Abstract
To elucidate the immunomodulation effects of dead lactobacilli, whole cells and gastrointestinal enzymatic hydrolysates of supernatants and precipitates from Lactobacillus paracasei subsp. paracasei NTU 101 and L. plantarum NTU 102 on RAW264.7 macrophages and splenocytes were investigated. Increased NO, COX-2 expression, IL-10 and IL-12 were observed in high-dose precipitates and whole cells of both strains after 24-h stimulation. All of the hydrolysates and whole cells from both strains induced lower pro-inflammatory cytokines (IL-1β and IL-6) than LPS. The supernatants activated cell division to the S phase or promoted advance to the G2/M phase. Regardless of the Lactobacillus strains, higher levels of TNF-α, IL-6, IL-10 and IL-12 in splenocytes were induced by the precipitates. Supernatant of NTU 101 increased the amounts of IFN-γ than precipitate in splenocytes. It shows that hydrolysates of NTU 101 induce the proliferations of macrophage and splenocyte and the release of IL-10 and IL-12 cytokines to modulate the innate and adaptive immune systems and inflammatory response.
Introduction
Lactic acid bacteria (LAB) have health-promoting attributes, such as improving intestinal tract health, enhancing the immune system, synthesising and enhancing the bioavailability of nutrients, reducing the symptoms of lactose intolerance, decreasing the prevalence of allergy in susceptible individuals, reducing the risk of certain cancers, hypocholesterolemic properties, antagonistic actions and restraining intestinal and food-borne pathogens (de Vrese & Schrezenmeir, Citation2008; Geier, Butler, & Howarth, Citation2006; Parvez, Malik, Ah Kang, & Kim, Citation2006). The immunomodulatory properties of LAB and LAB-containing foods such as fermented milk are currently under investigation. In particular, Lactobacillus and Bifidobacterium have been shown to be related to the enhancement of immune functions, such as macrophage and lymphocytic activation, antibody synthesis and T- and B-cell proliferation (Fujiwara, Inoue, Wakabayashi, & Fujii, Citation2004; Ko, Goh, Lee, Choi, & Kim, Citation1999; Nonaka et al., Citation2008).
In a previous study of L. paracasei subsp. paracasei NTU 101 and L. plantarum NTU 102, two Taiwan-native strains of LAB isolated from human faeces and homemade Korean-style cabbage pickle, we found that both strains resist gastric juice and bile salts in the natural environment and show ‘probiotic’ characteristics that are effective in reducing cholesterol in the blood and liver (Chiu, Lu, Tseng, & Pan, Citation2006; Lin, Chiu, & Pan, Citation2004). In addition, NTU 102 enhances the cellular and humoral immune responses, such as phenol oxidase (PO) and superoxide dismutase (SOD) activities and clearance efficiency against Vibrio alginolyticus in Litopenaeus vannamei (Pacific white shrimp) (Chiu, Guu, Liu, Pan, & Cheng, Citation2007) and higher atherosclerosis-preventing activity of fermented milk–soymilk supplemented with Momordica charantia (Tsai, Chu, Lee, & Pan, Citation2009). Tsai, Cheng, Liao, and Pan (2010) also showed that intake of food with Lactobacillus enhances CD (Cluster designation) 4+ T- and B-cell proliferation, and increases IL-1β, IL-10, IL-12, IFN-γ and TNF-α mRNA expressions in Peyer's patches, which could enhance immunosurveillance to prevent intestinal infections or other intestinal pathologies. Lactobacillus paracasei subsp. paracasei NTU 101 and L. plantarum NTU 102 are used as starters to ferment soy skim milk. LAB-fermented soy skim milk reduces the gastric lesion index and activity of lipid peroxides (LPOs) in the gastric mucosa and serum (Liu et al., Citation2009). Further, administration of the fermented soy skim milk enhances SOD activity and prostaglandin E2 (PGE2) synthesis. Therefore, LAB-fermented soy skim milk (109 CFU/day) protects against gastric injury (Liu et al., Citation2009).
The role of Lactobacillus in stimulating the primary non-specific immune response has been noted previously by the increase in macrophage phagocytic activity (Donnet-Hughes, Rochat, Serrant, Aeschlimann, & Schiffrin, Citation1999; Perdigon, Maldonado Galdeano, Valdez, & Medici, Citation2002) and effects on dendritic cells (DCs) maturation or alter Th1/Th2 balance in vivo intestinal epithelium (Delcenserie et al., Citation2008). Our research has shown that orally administered, live lactobacilli possess many probiotic functions such as immunomodulation and gastrointestinal protection (Liu et al., Citation2009). However, it is not clear whether dead lactobacilli have the same effects or not. To examine the probiotic functions of dead lactobacilli, we studied the effects of heat-killed whole cells and enzymatically hydrolysed cells of two Lactobacillus strains on the induction of cytokines, expression of cyclooxygenase-2 (COX-2) and cell cycle transition of murine RAW264.7 macrophages. We also used an in vitro splenocytic proliferation assay to evaluate the modulatory effects of the bacterial hydrolysates on splenic of C57BL/6J mice.
Materials and methods
Strains
Lactobacillus paracasei subsp. paracasei NTU 101 and L. plantarum NTU 102, isolated from infant faecal and Korean-style cabbage pickles (Chiu et al., Citation2006; Lin et al., Citation2004), were stored at −80°C refrigerator.
Culture condition
All strains were grown in MRS broth (Difco, Sparks, MD, USA) at 37°C until the late log phase. The pellets were collected by centrifugation at 5000×g for 20 min at 4°C, washed twice with phosphate buffered saline (PBS, pH 7.2) and stored at −20°C until used for hydrolysis with digestive enzymes.
Preparation of antigenic stimulant
Bacterial cell hydrolysates were prepared using the procedure described by Kim et al. (Citation2007a). One gram of whole cell was suspended in 4 mL PBS (0.1 M, pH 6.0) and adjusted to pH 2.0 using 1 M hydrochloric acid. One millilitre of freshly prepared pepsin (E.C. 3.4.23.1, Sigma Chemical Co., St. Louis, MO, USA; 10 mg/mL in 0.01 M HCl) was added and gentle shaken at 37°C for 6 h. After pepsin treatment, 2 mL of phosphate buffer (0.2 M, pH 6.8) was added to the mixture. Then, the pH was aseptically readjusted to pH 6.8 using 1 M NaOH. The mixture was additionally treated with 1 mL freshly prepared pancreatin from porcine pancreas (Sigma, 50 mg/mL in 0.2 M phosphate buffer, pH 6.8) and shaken for 14 h at 37°C in a water bath, and heated for 10 min at 72°C to inactivate the pancreatin activity. The precipitate (insoluble fraction) and supernatant (soluble fraction) from the mixture were collected by centrifugation at 10,000×g for 20 min at 4°C and then lyophilised. For the preparation of tissue culture, the precipitates, supernatants and undigested whole cells were diluted with Dulbecco's modified Eagle's medium (DMEM; Sigma) to the desired concentration and were then heated at 95°C for 30 min.
Macrophage culture and cell viability
The RAW 264.7 murine macrophage cell line (ATCC No. TIB71) was obtained from the Bioresource Collection and Research Centre in Taiwan. The cells were grown in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco, Grand Island, NY, USA), penicillin (100 U/mL) and streptomycin (100 µg/mL) (Gibco) at 37°C in a 5% CO2 humidified incubator. The cells were cultured in triplicate at a density of 5×105 cells/mL in 24-well tissue culture plates with various concentrations of whole bacterial cells, precipitates or supernatants of hydrolysed bacterial cells. Lipopolysaccharide (LPS, from Escherichia coli O26:B6, Sigma) at 1.0 µg/mL was utilised as a positive control. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) assay was performed according to the method of Mosmann (Citation1983). After 24, 48 and 72 h, the culture medium was removed and analysed for nitric oxide (NO) and cytokines. Sterile filtered MTT solution (40 µL, 5 mg/mL) in PBS (pH 7.4) was added to each well. After 4 h, unreacted dye was removed, and the insoluble formazan crystals were dissolved in 50 µL/well dimethyl sulphoxide (DMSO) and measured spectrophotometrically at 570 nm in a Bio-Rad Microplate Reader (BioRad, Hercules, CA, USA). The relative cell viability (%) related to control wells containing cell culture medium without samples was calculated by A570 nm[sample]/A570 nm[negative control]×100.
Nitric oxide determination
The levels of NO from the culture supernatants were determined via the Griess reaction (Ding, Nathan, & Stuehr, Citation1988). Eighty microlitres of culture supernatant was mixed with 80 µL of 1% sulphanilamide (Sigma) in H3PO4 and 80 µL of 0.1% aqueous N-(1-naphthyl)-ethylenediamine dihydrochloride (Sigma) solution for 5 min at room temperature and absorbance was measured at 550 nm. Nitrite concentrations were calculated on the basis of a standard curve prepared using sodium nitrite (Sigma).
Western blot analysis of COX-2 protein
The RAW264.7 cells were cultured in 6-well plates and pre-treated with DMEM only, the precipitates or supernatants of hydrolysed cells, undigested whole cells (10, 25, 50 or 100 µg/mL) or treated with LPS (1.0 µg/mL). After 24 h of incubation, the medium was decanted and the cells were scraped out. The cell suspensions were collected by centrifugation at 1000 ×g for 5 min at 4°C. The pellets were collected and washed with PBS. Then, the suspension was centrifuged at 10,000×g for 20 s at 4°C. The pellets were collected and 150 µL of modified Pro-prep™ solution (Pro-prep™, iNtRON, Gyeonggi-Do, Korea) were added to cell lyses for 25 min on the ice. The cell lysates were centrifuged at 10,000×g for 5 min at 4°C and the supernatant was transferred to a new microcentrifuge tube and the protein content was determined by the BCA protein assay kit (Pierce, Rockford, IL, USA). Forty microlitres of cell lysates was boiled in sample buffer (40% glycerol, 5% β-mercaptoethanol, 10% SDS, 0.33 M Tris, 0.05% bromophenol blue, pH 6.8) for 5 min. Cell lysates containing 10 µg of total proteins were subjected to 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane and blotted with anti-COX-2 or β-actin antibody. β-Actin was used as the internal control. Quantification of COX-2 protein expression was performed by ECL chemiluminescent detection analysis (UVP GelDoc-It, Upland, CA, USA). Images were analysed by ImageJ (National Institutes of Health, Bethesda, MD, USA). The values were expressed as a percentage of maximal band intensity in the culture treated with LPS alone.
Cell cycle analysis
Cell cycle distribution was analysed by flow cytometry. One millilitre of RAW 264.7 cells suspension (1×107 cells/mL) was fixed in cold 70% ethanol for 2 h and then centrifuged at 300×g for 5 min. The pellet was re-suspended in PBS containing 40 µg/mL of RNase A, 0.1% Triton X-100 and 20 µg/mL of propidium iodide (PI, Sigma) in 4°C at least for 30 min. The stained cells were then filtered through a nylon filter (35 µm) and analysed by Fluorescence Activated Cell Sort (FACS, Cytomic™ FC500 Flow cytometry CXP, CA, USA) for relative DNA content based on red fluorescence. DNA histograms were analysed according to the mathematical model of Fried, Perez, and Clarkson (Citation1976). The percentage of cells in each stage of cell cycle was determined using CXP software program.
In vitro splenocyte proliferation assay
Spleens from 16-week-old C57BL/6J female mice were collected under aseptic condition and minced by scissors with 1X Hank's balanced salt solution. (HBSS, HyClone® Thermo Sci., Logan, UT, USA). The homogeneous cell suspension was passed through a fine steel mesh and settled. After depletion of erythrocytes, splenocytes (1×106 cells/100 µL per well) were cultured with various concentrations of precipitates or supernatants from two strains in RPMI-1640 (Roswell Park Memorial Institute) medium (HyClone®) supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin and 100 µg/mL streptomycin (Pen Strep, Invitrogen, NY, USA) in 96 well plate for 24 h. Then, the plates were centrifuged at 400×g for 10 min and supernatants were collected for cytokine (TNF-α, IL-1β, IL-4, IL-6, IL-12, IL-10, IL-2 and IFN-γ) measurements.
Cytokine measurement
The supernatants of macrophage RAW 264.7 and splenocytes were collected for assay. TNF-α and IL-6 in the cell culture supernatants were quantified via the enzyme-linked immunosorbent assay (Dong, Azcona-Olivera, Brooks, Linz, & Pestka, Citation1994). The plates were coated overnight at 4°C with 100 µL of 1 µg/mL rat anti-mouse TNF-α or IL-6 antibodies (Endogen, Rockford, IL, USA). The plates were then washed three times with 0.01 M PBS (pH 7.4) containing 0.2% (v/v) Tween 20 in PBS (PBST). The plates were incubated for 1 h at room temperature with 200 µL of 0.01 M PBS containing 3% (w/v) bovine serum albumin (Sigma), and subsequently washed three times with PBST. Standard cytokines or samples were added in 50 µL aliquots per well and incubated for 1 h at 37°C. The plates were washed four times with PBST and incubated for 1 h with 50 µL at a concentration of 500 ng/mL of biotinylated rat anti-mouse IL-6 or TNF-α antibody and washed four times with PBST. Fifty microlitres of streptavidin-horseradish peroxidase conjugate (Pierce, Rockford, IL, USA) was added to each well, and the plates were incubated for 30 min and washed five times with PBST. Bound peroxidase conjugate was detected via the addition of 100 µL of tetramethylbenzidine and hydrogen peroxide solution (Pierce). The reaction was halted via the addition of 100 µL of 1 M sulphuric acid and absorbance was measured at 450 nm using a Bio-Rad Microplate Reader (BioRad). The cytokine concentrations were quantified on the basis of a linear dose–response standard curve. IL-4, IL-1β, IL-12, IL-10, IL-2 and IFN-γ were determined using DuoSet mouse IL-4, IL-1β, IL-12, IL-10, IL-2 and IFN-γ kits (R&D systems, Abingdon, UK) according to the manufacturer protocol, respectively.
Statistical analysis
The experimental data were subjected to an analysis of variance for a completely random design using a Statistical Analysis System (2008) version, SAS Institute, Cary, NC, USA). Data are expressed as mean±standard deviation with triplication for each experiment. Duncan's multiple range tests were used to determine the difference among means at the level of α = 0.05 and 0.01.
Results
Effect of the bacterial strains on morphology, proliferation and nitric oxide production in macrophages
When RAW264.7 cells were cultured with DMEM medium alone for 48 and 72 h, most cells were small and round (A, B). However, the LPS treatment induced a morphological transformation to dendritic-like cells (C, D). When RAW cells were treated with heat-killed whole cells or precipitates of the two Lactobacillus strains at 100 µg/mL, morphological alterations were also clearly observed in association with macrophage change like LPS treatment in E–L. Supernatant-treated cells had the same morphology as control cells (M–P). The relative cell viabilities of macrophages stimulated by whole cells and precipitates of L. paracasei subsp. paracasei NTU 101 and L. plantarum NTU 102 were decreased with increasing concentrations from 91.4–99.9% (10 µg/mL) to 22.8–66.4% (100 µg/mL) in 48- and 72-h incubation (p<0.01, ). While, the viabilities of macrophage stimulated by supernatants of both strain were significantly increased (>10 µg/mL) by a factor of 1.6 compared with that of the control group and had no dose-dependent effect (p<0.01). It shows that the whole cells and precipitates of both strains may possess the anti-proliferation effect on macrophage RAW 264.7.
Figure 1. Morphology of RAW264.7 cells cultured for 48 and 72 h after the addition of Dulbecco's modified Eagle's medium only (A and B), lipopolysaccharide at 1 µg/mL (C and D), whole cell (100 µg/mL) of Lactobacillus paracasei subsp. paracasei NTU 101 (E and F) and Lactobacillus plantarum NTU 102 (G and H), precipitate (100 µg/mL) of L. paracasei subsp. paracasei NTU 101 (I and J) and L. plantarum NTU 102 (K and L), and supernatant (100 µg/mL) of L. paracasei subsp. paracasei NTU 101 (M and N) and L. plantarum NTU 102 (O and P). Scale bar: 50 µm.
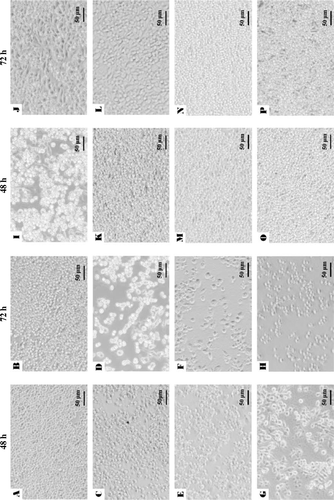
Table 1. Effect of whole cells and hydrolysates on cell viability in macrophage RAW 264.7 incubated for 24, 48 and 72 h.
The results of NO production in macrophages induced by L. paracasei subsp. paracasei NTU 101 and L. plantarum NTU 102 at 24, 48 and 72 h are summarised in . The release of NO markedly increased by treated with 25 µg/mL of precipitates and whole cells at 48 and 72 h, and increased in a time-dependent manner, but they were lower than that of stimulated by LPS (p<0.01). Additional, NO production was less intense in supernatant-treated macrophages at 24 and 48 h. Levels of NO in macrophages treated with 100 µg/mL of precipitates and whole cells (from 14.66 to 30.76 µM) were higher than that of supernatant-treatment (from 6.10 to 20.09 µM) ().
Table 2. Effect of whole cells and hydrolysates on NO production (µM) in macrophage RAW 264.7 incubated for 24, 48 and 72 h.
Effect of the bacterial strains on release of cytokines from macrophages RAW264.7
RAW264.7 cells produced lower amounts of TNF-α, IL-6, IL-1β, IL-12 and IL-10 in DMEM medium spontaneously. Increase of pro- and anti-inflammatory cytokines was observed in macrophages treated with whole cells and precipitates of NTU 101 (p<0.01, A) and NTU 102 (p<0.01, B). Further, upon treatments with high concentrations (>50 µg/ml) of whole cells and the precipitates (>25 µg/mL) by NTU 101, the release of IL-12 increased (p<0.01, A). IL-6 production was induced to a higher extent by whole cells of NTU 101 than those of NTU 102. The highest level of IL-6 induction was found in macrophages treatment with LPS (2720 pg/mL, p<0.01), whereas the induction of this cytokine was weak or barely detectable with supernatants of both bacterial species and untreated cells. Further, the TNF-α-induction activity by stimulated with whole cells in RAW 264.7 was higher than that of precipitates in both species. The secretions of IL-1β in RAW 264.7 induced by supernatants from both strains were significantly lower than other treatments in . But in all treatments, the releases of IL-1β were obviously lower than that of LPS.
Figure 2. Cytokines secretion from murine macrophages RAW 264.7 induced by hydrolysates of Lactobacillus paracasei subsp. paracasei NTU 101 (A) and Lactobacillus plantarum NTU 102 (B). Cytokines TNF-α, IL-1β, IL-6, IL-12 and IL-10 were analysed by ELISA. Macrophages (1×105/well) were cultured with whole cell or bacterial hydrolysates from 0 to 100 µg/mL for 24 h and the supernatants were collected to analyse the cytokines. -⋄-: Supernatant, -□-: Precipitate, -Delta;-: Whole cell, -•- LPS. Each value is expressed as a mean±standard deviation (n=3). Means with asterisk were significantly different compared with supernatant group in each concentration (*p<0.01).
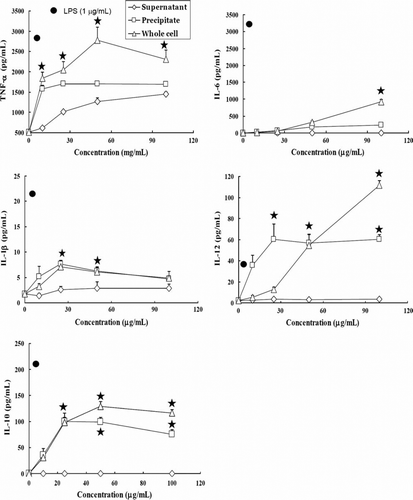
IL-12 (Th1 immune response cytokine) was present at a very low concentration or was undetectable in the treatment with supernatants of both strains, and IL-12 production induced by precipitates and whole cells was dose-dependent (p<0.01, A,B). High concentrations (>25 µg/mL) of precipitates from both strains can stimulate higher amounts of IL-12 than LPS. IL-10 (anti-inflammatory cytokine) production reflected a dose-dependent increase with higher concentrations (>10 µg/mL) by whole cells and precipitates in both strains (p<0.01, A,B), but the IL-10 production induced by whole cells was higher than that induced by precipitates. As shown in , the ratio of IL-12/IL-10 showed the balance of the Th1/Th2 immunomodulatory pathway; the ratio in the LPS treatment was 0.234, lower than that with different concentrations of precipitates and whole cells of NTU 101 and NTU 102 (about 0.39–3.22 and 0.36–1.86, respectively). The ratio of IL-12/IL-10 was significantly higher in macrophages treated with NTU 101 than with NTU 102 at high concentrations (p<0.01).
Table 3. The ratio of IL-12/IL-10 in supernatant from macrophages RAW 264.7 induced by Lactobacillus paracasei subsp. paracasei NTU 101 and L. plantarum NTU 102 precipitates of bacterial hydrolysates or whole cells incubated for 48 h.
Effect of the bacterial strains on COX-2 expression in macrophages RAW 264.7
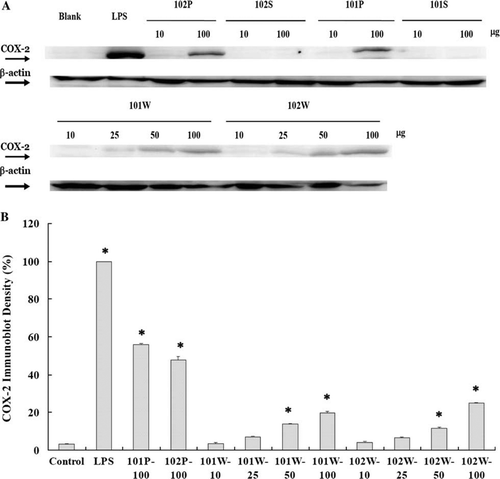
Western blot analysis showed that COX-2 expression was the highest in the LPS treatment and lower for precipitates and whole cells of the two strains; the expression was dose-dependent (A). The immunoblot density of COX-2 expression in macrophage is shown in B. Compared with the control groups, the precipitates of NTU 101 (101P) and NTU 102 (102P) at 100 µg/mL induced significantly higher expression of COX-2 (55.89% and 47.76%, respectively, p<0.01). However, the COX-2 expressions of all treatments were significantly lower than that of LPS (p<0.01).
Figure 3. Western blot analyses of COX-2 and β-actin expressions (A) and percentage of immunoblot density of COX-2 (B) in macrophages stimulated with Lactobacillus paracasei subsp. paracasei NTU 101 and L. plantarum NTU 102 hydrolysates. Macrophages (5×105/well) were cultured with whole cell (W) or bacterial supernatant (S) and precipitate (P) hydrolysates for 24 h. Immunoblot density (%) = sample band intensity/LPS band intensity×100%. Each value is expressed as mean±standard deviation (n=3). Means with asterisk were significantly different compared with control group (*p<0.01).
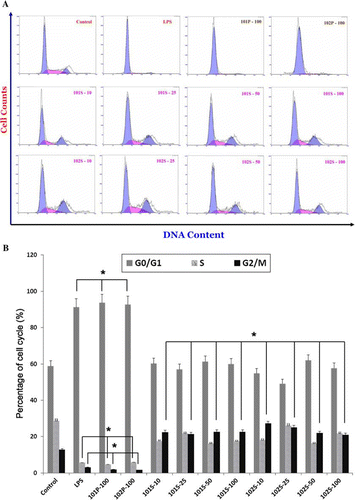
Effect of the bacterial strains on cell cycle transition of RAW264.7 macrophages
Flow cytometric analysis of the nuclear DNA content of macrophages was performed after staining with PI to determine their cell cycle transition. In the cell viability analysis, we found that the values of higher dosages (>50 µg/mL) in 101P, 101W, 102P and 102W for 48-h incubation were decreased (45.9–75.7%). But the values of 101S and 102S (157.7–176.8%) were increased at dosages higher than 25 µg/mL. For this reason, we used 10, 25, 50 and 100 µg/mL supernatants and precipitates of lactobacilli hydrolysates to evaluate whether these bacteria modulate cell cycle progression and distribution of RAW264.7 cells for 24-h incubation. Supernatants at different concentrations activated cell division from the G0/G1 phase (blue solid) to the S phase (pink solid) or promoted advance to the G2/M phase (blue histogram) in A. The increase in the population of G2/M-phase cells stimulated by supernatants of NTU 101 and NTU 102 ranged from 20.8% to 27.1%, which was higher than that induced by the control (12.7%) in B (p<0.01). It also demonstrates that precipitates of NTU 101 and NTU 102 as well as LPS treatment-induced cell cycle arrest at the G0/G1 phase (shown only at 100 µg/mL; 93.7%, 92.7% and 91.3%, respectively, p<0.01).
Figure 4. Histograms representing distributions of RAW 264.7 cells stimulated with Lactobacillus paracasei subsp. paracasei NTU 101 and L. plantarum NTU 102 hydrolysates (A). The graphic symbol of G0/G1 phase was blue solid; S phase was pink solid and G2/M phase was blue histogram. Quantification of the cell cycle distribution and the percentage of the distinct cell cycle phases in macrophages stimulated with lactobacilli hydrolysates were gated and calculated using the CXP software (B). (Macrophages (5×105/well) were cultured with bacterial supernatant (S) and precipitate (P) hydrolysate for 24 h. Each value is expressed as mean±standard deviation (n=3). The symbol 101S-10, -25, -50 or -100 represented the concentration of 10, 25, 50 and 100 µg/mL, respectively. It is the same as in 101P, 102S and 102P. Means with asterisk at percentages of G2/M phase were significantly different in decrease or increase with respect to control groups in (B) (*p<0.01).
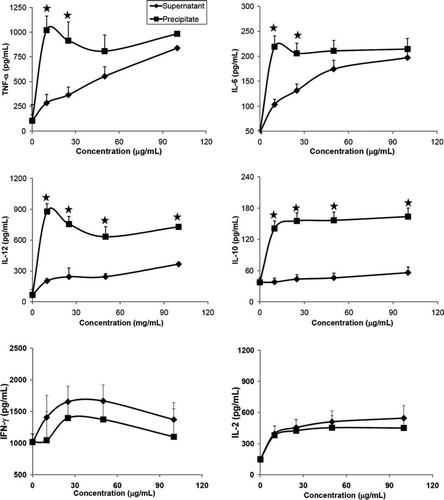
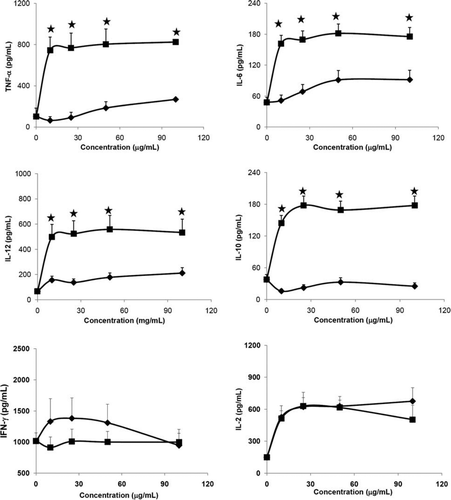
Effect of the bacterial strains on cytokines secretion of splenocytes in vitro
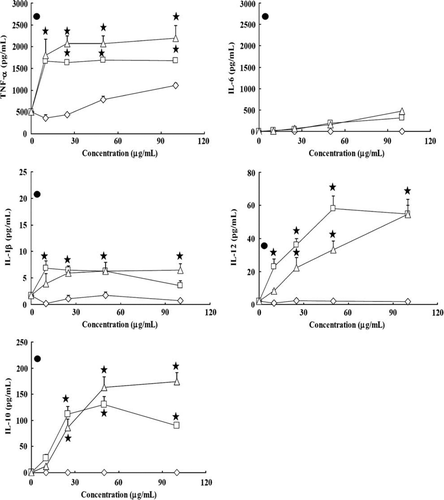
To investigate the effects of the tested hydrolysates on the growth of splenocytes, six 4-month-old C57BL/6 mice were used. For isolation of splenocytes, the spleen was aseptically removed and incubated with various concentrations of different supernatants and precipitates hydrolysates from Lactobacillus for 24 h, and supernatants of culture medium were collected for cytokine measurements. The amounts of IL-1β and IL-4 in splenocytes that were stimulated by all tested samples were undetectable. The other cytokines induced by the lactobacilli are indicated in A,B. Regardless of the Lactobacillus strains, higher amounts of IL-10, and IL-12 were induced by precipitates than supernatants (p<0.01). Amounts of TNF-α and IL-6 were induced dose-dependently by the supernatant of NTU 101 (A) than by the NTU 102 (B). While the IFN-γ induced by supernatants of NTU 101 were slightly increased than that of precipitates (A,B, p<0.01).
Figure 5. Cytokines secretion from splenocytes of C57BL/6J female mice induced by hydrolysates of Lactobacillus paracasei subsp. paracasei NTU 101 (A) and Lactobacillus plantarum NTU 102 (B). Cytokines TNF-α, IL-6, IL-10, IL-12, IL-2 and IFN-γ were analysed by ELISA. Splenocytes (1×106 cells/100 µL per well) were cultured with precipitates or supernatants of bacterial hydrolysates from 10 to 100 µg/mL in RPMI-1640 for 24 h and the supernatants were collected. -♦-: Supernatant, -▪-: Precipitate. Each value is expressed as a mean±standard deviation (n=3). Means with asterisk were significantly different compared with supernatant group in each concentration (*p<0.01).
Discussion
Lactobacillus species are non-pathogenic, Gram-positive LAB found in the normal gut microflora of mammals and humans. Some strains not only modulate mucosal immune functions upon oral administration (e.g., lipoteichoic acid in wild-type Lactobacillus strains, L. plantarum NCIMB8826 and Gram-positive strains) but also have immunostimulatory properties and provide a strategy to influence cytokine expression and immune responses (Dogi, Weill, & Perdigón, Citation2010; Grangette et al., Citation2005; Maassen et al., Citation2000). Some enhance the Th1 response through the release of pro-inflammatory cytokines such as IL-1β, TNF-α, IL-12 and IL-18, whereas others are anti-inflammatory cytokines such as IL-10 and TGF-β (Hessle, Andersson, & Wold, 2000; Matsuguchi et al., Citation2003; Miettinen et al., Citation1998). Their effects are strain-dependent and correlate with the specific Lactobacillus-induced cytokine profile. Several studies have also shown that heat-killed Bifidobacterium and Lactobacillus species and their cellular components stimulate the production of hydrogen peroxide, NO and various cytokines, such as IL-6 and TNF-α, in macrophage cell lines (Kim et al., Citation2007a, Citation2007b; Marcinkiewicz et al., Citation2007; Park et al., Citation1999).
In our previous study, DC activation in adaptive immune responses was induced by the administration of L. paracasei subsp. paracasei NTU 101 after 6 weeks (Tsai et al., Citation2008). The influence of Lactobacillus on lymphocytic proliferation and immunostimulation was evaluated by a stimulation index. The results suggest that lymphocytic proliferation is time-dependently affected by L. paracasei subsp. paracasei NTU 101. Further, the role of Lactobacillus paracasei subsp. paracasei NTU 101 in gut mucosal immunity was investigated in the Peyer's patches and spleen of BALB/c mice. After feeding with Lactobacillus, the percentage of CD4+ T cells in both the Peyer's patches and the spleen significantly increased; higher levels of intestinal IgA+-producing cells in the lamina propria were induced in the Peyer's patches. This study shows that oral administration of lactobacilli promotes greater intestinal IgA production, which can enhance immunosurveillance to prevent intestinal infections or other intestinal pathologies (Tsai et al., Citation2010).
When RAW264.7 cells were cultured with LPS, precipitates or heat-killed whole cells of the two Lactobacillus strains in the present study, we found that cell proliferation was suppressed, a change in cellular morphology, more release of NO and expression of COX-2 were induced. The strong response in precipitates or heat-killed whole cells to macrophage may result from the undigested cell membrane or intact bacterial cells components in lactobacilli such as peptidoglycan, teichoic acid and exopolysaccharide (Amrouche Boutin, Prioult, & Fliss, Citation2006). The survival and colonisation of orally administered probiotics in the gastrointestinal environment is generally poor, and these bacteria are susceptible to the low acidic pH in the stomach, bile acid and pancreatic juice within the small intestine (Chung, Kim, Chun, & Ji, Citation1999; Kimoto-nira et al., Citation2007).
Many studies have demonstrated the enhanced secretion of several cytokines via the stimulation of macrophages with live, glutaraldehyde-fixed and heat-killed bacteria (Cross, Ganner, Teilab, & Fray, Citation2004; Kimoto, Mizumachi, Okamoto, & Kurisaki, 2004; Miettinen, Voupio-Varkila, & Varkila, Citation1996). Therefore, we evaluated the immunomodulatory functions of heat-killed whole cells or components of lactobacilli in macrophages. Hydrolysates of two Lactobacillus strains were treated with pepsin at pH 2.0 and pancreatin at pH 6.8 to imitate the human gastrointestinal environment. The results are consistent with those of previous studies, showing that the production of several cytokines can be increased significantly by exposing RAW264.7 cells to heat-killed whole cells in vitro (Foligne et al., Citation2007; Tejada-Simon & Pestka, Citation1999).
NO is an important macrophage mediator by acting as a reactive oxygen and nitrogen intermediate during oxygen-dependent phagocytosis (Park et al., Citation1999). Generation of oxidative stress and NO are closely related in the inflammatory responses in cells. The productions of NO, IL-6 and TNF-α were dose-dependently increased with high concentrations of heat-killed whole cells of NTU 101 for 24 and 48 h. However, RAW264.7 cells exposed to supernatants of the two strains showed weaker responses. Several LAB strains reportedly have stimulatory properties on macrophages and natural killer cells in vitro. Most strains can induce both pro- and anti-inflammatory cytokines. IL-12 stimulates T cells and natural killer cell cytotoxicity, and IFN-γ production to promote the Th1 pathway. IL-10 inhibits the stimulation of IL-12 in T cells and decreases the release of pro-inflammatory cytokines from macrophages (Hessle, Andersson, & Wold, 1999; Hessle et al., Citation2000). The secretions of cytokines (IL-12 and IL-10) in the macrophage responses to various strains in this study are therefore consistent with the findings of previous studies.
The ratio of IL-12/IL-10 in macrophages stimulated by the bacterial components was higher than that stimulated by LPS (), indicating that the two strains essentially enhance Th1-type immunisation, which is consistent with the findings from Lactococcus and Lactobacillus strains (Bleau, Savard, & Lamontagne, Citation2007; Hessle et al., Citation1999; Kimoto et al., Citation2004). Our findings suggest that non-viable whole cells treated with gastrointestinal enzymes can increase NO production, enhance the production of several cytokines (including the anti-inflammatory cytokine), and modulate the host immune reactions. IL-1β is an important mediator of the inflammatory response and involved in a variety of cellular activities, including the induction of COX-2, cell proliferation, differentiation and apoptosis (Fogal & Hewett, Citation2008). In our results, COX-2 was found to be expressed with high concentrations of the bacterial components, but this expression was about half that induced by the LPS treatment. The results of COX-2 expression were consistent with the amounts of IL-1β stimulated by LPS, precipitates or whole cells in RAW 264.7. COX-2 is responsible for formation of important biological mediators including prostaglandins, prostacyclin and thromboxane. Pharmacological inhibition of COX-2 can provide relief from the symptoms of inflammation and pain. The COX-2 expression may be derived from induced NO and IL-1β (Cuzzocrea & Salvemini, Citation2007).
The cell cycle is a series of events in a cell leading to its division and duplication (replication), consisting of three populations: the G0/G1- and G2/M-phase populations and the S-phase population. The activation of each phase depends on the proper progression and completion of the previous one (Smith & Martin, Citation1973). In our study, we used hydrolysates of lactobacilli to investigate their effect on the RAW264.7 cell cycle in terms of the percentage of cells advancing from the G0/G1 phase to the G2/M phase. Notably, the cell cycle analysis of cells treated with supernatants for 24 h demonstrated a significantly increased G2/M-phase ratio compared with that induced by precipitates or the LPS treatment. However, the supernatants enhanced the proliferation of macrophages were showed in the results of viability and cell cycle distribution as well as untreated cells. They also demonstrated the similar amounts of NO and pro-inflammatory cytokines secretion.
According to their immunomodulatory effects, oral administration of heat-killed lactobacilli acts as an inducer of macrophage functions, followed by express IL-10 and IL-12 cytokine release. Our results indicate that heat-killed lactobacilli hydrolysates have potential therapeutic applications in modulation of inflammatory response and non-specific immunity in vitro.
Acknowledgements
We also thank Dr. Shu-Fen Lu and Mr. Yan-Zhen Liu, the staff of the Taiwan Mouse Clinic (NSC 98-3112-B-001-041) which is funded by the National Research Program for Genomic Medicine (NRPGM) at the National Science Council (NSC) of Taiwan for technical support in providing the μCT system.
References
- Amrouche , T. , Boutin , Y. , Prioult , G. and Fliss , I. 2006 . Effects of bifidobacterial cytoplasm, cell wall and exopolysaccharide on mouse lymphocyte proliferation and cytokine production . International Dairy Journal , 16 : 70 – 80 .
- Bleau , C. , Savard , R. and Lamontagne , L. 2007 . Murine immunomodulation of IL-10 and IL-12 induced by new isolates from avian type 2 Lactobacillus acidophilus . Canadian Journal of Microbiology , 53 : 944 – 956 .
- Chiu , C.H. , Lu , T.Y. , Tseng , Y.Y. and Pan , T.M. 2006 . The effects of Lactobacillus-fermented milk on lipid metabolism in hamsters fed on high-cholesterol diet . Applied Microbiology and Biotechnology , 71 : 238 – 245 .
- Chiu , C.H. , Guu , Y.K. , Liu , C.H. , Pan , T.M. and Cheng , W. 2007 . Immune responses and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum . Fish Shellfish Immunology , 23 : 364 – 377 .
- Chung , H.S. , Kim , Y.B. , Chun , S.L. and Ji , G.E. 1999 . Screening and selection of acid and bile resistant bifidobacteria . International Journal of Food Microbiology , 47 : 25 – 32 .
- Cross , M. , Ganner , A. , Teilab , D. and Fray , L. 2004 . Patterns of cytokine induction by Gram-positive and Gram-negative probiotic bacteria . FEMS Immunology and Medical Microbiology , 42 : 173 – 180 .
- Cuzzocrea , S. and Salvemini , D. 2007 . Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes . Kidney International , 71 : 290 – 297 .
- Delcenserie , V. , Martel , D. , Lamoureux , M. , Amiot , J. , Boutin , Y. and Roy , D. 2008 . Immunomodulatory effects of probiotics in the intestinal tract . Current Issues in Intestinal Microbiology , 10 : 37 – 54 .
- de Vrese , M. and Schrezenmeir , J. 2008 . Probiotics, prebiotics, and synbiotics . Advances in Biochemical Engineering/Biotechnology , 111 : 1 – 66 .
- Ding , A.H. , Nathan , C.F. and Stuehr , D.J. 1988 . Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages . The Journal of Immunology , 141 : 2407 – 2412 .
- Dogi , C.A. , Weill , F. and Perdigón , G. 2010 . Immune response of non-pathogenic Gram (+) and Gram(−) bacteria in inductive sites of the intestinal mucosa study of the pathway of signaling involved . Immunobiology , 215 : 60 – 69 .
- Dong , W. , Azcona-Olivera , J.L. , Brooks , K.H. , Linz , J.E. and Pestka , J.J. 1994 . Elevated gene expression and production of interleukins 2, 4, 5, and 6 during exposure to vomitoxin (deoxynivalenol) and cycloheximide in the EL-4 thymoma . Toxicology and Applied Pharmacology , 127 : 282 – 290 .
- Donnet-Hughes , A. , Rochat , F. , Serrant , P. , Aeschlimann , J.M. and Schiffrin , E.J. 1999 . Modulation of nonspecific mechanisms of defense by lactic acid bacteria: Effective dose . Journal of Dairy Science , 82 : 863 – 869 .
- Fogal , B. and Hewett , S.J. 2008 . Interleukin-1beta: A bridge between inflammation and excitotoxicity? . Journal of Neurochemistry , 106 : 1 – 23 .
- Foligne , B. , Nutten , S. , Grangette , C. , Dennin , V. , Goudercourt , D. Poiret , S. 2007 . Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria . World Journal of Gastroenterology , 13 : 236 – 243 .
- Fried , J. , Perez , A.G. and Clarkson , B.D. 1976 . Flow cytofluorometric analysis of cell cycle distributions using propidium iodide: Properties of the method and mathematical analysis of the data . The Journal of Cell Biology , 71 : 172 – 181 .
- Fujiwara , D. , Inoue , S. , Wakabayashi , H. and Fujii , T. 2004 . The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance . International Archives of Allergy and Immunology , 135 : 205 – 215 .
- Geier , M.S. , Butler , R.N. and Howarth , G.S. 2006 . Probiotics, prebiotics and synbiotics: A role in chemoprevention for colorectal cancer? . Cancer Biology and Therapy , 5 : 1265 – 1269 .
- Gong , G. , Qin , Y. and Huang , W. 2011 . Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis C.H. Wright in vitro and in vivo . Phytomedicine , 18 : 456 – 463 .
- Grangette , C. , Nutten , S. , Palumbo , E. , Morath , S. , Hermann , C. Dewulf , J. 2005 . Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids . Proceedings of the National Academy of Sciences of the United States of America , 102 : 10321 – 10326 .
- Hessle , C. , Andersson , B. and Wold , A.E. 1999 . Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production . Clinical and Experimental Immunology , 116 : 276 – 282 .
- Hessle , C. , Andersson , B. and Wold , A.E. 2000 . Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while Gram-negative bacteria preferentially stimulate IL-10 production . Infection and Immunity , 68 : 3581 – 3586 .
- Kim , D.W. , Cho , S.B. , Lee , H.J. , Chung , W.T. , Kim , K.H. , Hwangbo , J. Nam , I.S. 2007a . Comparison of cytokine and nitric oxide induction in murine macrophages between whole cell and enzymatically digested Bifidobacterium sp. obtained from monogastric animals . The Journal of Microbiology , 45 : 305 – 310 .
- Kim , D.W. , Cho , S.B. , Yun , C.H. , Jeong , H.Y. , Chung , W.T. Choi , C.W. 2007b . Induction of cytokines and nitric oxide in murine macrophages stimulated with enzymatically digested Lactobacillus strains . The Journal of Microbiology , 45 : 373 – 378 .
- Kimoto , H. , Mizumachi , K. , Okamoto , T. and Kurisaki , J. 2004 . New Lactococcus strain with immunomodulatory activity: Enhancement of Th1-type immune response . Microbiology and Immunology , 48 : 75 – 82 .
- Kimoto-nira , H. , Mizumachi , K. , Nomura , M. , Kobayashi , M. , Fujita , Y. Okamoto , T. 2007 . Lactococcus sp. as potential probiotic lactic acid bacteria . Japan Agricultural Research Quarterly , 41 : 181 – 189 .
- Ko , E.J. , Goh , J.S. , Lee , B.J. , Choi , S.H. and Kim , P.H. 1999 . Bifidobacterium bifidum exhibits a lipopolysaccharide-like mitogenic activity for murine B lymphocytes . Journal of Dairy Science , 82 : 1869 – 1876 .
- Lin , F.M. , Chiu , C.H. and Pan , T.M. 2004 . Fermentation of a milk-soymilk and Lycium chinense Miller mixture using a new isolate of Lactobacillus paracasei subsp. paracasei NTU101 and Bifidobacterium longum . Journal of Industrial Microbiology and Biotechnology , 31 : 559 – 564 .
- Liu , C.F. , Hu , C.L. , Chiang , S.S. , Tseng , K.C. , Yu , R.C. and Pan , T.M. 2009 . Beneficial preventive effects of gastric mucosal lesion for soy-skim milk fermented by lactic acid bacteria . Journal of Agricultural and Food Chemistry , 57 : 4433 – 4438 .
- Maassen , C.B. , van Holten-Neelen , C. , Balk , F. , den Bak-Glashouwer , M.J. , Leer , R.J. Laman , J.D. 2000 . Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains . Vaccine , 18 : 2613 – 2623 .
- Marcinkiewicz , J. , Ciszek , M. , Bobek , M. , Strus , M. , Heczko , P.B. Kurnyta , M. 2007 . Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria . International Journal of Experimental Pathology , 88 : 155 – 164 .
- Matsuguchi , T. , Takagi , A. , Matsuzaki , T. , Nagaoka , M. , Ishikawa , K. Yokokura , T. 2003 . Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through toll-like receptor 2 . Clinical and Diagnostic Laboratory Immunology , 10 : 259 – 266 .
- Miettinen , M. , Matikainen , S. , Vuopio-Varkila , J. , Pirhonen , J. , Varkila , K. Kurimoto , M. 1998 . Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells . Infection and Immunity , 66 : 6058 – 6062 .
- Miettinen , M. , Voupio-Varkila , J. and Varkila , K. 1996 . Production of human tumor necrosis factor alpha, interleukin-6, and interleukin 10 is induced by lactic acid bacteria . Infection and Immunity , 64 : 5403 – 5405 .
- Mosmann , T. 1983 . Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays . The Journal of Immunological Methods , 65 : 55 – 63 .
- Nonaka , Y. , Izumo , T. , Izumi , F. , Maekawa , T. , Shibata , H. Nakano , A. 2008 . Antiallergic effects of Lactobacillus pentosus strain S-PT84 mediated by modulation of Th1/Th2 immunobalance and induction of IL-10 production . International Archives of Allergy and Applied Immunology , 145 : 249 – 257 .
- Park , S.Y. , Ji , G.E. , Ko , Y.T. , Hoo , H.K. , Ustunol , Z. and Pestka , J.J. 1999 . Potentiation of hydrogen peroxide, nitric oxide, and cytokine production in RAW 264.7 macrophage cell exposed to human and commercial isolates of Bifidobacterium . International Journal of Food Microbiology , 46 : 231 – 241 .
- Parvez , S. , Malik , K.A. , Ah Kang , S. and Kim , H.Y. 2006 . Probiotics and their fermented food products are beneficial for health . Journal of Applied Microbiology , 100 : 1171 – 1185 .
- Perdigon , G. , Maldonado Galdeano , C. , Valdez , J.C. and Medici , M. 2002 . Interaction of lactic acid bacteria with the gut immune system . European Journal of Clinical Nutrition , 56 : S21 – S26 .
- Smith , J.A. and Martin , L. 1973 . Do cells cycle? (Cell kinetics/control of growth/DNA replication/cell culture) . Proceedings of the National Academy of Sciences of the United States of America , 70 : 1263 – 1267 .
- Tejada-Simon , M.V. and Pestka , J.J. 1999 . Proinflammatory cytokine and nitric oxide induction in murine macrophages by cell wall and cytoplasmic extracts of lactic acid bacteria . Journal of Food Protection , 62 : 1435 – 1444 .
- Tsai , T.Y. , Chu , L.H. , Lee , C.L. and Pan , T.M. 2009 . Atherosclerosis-preventing activity of lactic acid bacteria-fermented milk-soymilk supplemented with Momordica charantia . Journal of Agricultural and Food Chemistry , 57 : 2065 – 2071 .
- Tsai , Y.T. , Cheng , P.C. , Fan , C.K. and Pan , T.M. 2008 . Time-dependent persistence of enhanced immune response by a potential probiotic strain Lactobacillus paracasei subsp. paracasei NTU 101 . International Journal of Food Microbiology , 128 : 219 – 225 .
- Tsai , Y.T. , Cheng , P.C. , Liao , J.W. and Pan , T.M. 2010 . Effect of the administration of Lactobacillus paracasei subsp. paracasei NTU 101 on Peyer's patch-mediated mucosal immunity . International Immunopharmacology , 10 : 791 – 798 .