISA for determination of OP pesticides was reported (Alcocer et al., 2000). Recently, an ELISA for broadly selective detection of OP pesticides was developed with IC50 values in the range of 20–59 ng mL−1 for four pesticides (Wang et al., Citation2010).
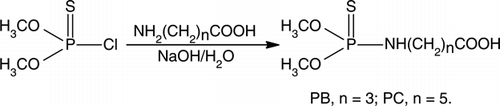
In this article, generic haptens with different spacer-arms were prepared from O,O-dimethyl phosphorothioate chlorine and amino acid (). The 12 OP pesticides were detected by the class-specific polyclonal antibodies. In addition, for three pesticides, PM, fenitrothion and fenthion, the IC50 values were lower than 0.80 mg L−1 and IC15 were lower than 0.07 mg L−1. It is the first time reported that multi-residue determination by direct competitive ELISA was established based on the class-specific antibodies.
Experimental
Materials and instrumentation
Horseradish peroxidase (HRP), keyhole limpet hemocyanin (KLH), Freunds complete and incomplete adjuvants, N-hydroxysuccinimide (NHS), N,N-dicyclohexyl carbodiimide (DCC), 3,3,5,5-tetramethyl benzidine (TMB) were and HRP-labelled goat anti-rabbit IgG secondary antibody purchased from Sigma (USA). Protein A-sepharose 4B was purchased from Amersham (Uppsala, Sweden). 6-(O,O-dimethylthiophosphorylamino)-hexanoic acid active ester (PCE) was prepared in our laboratory. 4-Amino butyric acid, PM, fenitrothion, fenthion, plondrel, azinphos-ethyl, azinphos-methyl, actellic, phosmet, methidathion, chloramine phosphorus, dimethoate, dichlorvos, chlorpyrifos, ronnel, iodfenphos, demeton, famphur, etrimfos, glyphosate, diazinon, malathion, bayrusil and methamidophos were purchased from Sigma (USA). All solvents were analytical grade.
NMR spectra were recorded on AV-300 spectrometers (Bruker, Rheinstetten, Germany), and the chemical shifts were expressed in parts per million (ppm) using tetramethylsilane (TMS) as an internal standard. Infrared spectroscopy (IR) spectra were performed using a Bruker Vector 22 spectrometer. Mass spectra were obtained on a LCQ Advantage HPLC-MS (ThermoFinnigan, SanJose, CA). Micro-well plates were from Nunc (Roskilde, Denmark) and the micro-plate washer was from Bio-Rad (Hercules, USA). Purified water was obtained using a Millipore Milli-Q water system (Millipore, Bedford, MA). Immunosorbent assay absorbance was read with a Multiskan Spectrum purchased from Thermo (Labsystems, Vantaa, Finland) in dual wave length mode (450–650 nm).
Solutions
The following solutions were used: Phosphate-buffered saline (PBS, the concentration of phosphate was 0.01 mol L−1, pH 7.5), PBS with 0.05% Tween 20 (PBST), coating buffer (CB: 50 mmol L−1, sodium carbonate buffer, pH 9.6), TMB substrate solution (prepared by adding 3.3 mg TMB in 250 µL dimethyl sulphoxide to 25 mL of phosphate citrate buffer (0.1 mol L−1, citric acid and 0.2 mol L−1 Na2HPO4, pH4.3) containing 3.25 µL of a 30% H2O2 solution), and stopping solution (1.25 mol L−1 sulphuric acid).
Hapten synthesis
Synthesis of 4-(O,O-dimethyl-thiophosphorylamino)-butyric acid (PB)
4-Amino butyric acid (103 mg, 1 mmol) was added in the solution of 2.5 mol L−1 NaOH in ice bath. 605.2 µL O,O-dimethylthiophosphoryl chlorine was added dropwise and pH was adjust to 10.0 by 2.5 mol L−1 NaOH. The mixtures were washed by hexane/diethyl ester (7/1) to remove impurities and then pH was adjusted to 2.0 by 1.0 mol L−1 HCl. The mixtures were extracted by diethyl ether (3×25 mL) and the combined organic layers were dried by Na2SO4. The residues were purified by column chromatography (CH2Cl2/CH3OH: 50/1), products were given as white solids in 61.8% yields. R f =0.42, ESI-MS: m/z 226.31 [M–H]−, (C6H14O4NPS Mr=227), IR (KBr, cm−1): 3306 (–OH), 3000–2850 (–CH), 2850–2815 (–OCH3), 1710 (–C = O), 636 (–P = S), 31P-NMR (δ, CDCl3): 76.474.
Synthesis of 4-(O,O-dimethyl-thiophosphorylamino)-butyric acid active ester (PBE)
In 50 mL flask, PB (456 mg, 2 mmol) and NHS (254 mg, 2.2 mmol) were dissolved in 20 mL CH2Cl2. The mixtures were stirred for 0.5 h in ice bath. Under N2 atmosphere, the solution of DCC (454 mg, 2.2 mmol) in 5 mL flesh distilled THF was added. The mixtures were stirred overnight at room temperature. After white solid was removed by filtration, the residues were washed by NaCl (3×20 mL). After dried by Na2SO4, the solvents were concentrated by reduced pressure. The residues were purified by column chromatography (CH2Cl2/CH3OH: 80/1) and products were obtained in 23% yields as white solids. ESI-MS: m/z 347.19 [M + Na]+, (C10H17O6N2PS, Mr = 324), IR (KBr, cm−1): 3382 (–NH), 3000–2850 (–CH), 2850–2815 (–OCH3), 1780–1740 (–C = O), 750–580 (P = S). 31P-NMR (δ, CDCl3): 76.903.
Synthesis of immunogen, coating antigen and enzyme tracer
Immunogen, coating antigen and enzyme tracer were conjugated by haptens with KLH, OVA and HRP by active ester method, respectively. The coupling method was as below in example of PB-KLH. 2 mg PBE was dissolved in 0.1 mL DMF as solution 1 and 10 mg KLH in 2 mL potassium phosphate buffer (pH 8.3) as solution 2. In ice bath, solution 1 was slowly added in solution 2 and the mixtures were stand at 4°C overnight. The mixtures were dialysed for three days, dried as a white powder and stored at −20°C.
Antibody production
Antibodies were produced in rabbits as described by Wang, Allan, Skerritt, and Kennedy (Citation1998). Four white female New Zealand rabbits (two rabbits per immunogen) were immunised by intradermal and intramuscular injections of haptens conjugated to KLH. The antisera were purified by Protein A-Sepharose 4B affinity chromatography. The IgG fraction was isolated and used for the immunosorbent assay as described below.
Development of direct competitive ELISA
Micro-well plates were coated with purified antibodies at 1 µg per well in 100 µL CB, and then incubated overnight at room temperature. The plates were washed three times with 10 mmol L−1 PBST and unbound active sites were blocked with 200 µL of 1% BSA in PBS per well for 1 h. After the plates had been washed four times, 100 µL of the standards in PBS (or diluted sample solution) and 100 µL HRP-haptens in PBS were added to each well and the mixtures were incubated for 1 h at room temperature. After five times wash, 150 µL of TMB substrate solution was added to each well. The enzymatic reaction was stopped after 30 min by adding 2.5 mol L−1 H2SO4 (50 µL per well). The absorbance was then read in dual-wavelength mode (450 nm as the test and 650 nm as the reference).
Assessment of direct competitive ELISA
Direct competitive ELISA protocol and its detection equation, sensitivity (limit of detection, LOD), precision (intra- and inter-assay variability) and accuracy (spiked recovery test) were determined.
Cross-reactivitiy of organophosphorus pesticides
The optimised assays were applied to cross-reactivity (CR) studies by using the standard solution of 23 OP pesticides. The CR was determined by dividing the IC50 of the cross-reactant by the IC50 of PM (assigned as 100%) and multiplying by 100 to obtain a percent figure.
Sample extraction
Organophosphorus pesticides (OP) have been widely used in agriculture and into the waters by various ways. Three different kinds of water (drinking water, pure water and river water) were chosen to evaluate the performance of the direct competitive ELISA. Pure water was obtained using a Millipore Milli-Q water system, drinking water was obtained from local, river water were from the river in our university and purified by filtration before detection.
Direct competitive ELISA for the spiking study of river waters: After simple filtration, river water was spiked with PM dissolved in methanol at three different levels (0.15, 0.30 and 0.45 mg L−1). The spiked samples were diluted by PBS and directly analysed by ELISA without treatment.
Results and discussion
Hapten design and conjugate identification
Haptens were designed according to O,O-dimethyl phosphorothioate as generic structure of OP pesticides. The spacer-arms length (4-carbon and 6-carbon) were studied because the structure of the protein conjugate could be affected by the length of the spacer-arm. All compounds were identified by MS, IR and 31P-NMR. Verification of the protein conjugates was measured by the UV–Vis spectrum (200–800 nm).
For multi-residue detection of OP pesticides, generic haptens were designed similarly to those used in earlier reports (). However, the structure of generic hapten was introduced by a phenyl group, benefiting the production of the antibody. As a result, the average IC50 89 ng mL−1 of the 12 OP pesticides was reported as the most ideal results until now (Piao et al., 2009). In our studies, generic haptens were linear molecules and it was more difficult for them to cause immune response.
Determination of the affinity of the antibodies
Antisera titre was used to represent the affinity of the antibodies and was monitored using an indirect ELISA (Zhang et al., Citation2006). Antisera titration was optimised to give absorbance values ranging from 0.7 to 1.2 in the absence of analyte using a checkerboard titration. In the procedure, the concentration of coating antigen was 5 mg L−1 and the enzyme tracer was diluted 1:10,000 in PBST. shows the results of the combination of immunogen and coating antigen. Generally, the titre of coating antigen and homologous immunogen was higher than that of heterologous combination. For PB and PC immunogen, the titres of Ab-1 and Ab-4 were better and chosen to further detection.
Table 1. Titres of antisera with different combination of immunogen and coating antigen.
Development of standard curve of PB and PC for methyl parathion
In direct competitive ELISA, PM was chosen as the analyte. After chessboard titration, the antibody concentration was chosen as 10 µg mL−1, the enzyme tracer of PB and PC were diluted in 1:6000 and 1:2000, respectively. The effects of ionic strength, pH value and organic solvent were studied. Optimisation of the assay is depended on a low IC50 value. The effect of ionic strength was evaluated using distilled water, and PBS with concentrations between 0.01 and 0.05 mol L−1 (five concentrations in total) during the antigen-antibody reaction. The results showed the IC50 was increased gradually when ionic concentration was increased. As a result, 0.01 mol L−1 PBS was chosen as the optimum reaction environmental solution. After the optimisation of the ionic strength, the effect of pH value was estimated from pH 4.5–9.5. It was found that the IC50 was lowest when pH value was 7.0. It was indicated that the enzyme reaction and the binding of antigen and antibody reaction was suitable at neutral conditions. Finally, the concentration of methanol was studied. It was shown that the IC50 was increased when methanol concentrations were high. It was concluded that methanol concentration was obviously interfered with the ELISA and no methanol was added in further studies.
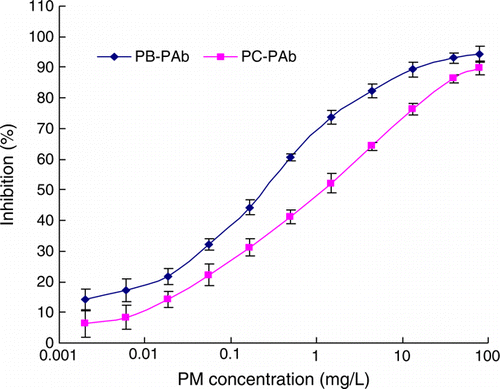
Under optimum conditions, the standard inhibition curves of Ab-1 and Ab-4 were made PM as objective shown in . The linear equation for Ab-1 was y=10.798Ln(x) + 65.316 with R 2 of 0.9919, IC50 was 0.25 mg L−1, while IC15 was 0.0021 mg L−1. For Ab-4, the linear equation was y=9.4625Ln(x) + 49.751 with R 2 of 0.9944, IC50 was 1.4 mg L−1 and IC15 was 0.14 mg L−1. Although IC50 was higher than that in the published paper for PM individually, it was better than the earlier reported for multi-residue detection (Alcocer et al., Citation2000; Jang et al., Citation2002).
Cross-reactivity of organophosphorus pesticides
Twenty-three commonly used OP pesticides were studied and their CRs with Ab-1 and Ab-4 were shown in . Generally, the effect of Ab-1 was better than that of Ab-4. Twelve pesticides were detected by Ab-1, while only six pesticides were detected by Ab-4. Ten OP pesticides (chlorpyrifos, iodfenphos, demeton, famphur, etrimfos, glyphosate, diazinon, malathion, bayrusil and methamidophos) were not identified neither by Ab-1 nor by Ab-4. In details, for Ab-1, the IC50 values were estimated to be 0.25 mg L−1 for PM, 0.65 mg L−1 for fenitrothion and 0.80 mg L−1 for fenthion. For Ab-4, the corresponding IC50 values were obviously higher. It was probably because that the six-carbon spacer was too longer to embed in the protein and it was difficult to fully expose the antigen determinant. The method was developed using PM as the analyte, so CRs of the similar structure pesticides were relatively high (line 1–3), however, the results were not satisfactory for pesticide with the structure of the phenyl group which was substituted with chlorine (line 13–15). It was indicated that chlorine atom played an important role in the reaction of antibody and antigen. Fortunately, the pesticides with diethyl phosphorothioate group were identified by Ab-1 (line 5 and 10).
Table 2. Cross-reactivity (CR) with organophosphate pesticides.
Assay precision
The variability of intra- and inter-assay was assessed to precision of direct competitive ELISA protocol. The intra-assay variability was given by the average of 22 same wells. The inter-assay variability was obtained by the average of three same times at seven different days. The results were shown in . The variation coefficient of intra- and inter-assay in different concentration was in the range from 0.02 to 0.71% and 0.34 to 24.4%, respectively. It was suggested that precision of direct competitive ELISA was good.
Table 3. Variability of intra- and inter-assay of the ELISA curve for methyl parathion (PM).
Matrix interferences and removal
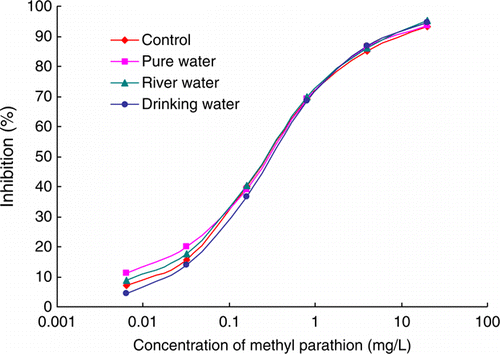
In this study, three different kinds of water (pure water, drinking water and river water) were chosen to estimate matrix interference. The standard inhibition curves of PM were prepared in these samples and in 0.01 mol L−1 PBS as a control. Different dilution ratios with PBS were tested for reducing matrix effects. Pure water, drinking water and river water were diluted with PBS by 2, 5 and 10 folds, respectively. Standard inhibition curves of different matrices were shown in . It was obviously that the four curves were well correlated. It indicated that the matrix interferences were fully removed by the simple dilution method.
Figure 4. Standard inhibition curves of methyl parathion (PM) in 0.01 mol L−1 PBS (diamonds) and different matrices by direct competitive ELISA (n=3). Pure water (squares), drinking water (circles) and river water (triangles) were diluted with PBS by 2, 5 and 10 folds with 0.01 mol L−1 PBS, respectively.
Spiked recovery test of assay
After the removal of matrix effect, spiking and recovery studies were carried out. Recoveries of three samples were tested by three spiked concentration of 0.15, 0.3 and 0.45, respectively. The results were shown in . It was found that reliable recoveries were obtained in drinking water, pure water and river water. The coefficient of variation in all cases was not more than 13.06%.
Table 4. Recovery studies of methyl parathion (PM) from spiked water samples.
Conclusion
In summary, the class-specific antibodies were raised against generic haptens with the common structure of OP pesticides with different spacer-arms. A direct competitive ELISA for multi-residue determination of OP pesticides was developed. Twelve organophosphate pesticides were determined using Ab-1. In addition, IC50 values of PM, fenitrothion and fenthion were 0.25, 0.65 and 0.80 mg L−1, respectively. CRs of five OP pesticides were over 15% with IC15 in range from 0.002 to 0.8 mg L−1. Although the sensitivity for the compounds was not as great as that reported for individual OP pesticides, it had successfully provided a screening assay for many OP pesticides more effective than reported before.
Acknowledgements
The authors are grateful for financial supports from the Ministry of Science and Technology of the People's Republic of China (No. 2009BADB9B03) and Tianjin University of Science and Technology (Project 20080402).
References
- Alcocer , M.J.C. , Dillon , P.P. , Mamming , B.M. , Doyen , C. , Lee , H.A. Daly , S.J. 2000 . Use of phosphonic acid as a generic hapten in the production of broad specificity anti-organophosphate pesticide antibody . Journal of Agricultural and Food Chemistry , 48 ( 6 ) : 2228 – 2233 .
- Banks , J.N. , Chaudhry , M.Q. , Haverly , M. , Watkins , T. and North Way , B.J. 1998 . Production and characterisation of polyclonal antibodies to the common moiety of some organophosphorus pesticides and development of a generic type ELISA . Food and Agricultural Immunology , 10 ( 4 ) : 349 – 361 .
- Brun , E.M. , Garcés , G.M. , Escuín , E. , Morais , S. , Puchades , R. and Maquieira , Á. 2004 . Assessment of novel diazinon immunoassays for water analysis . Environmental Science and Technology , 38 ( 4 ) : 1115 – 1123 .
- Hassal , K.A. 1990 . The biochemistry and uses of pesticides , London : Macmillan .
- Jang , M.S. , Lee , S.J. , Xue , X. , Kwon , H.M. , Ra , C.S. Lee , Y.T. 2002 . Production and characterization of monoclonal antibodies to a generic hapten for class-specific determination of organophosphorus pesticides . Bulletin. Korean Chemical Society , 23 ( 8 ) : 1116 – 1120 .
- Josep , V.M. and Angel , M. 1999 . Development of monoclonal ELISAs for Azinphos-methyl. 2. assay optimization and water sample analysis . Journal of Agricultural and Food Chemistry , 47 : 1285 – 1293 .
- Kolosova , A.Y. , Park , J.H. , Eremin , S.A. , Park , S. , Kang , S. Shim , W. 2004 . Comparative study of three immunoassays based on monoclonal antibodies for detection of the pesticide parathion-methyl in real samples . Analytica Chimica Acta , 511 : 323 – 331 .
- Lee , J.K. , Ahn , K.C. , Stoutamire , D.W. , Gee , S.J. and Hammock , B.D. 2003 . Development of an enzyme-linked immunosorbent assay for the detection of the organophosphorus insecticide acephate . Journal of Agricultural and Food Chemistry , 51 : 3695 – 3703 .
- Liang , Y. , Liu , J.X. , Liu , Y. , Yu , X.Y. and Fan , M.T. 2008 . Synthesis of three haptens for the class-specific immunoassay of O,O-dimethyl organophosphorus pesticides and effect of hapten heterology on immunoassay sensitivity . Analytica Chimica Acta , 615 : 174 – 183 .
- Liang , Y. , Liu , Y. , Zhu , J.F. , Fan , M.T. and Liu , X.J. 2008 . Production of broad specificity anti-methoxyorganophosphorus pesticides antibody . Chinese Journal of Analytical Chemistry , 36 ( 5 ) : 647 – 652 .
- Piao , Y.Z. , Kim , Y.J. , Kim , Y.A. , Lee , H.S. , Hammock , B.D. and Lee , Y.T. 2009 . Development of ELISAs for the class-specific determination of organophosphorus pesticides . Journal of Agricultural and Food Chemistry , 57 : 10004 – 10013 .
- Schenck , F. , Wong , J. , Lu , C. , Li , J. , Holcomb , J.R. and Mitchell , L.M. 2009 . Multiresidue analysis of 102 organophosphorus pesticides in produce at parts-per-billion levels using a modified QuEChERS method and gas chromatography with pulsed flame photometric detection . Journal of Association of Official Analytical Chemists International , 92 ( 2 ) : 561 – 573 .
- Spinks , C.A. 2000 . Broad specificity immunoassay of low molecular weight food contaminants: New paths to Utopia . Trends in Food Science & Technology , 11 ( 6 ) : 210 – 217 .
- Wang , S. , Allan , R.D. , Skerritt , J.H. and Kennedy , I.R. 1998 . Development of a class-specific competitive ELISA for the benzoylphenylurea insecticides . Journal of Agricultural and Food Chemistry , 46 : 3330 – 3338 .
- Wang , C.M. , Li , X.B. , Liu , Y.H. , Guo , Y. , Xie , R. Gui , W. 2010 . Development of a mab-based heterologous immunoassay for the broad-selective determination of organophosphorus pesticides . Journal of Agricultural and Food Chemistry , 58 : 5658 – 5663 .
- Zhang , Q. , Li , T.J. , Zhu , X.X. , Xu , L. , Liu , F. Hu , B. 2006 . Determination of N-methylcarbamate insecticide metolcarb by enzyme-linked immunosorbent assay . Chinese Journal of Analytical Chemistry , 34 ( 2 ) : 178 – 182 .