Abstract
Inhalation of olive pollen is a major cause of allergic respiratory diseases in Mediterranean region. Using bags attached to the branches, pollens were collected from 36 olive cultivars grown in Jordan just before bloom. Proteins were extracted from pollens and analysed by SDS-PAGE and immunoblotting using antisera collected from patients with history of olive pollen-allergy. Immunoblot results showed that 25 cultivars contained one or two allergenic proteins in the range of 15–72 kDa with majority of the proteins corresponding to 15–20 kDa, while remainders did not exhibit any allergenic protein. Seven cultivars exhibited three allergenic proteins and only two cultivars exhibited four allergenic proteins and thus, were considered potentially hyperallergenic cultivars. This study highlights the great variability in the antigenic and allergenic composition of Olea europaea pollen extracts which may serve as guidance for selecting the olive tree cultivars that could be planted in urban areas.
1. Introduction
Olive tree (Olea europaea) has been considered as one of the earliest domesticated fruit trees. It was spread from the Middle East towards the west of Europe approximately 6000 years ago (Alche et al., Citation2007). Recently, it became one of the most important fruit trees cultivated in the Mediterranean region, parts of California in USA, and other countries of the world (Cardaba et al., Citation2007). Fruits and oil are the two most important products of this tree (Carnes et al., Citation2002). The olive family (Oleaceae) known to be composed of 600 cultivars belonging to 30 genera and is found in all continents. In Jordan, olive tree is one of the most important fruit trees, supporting the national economy by more than 100 million Euros annually. The cultivated area currently is about 1.28 million dunums (1 dunum equal to a 1000 m2), which is equivalent to about 72% of the area planted with fruit trees, and about 36% of the total cultivated area in Jordan with an estimated number of more than 17 million olive trees (Annual Report, Jordan Ministry of Agriculture, Citation2008).
As olive trees are widely distributed in Mediterranean Europe, Middle East and other parts of the world, their flower pollen grains are recognised as one of the major causes of respiratory allergy called ‘olive pollinosis’. In addition, olive pollens were recognised as a causative agent of conjunctival symptoms and were reported to exacerbate asthma in patients from late April to early June (Quiralte et al., Citation2002). The threshold level of Olea europaea pollen grains required to elicit symptoms of seasonal allergic rhinitis is extremely high, which is around 167 pollen grains/cubic meter (Brito et al., Citation2011). Nevertheless, levels of 5000 pollen grains/cubic meter were reported in southern parts of Spain in mid May causing severe rhinitis and bronchial asthma and therefore, it is recognised as the second cause of pollinosis only after grass pollen (Lombardero et al., Citation2002; Quiralte et al., Citation2007). However, its prevalence is unlikely to be homogeneous over the entire area because olive pollen grains differ from many allergenic pollen grains of other trees and shrubs. In addition, there are many different cultivars of olive trees that produce pollen grains differ in terms of their allergenicity, and the amount of released pollens (Lombardero et al., Citation2002). As a result, the prevalence of allergenicity caused by olive pollen grains varies considerably.
Olive pollen grains extract has at least 20 protein bands with allergic activity (Cardaba et al., Citation2007; Quiralte et al., Citation2002) with the amount of each pollen protein varies among the different olive cultivars. The variation could be related to, genetic, environmental or physiological factors that play a role in the development of pollen grains and subsequently the formation of the pollen grain tubes (Carnes et al., Citation2002). Pollens collected from different olive tree cultivars in Spain showed qualitative and quantitative variation in the concentration of their allergic proteins (Carnes et al., Citation2002).
Currently, 11 allergens have been discovered and well characterised. They were given names Ole e 1 to Ole e 11 as per the current international nomenclature (Lahoz & Florido, Citation2007). Ole e 1 is by far considered the major allergen protein in the olive pollens and it was the first allergen to be discovered (in 1980s) and appeared as a double band acidic protein of 18–20 kDa. Later, it appeared that Ole e 1 has two variants; one of them is a non-glycosylated form of 18 kDa, while the second variant is a 20 kDa glycosylated polypeptide and could also exist as a 40 kDa dimmer (Lauzurica et al., Citation1988; Lombardero et al., Citation2002). This protein was incriminated in about 70% of patients with hypersensitivity to olive pollens (Cardaba et al., Citation2007; Kirmaz, Yuksel, Bayrak, & Yilmaz, Citation2005; Lombardero et al., Citation2002).
Some of the olive pollen allergic proteins are unique to olive pollens, while others may cross-react with proteins from other sources. Ole e 2 (profilin) a minor allergen of 14–16 kDa found not only in olive pollens, but also in many other sources like cherry, celery, peanut, pear, Timothy grass (Phleum pratense) pollens and Soybean, with an average mass of 15 kDa in these plants (Martinez et al., Citation2002). About 27% of patients suffering from olive pollen-allergy are sero-positive (IgE) for this antigen (Martinez et al., Citation2002). Ole e 3, Ole e 8 (calcium binding proteins) and Ole e 7 (lipid transfer protein) in addition to Ole e 2 are also remarkable allergenic components of olive pollen (Rodriguez et al., Citation2002). Ole e 5 (a member of superoxide dismutase family) and Ole e 4 were related to each other with average mass of 16 kDa, but differ in their allergenicity with Ole e 4 binds more to IgE than Ole e 5 as evidenced from band intensity of the immunoblotts (Carnes et al., Citation2002; Huecas, Villalba, & Rodriguez, 2001; Quiralte et al., Citation2002). Ole e 6 is an allergen without any known homologous counterpart as no protein sequence match was found in protein database (Quiralte et al., Citation2002; Rodriguez et al., Citation2002). Ole e 9, a polymorphic glycosylated protein (1.3-β-Glucanase), is a member of a family of hydrolytic enzymes common to higher plants and was reported to be involved in 65% of allergic patients to olive pollens (Huecas et al., Citation2001, Rodriguez et al., Citation2002). Ole e 10 (10 kDa) has been described as a new major allergen and shares IgE B-cell epitopes with proteins from different pollens (Quiralte et al., Citation2002, Citation2007). The incrimination of Ole e 10 in the development and exacerbation of asthma processes has been recently proposed (Barral, Batanero, Villalba, & Rodriguez, Citation2005). Furthermore, the protein profile of olive pollen displays several other high molecular weight polypeptides. Huecas and colleagues (2001) identified several allergic proteins of 42, 45–47, 60, 65 and 70 kDa.
The aim of this study was to investigate the allergenic and antigenic composition of olive pollen obtained from 36 different olive cultivars commonly grown in Jordan to identify the most allergenic olive tree cultivars that correlates with high titre IgE antibodies found in serum of patients suffering from olive pollens allergy and to identify the olive cultivars with hypoallergic pollens as well.
2. Materials and methods
2.1 Samples collection
Pollen grains were collected from 36 different cultivars of olive trees grown in Jordan using paper bags attached to the flowering branches of the olive trees, which were left for few days to collect only mature pollens from olive cultivars that were accurately identified by an olive expert. Pure pollens from flowers of 36 olive tree cultivars were collected and kept frozen at –70° C until used. The cultivars used in the study were; Mary Abu Shokh, Nabali Muhasan 1, Telmesani, Daibli, Ayvolik, Barouni, Shami, Picholine, Sorani1, Sigwase, Rasei, Nasouhi Jaba2, Canino, Yonani, Manzanillo, Abu Satl, Ascolano, Jekar, Of the Pegon, Itana, Frantoio2, Jlott, Cipressino, Mission 2, Sorani2, Khodeiry, Urmagic, Leccino, Touffahi, San Francesco, Coratina, Barnea (K18), Kfari Romi, Kfari Baladi, Qanabisi and Arabi Altafilah.
2.2 Allergen extracts
Pollen grains extracts were prepared following the protocol described by Carnes et al. (Citation2002) with brief modifications as following; 6 g of pollen were diluted in 1:5 wt/vol 0.1M phosphate-buffered saline (PBS), pH 7.4 containing 0.01% SDS and stirred for 4 h at 4° C. The extracts were centrifuged (16,000×g for 20 min at 4° C) and the supernatants were collected. Precipitates were again reconstituted in 1:5 wt/vol in 0.1M PBS pH 7.4 containing 0.01% SDS and extracted overnight at 4° C with continuous stirring. Afterwards, the extracts were centrifuged as above and the supernatants from both extractions were mixed, filtered and dialysed against double distilled water for 20 h in dialysis bags of a 6–8 kDa MW cutoff (Spectra, USA). The extracts were then filtered and kept frozen at –20° C until used. The protein content of each extract was determined by the Bradford protein assay following the manufacturer's instructions (Bio-Rad, USA).
2.3 Patient population
Serum was collected from 18 individuals (six males and 12 females) from northern part of Jordan with clinical symptoms typical for pollens allergy that peaks during the olive bloom and pollination. The symptoms exhibited by allergic individuals include the following; sneezing, coughing, runny noses, watery eyes, short breathing, redness, itching or asthma. The serum was collected and stored at –20° C. A serum pool was prepared with individual sera and was used for immunoblotting.
The study was approved by the Jordan University of Science and Technology committee for research on humans (IRP).
2.4 Electrophoresis
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli (Citation1970) in a Protean II cell vertical electrophoresis apparatus (Bio-Rad, USA). Separating and stacking gels containing 12% and 4% acrylamide, respectively, were used to separate the pollen proteins. Samples were denatured at 100° C for 2 min and centrifuged for 1 min at 1000×g. Equal amounts of pollen extract (10 µg) were mixed with 10 µl of reducing sample buffer and loaded onto the gels. Sigma pre-stained molecular weight standards were used to estimate the MW of allergens. Gels were run at a constant voltage (100 volt) until the tracking dye reached the end of the gel. Gels were either stained with Coomassiee Brilliant Blue R-250 or subjected to electroblotting.
2.5 Immunoblotting
Separated proteins were electrophoretically transferred from the SDS-PAGE gels onto 0.45 µm nitrocellulose membranes using a Trans-blot cell (Bio-Rad, USA). Immediately after electrophoresis, gels were equilibrated in Tris-Glycine electro-transfer buffer (0.025 M Glycine, 0.192 M Tris) for 15 min. Nitrocellulose membranes were also equilibrated in the same transfer buffer for 15 min before the transfer. Proteins were electro-blotted onto nitrocellulose membranes at 27 volts for 90 min.
Membranes were blocked with 15 ml PBS-Tween 20 (0.05%) containing 2% BSA for 1 h at room temperature followed by washing five times with 20 ml PBS-Tween 20 (0.05%). Membranes were incubated overnight at room temperature with 10 ml of 1:10 serum pool obtained from patients known to have allergy to olive pollen. Nitrocellulose membranes were washed five times with 20 ml PBS-Tween 20 (0.05%) and incubated with 15 ml of specific anti human IgE Alkaline-Phosphatase conjugate (Sigma-Aldrich, USA) diluted 1/2500 in PBS-Tween 20 (0.05%) for 2 h at 37° C. After a final washing step, bands were visualised by the addition of Diaminobenzidine (DAB) substrate. Colour development was stopped by rinsing the membranes with distilled water. Molecular weight of the protein bands were estimated using the Quantity One, 1 B software analysis version 4.6.3 from Bio-Rad.
3. Results
3.1 Protein content of the pollens
The 36 olive tree cultivars were arranged into four groups based on protein content of the pollens as shown in . Three of the cultivars (8%) contained very low protein content about 100 µg/ml of the extract while the majority, 21 cultivars (58%) contained from 100 to 200 µg/ml. The other nine cultivars (25%) contained between 200 and 300 µg protein per ml of extract and only three cultivars (8%) contained very high protein content (>300 µg/ml).
Table 1. Categories of the 36 olive cultivars based on the protein content of their pollens as determined by the Bradford protein assay.
3.2 Protein profiles following SDS-PAGE analysis
Analysis of SDS-PAGE gels of different olive pollen extracts revealed several protein bands from 15 to 95 kDa (). The SDS-PAGE profiles were divided into three groups with olive cultivars extracts exhibiting low/no protein bands, olive cultivars extracts exhibiting medium number of protein bands (5) and olive cultivars extracts exhibiting high number of protein bands (10). Results in shows a summary of the olive cultivars in each group.
Figure 1. SDS-PAGE (upper panel) and immunoblot analysis (lower panel) of pollens extracted from 36 olive cultivars grown in Jordan.
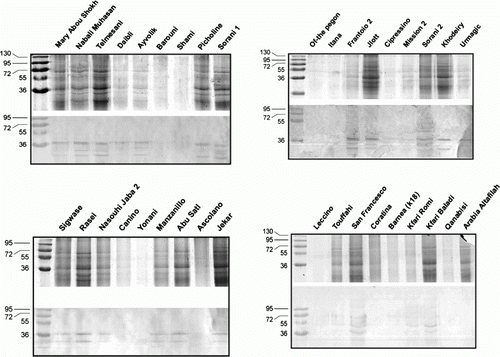
Table 2. Classification of 36 olive cultivars based on the number of protein bands exhibited SDS-PAGE gels.
3.3 Immunoblot analysis
The allergic profiles were studied by immunoblot analysis using pooled serum obtained from patients allergic to olive pollens. The allergic proteins recognised by the antiserum were mainly in the range of 17–72 kDa (). shows all the olive cultivars and the different allergens they possess. Eleven olive cultivars (Canino, Yonani, Ascolano, Pegon, Litana, Crpressino, Leccino, Coratina, Qanabisi, Arabi Altafilah and Shami) did not exhibit the presence of any allergen protein, while five cultivars (Mission 2, urmagic, Daibli, Baroni, Barnea [K18]) exhibited one allergen in each cultivar with protein corresponding to 36, 40 or 72 kDa. Eleven cultivars (Mary Abu Shokh, Touffahi, Kfari Romi, Kfari Baladi, Sigwase, Rasei, Nasouhi Jaba, Manzanillo, Abu Satl, Khodeiry, Jekar) exhibited two protein bands of 17, 25, 36, 40, 45, 55, 72 kDa for each cultivar, while the other seven cultivars (Nabali Muhasan 1, Telmisani, Ayvolik, Picholine, San Francisco, Frantoio 2, Jlott) each contained three allergic proteins corresponding to 17, 25, 30, 36, 40, 55, 72 kDa and thus were considered a major allergic cultivars among the 36 tested. Only two cultivars (Sorani 1 & Sorani 2) contained four allergic proteins corresponding to 17, 25, 30, 36 and 25, 36 40, 45 kDa, respectively, and thus by far, considered the most allergenic among the tested olive cultivars. Interestingly, Sorani 2 contained the highest amount of proteins in its pollen extract while Sorani 1 contained intermediate amount of pollen proteins. Nevertheless, more than one-third of the cultivars (Frantoio 2, Jlott, Sorani 1, Sorani 2, Nasouhi Jaba, Nabali Muhasan 1, Telmessani, Picholine, Rasei, Sigwase, Manzanillo, Abu Satl, and Jeker) contained two prominent bands corresponding to 25 and 36 kDa allergens.
Table 3. Olive cultivars and their content of allergen proteins as indicated by immunoblotting of extracted proteins and probed with a pool of human antiserum collected from diagnosed olive pollen allergy.
4. Discussion
Pollen proteins play an important role in pollen physiology (Alche et al., Citation2007); however, it is unknown whether the functions of these pollen proteins differ among various cultivars or whether the environment has an effect on their function. Biochemical and immunological studies of allergic proteins present in olive pollens showed that these proteins are polymorphic and vary greatly among different cultivars (Alche et al., Citation2007). The inhalation of olive pollen is one of the most common causes of pollen allergy in Mediterranean countries (Wheeler, Citation1992). In Jordan, there are more than 17 million olive trees covering about 36% of the entire agricultural land and many of these cultivars are planted without any prior knowledge on their allergic potential.
Generally, selection of the olive cultivars for planting in any region depends on climatic conditions, soil characteristics, availability of the cultivars and the general use of their fruits. It has been suggested that allergenicity of the olive pollen and probably of other trees depends on the cultivars (Besnard & Berville, Citation2000; Waisel et al., Citation1996). Therefore, prior knowledge of allergenic pollen producing olive tree cultivar is of great importance as to avoid planting such trees in urban areas or on street pavements, which is commonly practiced in some cities in the region including Jordan. In addition, the proper classification of trees in terms of allergenic potential might be of medical importance. Furthermore, reports from other countries such as Spain indicated that allergenicity of olive pollen might vary depending on the country of origin where the pollen protein profiles might vary depending salinity or other factors (Alche et al., Citation2007); however, no such data exist for the Mediterranean region.
In the present study, we evaluated the allergenic composition of olive pollens from 36 different cultivars collected from northern and middle parts of Jordan. Carnes et al. (Citation2002) reported that there are major differences in the allergenic composition of olive pollens collected from six different olive cultivars. Similarly, we also found great differences in the protein content of the pollen extracts of the 36 cultivars using SDS-PAGE and immunoblotting.
The olive cultivars were divided into four groups based on the amount of proteins extracted from 6 g of olive pollen (). Interestingly, the Manzanillo cultivar was among the cultivars that contained the highest amount of protein (340 µg/ml). Similar high protein content was also reported in Manzanillo cultivar by others (Carnes et al., Citation2002; Conde-Hernandez, Citation1994; Gonzalez-Quevedo, Citation1984) and was considered one of the most allergenic cultivars regardless of the place of origin or the climatic conditions it was grown. In addition, two other cultivars; Sigwase and Sorani 2 exhibited high protein content with 319 and 316 µg/ml, respectively. Immunoblotting experiments revealed that 11 cultivars did not show any antibody-reactive protein bands though all contained proteins as determined by Bradford protein assay. These 11 trees appeared to have no allergenic proteins or the proteins are extremely sensitive to heat or chemical denaturation received during the SDS-PAGE and the immunoblot analyses. As such, proteins from these cultivars could be considered non-allergenic or hypoallergenic compared to proteins from the other cultivars that are presumably considered resistant to both heat and chemical denaturation. Nevertheless, the other 25 cultivars contained 1–4 allergenic proteins ranging from 17 to 72 kDa (). Even though many proteins were identified in pollen extracts, each may represent a different form of the same protein as indicated by the closeness in the molecular weight, i.e. 36 vs. 40 kDa or 15 vs. 17/18 kDa.
Such variations might represent an adaptive advantage for the plant to different environments such as dryness, salinity or other adverse conditions (Alche et al., Citation2007). This could also explain the presence of several proteins in some cultivars as opposed to the absence of proteins in some other cultivars as the case in the 11 cultivars that apparently were devoid of any allergenic proteins. It is worth mentioning that six cultivars contained one protein each corresponding to 17 kDa. This protein could be any of the previously identified Ole e 1, Ole e 2, Ole e 4 or Ole e 5 proteins with molecular mass ranging from 16 to 20 kDa (Lauzurica et al., Citation1988; Carnes & Fernandez-Caldas, Citation2002). The other proteins had higher molecular weights ranging from 25 to 72 kDa (25, 30, 36, 40, 45, 55 and 72). Although some of these proteins seemingly had higher mass than those of Ole e 1, Ole e 2, Ole e 4 and Ole e 5, they might be variants of these proteins or simply dimmers especially those are in 36–45 kDa range (Rodriguez et al., Citation2002). Nevertheless, these proteins appeared similar to proteins reported in a study conducted by Huecas et al. (Citation2001) who reported the identification of allergic proteins in some olive cultivars with molecular mass range between 42 and 70 kDa. Furthermore, Carnes and Fernandez-Caldas (Citation2002) reported that a large proportion of the olive allergens are of 30 and 60 kDa. Similarly, our results indicate that olive pollen allergic proteins are highly variable among cultivars and from the region they were originated. In addition, Alche et al. (Citation2007) and Carnes and Fernandez-Caldas (Citation2002) has reported that pollen samples exhibit high batch to batch variability in several parameters such as protein profiles and the amounts of the allergen proteins. This might explain the absence of proteins in several of the cultivars, which may be dependent on certain environmental or processing conditions employed.
5. Conclusion
Olive cultivars have a great variation in the allergenic protein contents. Of 36 cultivars tested using sera from patients suffering possibly from olive pollen, four are considered hyperallergenic, 21 are moderately allergenic while 11 are hypoallergenic. The existence of less allergenic or hypoallergenic cultivars of olive tree would have a great importance in minimising exposure of public to olive pollen as olive trees are planted in urban areas; near residential houses and in the side of the street as an ornamental tree. Knowledge gained from this study would help select cultivars with low potential for allergenic pollen for planting near the residential areas or other parts of the region to prevent olive pollen induced allergic diseases.
Acknowledgements
The authors would like to acknowledge Deanship of Research at the Jordan University of Science and Technology for funding the project (Grant number 200/2008) in addition, the authors would like to thank Professor Arun K. Bhunia, from Purdue University for his critical reviewing of the manuscript.
Additional information
Notes on contributors
Alaa Al Bzour
King Saud University, Riyadh, Saudi ArabiaReferences
- Alche , J.D. , Castro , A.J. , Jimenez-Lopez , J.C. , Morales , S. , Zafra , A. Hamman-Khalifa , A.M. 2007 . Differential characteristics of olive pollen from different cultivars: Biological and clinical implications . Allergy , 17 : 69 – 75 .
- Barral , P. , Batanero , E. , Villalba , M. and Rodriguez , R. 2005 . Expression of the major olive pollen allergen Ole e 10 in the yeast Pichia pastoris: Evidence of post-translational modifications . Protein Expression and Purification , 44 : 147 – 154 .
- Besnard , G. and Berville , A. 2000 . Multiple origins for Mediterranean olives (Olea europaea L. sp. Europaea) based upon mitochondrial DNA polymorphisms . Academy of Science , 323 : 173 – 181 .
- Brito , F.F. , Mur Gimeno , P. , Carnes , J. , Martin , R. , Fernandez-Caldas , E. Lara , P. 2011 . Olea europaea pollen counts and aero allergen levels predict clinical symptoms in patients allergic to olive pollen . Annals of Allergy, Asthma & Immunology , 106 : 146 – 152 .
- Cardaba , B. , Llanes , E. , Chacartegui , M. , Sasre , B. , Lopez , E. Molla , R. 2007 . Modulation of allergic response by gene-environment interaction: Olive pollen allergy . Journal of Investigational Allergology & Clinical Immunology , 17 ( Suppl ) : 83 – 87 .
- Carnes , J. and Fernandez-Caldas , E. 2002 . Ole e 4 and Ole e 5, important allergens of Olea europaea . Allergy , 57 : 24 – 28 .
- Carnes , J. , Iraola , V. , Sastre , J. , Florido , F. , Boluda , L. and Fernandez-Caldas , E. 2002 . Allegenicity and immunochemical characterization of six cultivars of Ole europaea . Allergy , 57 : 313 – 318 .
- Conde-Hernandez , M. 1994 . Aislamiento caracterizacion y estandarizacion del extracto del polen de doce variedades de Olea europaea . (Doctoral dissertation, Sevilla, Espana). (In Spanish)
- Gonzalez-Quevedo , M.T. 1984 . Contribucion a la caracterizacion y estandarizacion de extractos alergenicos de polen de Olea europaea . (Doctoral dissertation, Sevilla, Espana). (In Spanish)
- Huecas , S. , Villalba , M. and Rodriguez , R. 2001 . Ole e 9, a major olive pollen allergen is a 1,3-β-glucanase . The Journal of Biological Chemistry , 276 : 27959 – 27966 .
- Kirmaz , C. , Yuksel , H. , Bayrak , P. and Yilmaz , O. 2005 . Symptoms of the olive pollen allergy: Do they really occur only in the pollination season? . Journal of Investigational Allergology & Clinical Immunology , 15 : 140 – 145 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Lahoz , C. and Florido , F. 2007 . Olive (Olea europea) pollen allergens-I. Immunochemical characterization by immunoblotting, CRIE and immunodetection by monoclonal antibodies . Allergology & Clinical Immunology , 17 : 53 – 55 .
- Lauzurica , P. , Gurbindo , C. , Maruri , N. , Galocha , B. , Diaz , R. Gonzalez , J. 1988 . Olive (Olea europea) pollen allergens-I. Immunological characterization by immunoblotting, CRIE and immunodetection by a monoclonal antibody . Molecular Immunology , 25 : 329 – 335 .
- Lombardero , M. , Obispo , T. , Calabozo , B. , Lezaun , A. , Polo , F. and Baber , D. 2002 . Cross-reactivity between olive and other species role of Ole e1-related species . Allergy , 57 : 29 – 34 .
- Martinez , A. , Asturias , A. , Monteseirin , J. , Moreno , V. , Garcia , A. Hernandez , M. 2002 . The allergenic relevance of profilin (Ole e2) from Olea europaea pollen . Allergy , 57 : 17 – 23 .
- Ministry of Agriculture Annual Report 2008 . Ministry of Agriculture . Jordan .
- Quiralte , J. , Florido , F. , Arias , M. , Gomez , A. , Saenz , B. Gonzalez , E. 2002 . Olive allergen-specific IgE responses in patients with Olea europaea pollinosis . Allergy , 57 : 47 – 52 .
- Quiralte , J. , Palacios , L. , Rodriguez , R. , Cardaba , B. , Arias de Saavedra , J.M. Villalba , M. 2007 . Modelling diseases; the allergens of Olea europaea pollen . Journal of Investigational Allergology & Clinical Immunology , 17 : 76 – 82 .
- Rodriguez , R. , Villalba , M. , Batanero , E. , Gonzalez , E.M. , Monsalve , R.I. Huecas , S. 2002 . Allergenic diversity of the olive pollen . Allergy , 57 : 6 – 16 .
- Waisel , Y. , Geller-Bernstein , C. , Keynan , N. and Arad , G. 1996 . Antigenicity of the pollen proteins of various cultivars of Olea europaea . Allergy , 51 : 819 – 825 .
- Wheeler , A.W. 1992 . Hypersensitivity to the allergens of the pollen from the olive tree (Olea europaea) . Clinical and Experimental Allergy , 22 : 1052 – 1057 .