Abstract
A novel bi-hapten immunogen was prepared by coupling amoxicillin and chlortetracycline to bovine serum albumin in turn. Then the immunogen was used to immunise New Zealand rabbits to produce the polyclonal antibody. The obtained antibody simultaneously cross-reacted with six penicillins (amoxicillin, penicillin G, ampicillin, penicillin V, carbencillin and sulbenicillin) and four tetracyclines (tetracycline, oxytetracycline, doxycycline and chlortetracycline) with crossreactivities in a range of 24–100%. Then a broad specificity enzyme linked immunosorbent assay was optimised for the simultaneous detection of the 10 drugs in milk. The limits of detection for these analytes were in a range of 0.4–3.7 ng/mL depending on the compound. The intra- and inter-assay recoveries of the 10 analytes from standards fortified blank milk ranged from 80.8 to 99.4% with coefficients of variation lower than 13%. This study provided a new strategy for the development of multi-analyte immunoassay through immunogen design.
1. Introduction
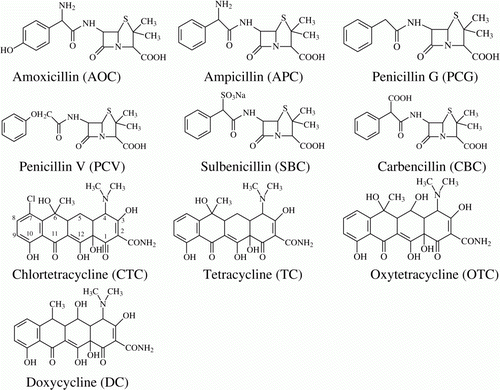
Penicillins (PCs) and tetracyclines (TCs) are the usually used drugs for the treatment of bacteria-induced diseases in animals, for example, the mastitis in dairy cow, due to their low toxicity and high/broad antibacterial activity. However, the residues of PCs and TCs in milk have been proven to cause allergic reactions to consumers (Sullivan, Wedner, Shatz, Yecies, & Parker, Citation1981) and accelerate the spreading of antimicrobial resistance (Chopra & Roberts, Citation2001; Jacoby, Citation2006; Roesch, Perreten, Doherr, Schaeren, Schallibaum, & Blum, Citation2006). Therefore, the Food and Drug Administration of USA has laid down the maximum residue limits (MRLs) for some PCs in milk, for example, PC, 5 ng/mL; ampicillin (APC), 10 ng/mL; and amoxicillin (AOC), 10 ng/mL. In addition, a MRL of 100 ng/mL for TCs in milk has also been set – as the sum of TC, oxytetracycline (OTC), doxycycline (DC) and chlortetracycline (CTC). The common PCs and TCs are shown in .
Therefore, it is important to monitor the residues of PCs and TCs in milk and the development of multi-analytes method for the determination of these drugs is the aim of many researchers. By now, HPLC (Bailón-Pérez, García-Campaña, del Olmo Iruela, & Cruces-Blanco, 2009; Kukusamude, Santalad, Boonchiangma, Burakham, Srijaranai, & Chailapakul, Citation2010), LC-MS/MS (Van Holthoon, Mulder, van Bennekom, Heskamp, Zuidema, & van Rhijn, Citation2010) and immunoassay (Benito-Pena, Moreno-Bondi, Orellana, Maquieira, & Amerongen, Citation2005; Miura, Kouno, & Kitagawa, Citation1981; Samsonova, Shchelokova, Ivanova, Rubtsova, & Egorov, Citation2005; Strasser, Usleber, Schneider, Dietrich, Bürk, & Märtlbauer, 2003; Yeh et al., Citation2008) have been the commonly used methods for residue determination of PCs. In addition, biosensor (Gustavsson, Bjurling, & Ase, Citation2002) and receptor-based technique (Janine & Michael, Citation2007) were also reported. For residue determination of TCs, the commonly used methods were HPLC (Cinquina, Longo, Anastasi, Giannetti, & Cozzani, 2003; Samanidou, Nikolaidou, & Papadoyannis, Citation2007; Spisso, de Oliveira e Jesus, Gonçalves de Araújo Júnior, & Monteiro, Citation2007), LC-MS/MS (Al-Mazeedi et al., Citation2010; Andersen, Roybal, Gonzales, Turnipseed, Pfenning, & Kuck, Citation2005; Jing et al., Citation2009) and immunoassay (Jeon & Paeng, Citation2008; Pastor-Navarro, Morais, Maquieira, & Puchades, 2007; Zhang, Lu, Liu, Zhao, & Xi, 2007). There has been only one paper reported for simultaneous determination of PCs and TCs (Gaugain-Juhel et al., Citation2009).
Compared with these instrumental methods, enzyme linked immunosorbent assay (ELISA) is a low cost and sensitive method capable of screening large amount of samples in a single test. Strasser et al. (Citation2003) have developed an ELISA to detect seven PCs (APC, PC, AOC, oxacillin, cloxacillin, dicloxacillin and nafcillin) based on the anti-APC antibody. Yeh et al. (Citation2008) have developed an ELISA to determine AOC and they reported the anti-AOC antibody could simultaneously recognised AOC, APC, PC, oxacillin and cloxacillin. Pastor-Navarro et al. (Citation2007) have developed an ELISA to detect OTC, TC and CTC based on the anti-OTC antibody. Zhang et al. (Citation2007) have developed an ELISA to detect TC and CTC based on the anti-TC antibody. However, there has been no ELISA method reported for the simultaneous detection of PCs and TCs.
For development of an ELISA to detect the two classes of drugs simultaneously, the class generic antibody of PCs and TCs can be incorporated into a single test, but those antibodies have to be prepared individually. An alternate way is to employ an antibody for simultaneously recognising PCs and TCs. However, there has been no paper reporting the production of such a broad-specific antibody.
The previous reports have shown the respective class-generic antibody of the two classes was obtained with a PCs drug or a TCs drug as the hapten (Pastor-Navarro et al., Citation2007; Strasser et al., Citation2003; Yeh et al., Citation2008; Zhang et al., Citation2007). Then we speculated that the broad-specific antibody for recognising both PCs and TCs might be obtained from an immunogen for simultaneously containing the class-generic hapten of PCs and TCs drugs. Based on the previous reports and the careful observation of their structures (), AOC and CTC were used to prepare a novel bi-hapten immunogen to produce the broad-specific polyclonal antibody. The obtained antibody was then used to develop an indirect competitive ELISA for the simultaneous detection of PCs and TC in milk. The details of the experiment are described in the following section.
2. Materials and methods
2.1. Reagents and chemicals
Standards of penicillin G potassium salt (PCG) (95.7%), amoxicillin anhydrous (AOC) (87%), ampicillin trihydrate sodium salt (APC) (86.6%), penicillin V potassium salt (PCV) (93.7%), OTC, DC, TC, CTC, bovine serum albumin (BSA), ovalbumin (OA) and Freund's adjuvants were all purchased from Sigma (St. Louis, MO, USA). Carbencillin disodium salt (CBC) was obtained from Sangon Biotech Co., Ltd (Shanghai, China). Sulbenicillin (SBC) was obtained from China Institute of Veterinary Drug Control (Beijing, China). The substrate tetramethylbenzidine (TMB) was purchased from Serva (Heidelberg, Germany). Other chemical reagents were all analytical grade or better from Beijing chemical company (Beijing, China).
2.2. Solutions
Standard stock solutions of the six PCs were prepared with ultrapure water (1 mg/mL) to be stable for two weeks at 4°C, and those of the four TCs were prepared with methanol (1 mg/mL) to be stable at least for 4 weeks at 4°C. Their working solutions with series concentrations (0.2, 0.5, 1, 2, 5, 10, 20, 50, 100 ng/mL) were prepared daily by diluting the stock solutions with PBS to be stable for one week at 4°C. PBS (pH 7.2) was prepared by dissolving 0.2 g KH2PO4, 0.2 g KCl, 1.15 g Na2HPO4 and 8.0 g NaCl in 1000 mL deionised water. Coating buffer was carbonate buffer (0.1M, pH 9.6). Substrate buffer was 0.1M citrate (pH 5.5). The substrate system was prepared by adding 200 µL 1% (w/v) TMB in DMSO and 64 µL 0.75% (w/v) H2O2 into 20 mL substrate buffer. Mcllvaine–EDTA solution (0.1 mol/L) was prepared by dissolving 11.8 g C6H8O7·H2O, 27.6 g Na2HPO4·12H2O and 37.2 g EDTA·12H2O in 1000 mL water and the pH value was adjusted to 3.8 with concentrated hydrochloric acid. Extraction solvent was a mixture of acetonitrile and Mcllvaine–EDTA solution (50:50, v/v).
2.3. Preparation of the bi-hapten conjugates
The preparation of the AOC conjugates (immunogen AOC-BSA and coating antigen AOC-OA) was according to a previous report (Yeh et al., Citation2008). The CTC conjugates were prepared as follows. Briefly, 2 mL of methanol/PBS (1:1, v/v) containing 20 mg CTC was added into 5 mL of PBS containing 70 mg BSA or 30 mg OA dropwise. Then 100 µL of 25% glutaraldehyde (GA) was added and the solution was stirred at room temperature for 4 h to prepare the immunogen (CTC-BSA) or coating antigen (CTC-OA). The two conjugates were dialysed against PBS for 3 days at 4°C and the dialysate was stored at −20°C before use.
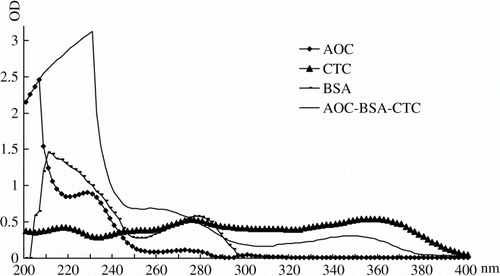
The bi-hapten immunogen was prepared by coupling AOC and CTC to BSA in turn and the preparation process is shown in . Firstly, AOC was coupled to BSA as the previous method and the coupling ratio of AOC to BSA was determined as a previous 2,4,6-trinitrobenzene sulfonic acid method (Sashidhar, Capoor, & Ramana, Citation1994). Secondly, CTC was coupled to AOC-BSA as described earlier(i.e. the conjugate AOC-BSA was regarded as an interim carrier protein) and the coupling ratio of CTC to AOC-BSA was determined. Thirdly, AOC, CTC, BSA and AOC-BSA-CTC were scanned on a UV spectrophotometer, respectively to identify the conjugation. Finally, the concentrations of the three immunogens (AOC-BSA, CTC-BSA and AOC-BSA-CTC) were diluted to 1 mg/mL (calculated as protein) for animal immunisation. The bi-hapten coating antigen (AOC-OA-CTC) was also prepared as the same method.
2.4. Production of the polyclonal antibodies
Fifteen New Zealand white rabbits were feed at Animal Experiment Center of College of Animal Science and Technology, Agricultural University of Hebei. The animal experiments were performed according to the Regulation Guideline for Experimental Animals issued by the Ministry of Science and Technology of China. The three immunogens were all used to produce the polyclonal antibodies. Fifteen New Zealand rabbits numbered R1–R15 were equally divided into five groups randomly. Rabbits R1–R3 were immunised with AOC-BSA, rabbits R4–R6 were immunised with CTC-BSA, rabbits R7–R9 were immunised with AOC-BSA-CTC, rabbits R10–R12 were immunised with AOC-BSA and CTC-BSA alternately and rabbits R13–R15 were immunised with the mixture of AOC-BSA and CTC-BSA (1:1, v/v). The rabbits were immunised with the emulsion of the immunogen (0.5 mg per animal, calculated as protein) in Freund's complete adjuvant at the first time and were boosted with the immunogen in Freund's incomplete adjuvant at a 3-week interval. After eight boosters, whole blood of each rabbit was obtained and the sera were collected. Finally, the IgG fraction was isolated using the saturated ammonium sulphate precipitation method to develop the ELISA.
2.5. Development and optimisation of the ELISA
A checkerboard procedure was used to determine the optimal dilutions of coating antigens and antibodies, in which the well with an absorbance of 1.2 was defined as the optimal dilution of coating antigen and antibody, respectively. Then the specificities of these antibodies for the drugs shown in were determined firstly by an indirect competitive ELISA method with AOC-OA or CTC-OA as the coating antigen. Briefly, the wells of a microtiter plate were coated with the coating antigen (100 µL/well). Then the plate was incubated overnight at 4°C and blocked with 1% foetal calf serum. The plate was washed three times with PBS, and then, 50 µL of the antibody dilution and 50 µL of standard solution (AOC, CTC or other analyte) with series concentrations were added to the wells (in triplicate) for incubation for 1 h at 37°C. After washes, 100 µL of horseradish peroxidase-labelled goat anti-rabbit IgG was added for incubation for 30 min at 37°C. After washes, 100 µL of TMB substrate system was added for incubation for 15 min at 37°C. Finally, the reaction was stopped by addition of 50 µL of sulphuric acid (2 mol/L), and the plate was read on an ELISA plate reader at 450 nm to obtain the OD values.
After that, AOC-OA-CTC, the mixture of AOC-OA and CTC-OA (1:1, v/v), and the antibodies of recognising both PCs and TCs were incorporated into several combinations to optimise the broad specificity ELISA as described earlier. The drugs shown in and several other drugs (cephalexin (CE), chloramphenicol (CP) and clenbuterol (CL)) were all determined by the optimised ELISA. The cross-reactivity (CR) towards these competitors were calculated as follows: CR (%)=100×IC50AOC or CTC/IC50competitor (the CRs for PCs were based on IC50 AOC and that of for TCs were based on IC50CTC). The half of inhibition concentrations (IC50) and the limits of detection (LODs) for these drugs were determined, which were defined as the concentrations of showing 50 and 10% of inhibition, respectively. The competitive inhibitory curves were developed by plotting the B/B 0 values (mean OD value of the standards divided by the zero standards) versus the concentrations (Log C).
2.6. Sample preparation
A volume of 5 mL of milk and 20 mL of extraction solvent were added into a centrifuge tube. The tube was vortexed for 5 min and left to stand for 15 min. Then, the tube was centrifuged at 4000 rpm for 15 min. The supernatant was transferred into a clean tube. The milk sample was extracted with another 20 mL of extraction solvent. The extracts were combined and evaporated to dryness under vacuum on a water bath at 50°C. The dry residue was reconstituted in 5 mL of PBS and the solution was filtrated with a 0.22 µm nylon Millipore filter for ELISA analysis.
Some blank raw milk samples milked from the controlled health cows were used for assessment of the matrix influence and the method recovery. The matrix-matched standards prepared with the extracts of blank milk sample were also used to develop the competitive curves. Recovery experiments were carried out to determine the accuracy (intra- or inter-assay recovery) and precision (coefficient of variation [CV]) of the method by fortification of these standards in blank milk at levels of 2–100 ng/mL (depending on the drug). Furthermore, 60 unknown raw milk samples milked from several farms were analysed with the proposed method.
3. Results and discussion
3.1. Bi-hapten immunogen
Yeh et al. (2008) have reported the antibody of AOC was cross-reactive with seven PCs and the antibody mainly recognised 6-aminopenicillanic acid and the benzene ring in their molecules. Therefore, AOC was chosen to prepare the bi-hapten immunogen. As for TCs, they contain the same core chemical structure and the differences are the substituents on the position of C5, C6 and C7 (). Our plan was to select one drug as the optimal hapten and link it with carrier at the carboxamide position, so the complexity of the structure far from the linking position is critical for the selectivity of the obtained antibody. After comprehensive comparison of the complexity around the three positions, CTC was selected as the optimal generic hapten of TCs to prepare the bi-hapten immunogen.
Then, AOC and CTC were coupled to BSA in turn by using glutaraldehyde method (). This method was successful for AOC (Yeh et al., Citation2008) but has not been reported for the preparation of CTC immunogen. By this means, the carboxamide group in CTC molecule was linked with the amidogen group in BSA with a 5-carbon chain as the space arm, that is, the core structure of TCs and the substituents in CTC as a general sophisticated structure is far from the carrier. Therefore, the antibody against such a CTC immunogen is supposed to recognise the four TCs. The previous report has proven that an immunogen with a long space arm between the hapten and the carrier can generate the antibody with high binding ability for the hapten (Franek et al., Citation2001). In the bi-hapten immunogen, AOC and CTC were all far from the carrier with a long 5-carbon chain as space arm, so the obtained antibody is supposed to recognise these PCs and TCs drugs simultaneously.
Figure 2. Preparation procedure of the bi-hapten conjugates by coupling amoxicillin and chlortetracycline to carrier in turn.
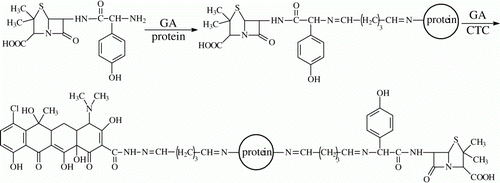
During the conjugation of CTC to BSA or CTC to AOC-BSA, the yellow colour was always present throughout the process of coupling, purification and dialysis. Furthermore, the UV scan diagram of AOC-BSA-CTC contained the characteristic peaks of BSA, AOC and CTC (). These two findings indicated AOC and CTC were coupled to BSA. The UV diagrams of AOC-BSA and CTC-BSA all contained the peaks of the respective hapten and the carrier (data not shown), indicating the two single-hapten immunogens were obtained. The coupling ratio of hapten/carrier is important for an ELISA method, because an immunogen with extremely high level of coupling ratio could not produce the high specificity antibody and the sensitivity of an ELISA with more substituted coating antigen was lower than that of with less substituted coating antigen (Franek, Diblikova, Cernoch, Vass, & Hruska, 2006). In this study, the coupling ratios in AOC-BSA and AOC-OA were 21 and 14, and were 19 and 13 in CTC-BSA and CTC-OA, respectively, and that of in AOC-BSA-CTC and AOC-OA-CTC were 18 (AOC):15 (CTC) and 11 (AOC):10 (CTC), respectively. These hapten densities are medium and appropriate.
3.2. Antibody performance
The antibodies showing the highest titre in each group (R2, R4, R8, R12 and R13) were selected for the subsequent experiments. Their specificities and sensitivities for these PCs and TCs were evaluated by indirect competitive ELISA.
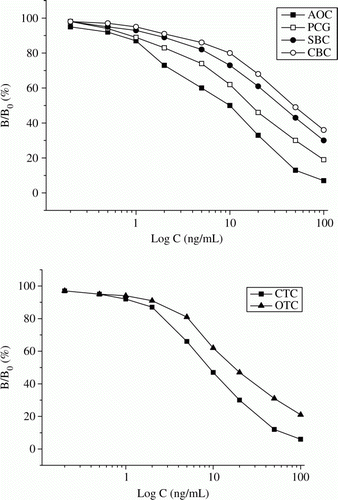
As shown in , antibody R2 only cross-reacted with the six PCs (APC, AOC, PCG, PCV, SBC and CBC) with CRs in the range of 31–100% and these results were similar to the previous report (Yeh et al., Citation2008). Antibody R4 only cross-reacted with the four TCs with CRs in the range of 19–100% and these results were better than that of the previous reports (Pastor-Navarro et al., Citation2007; Zhang et al., Citation2007). This means the use of AOC and CTC as the generic hapten of PCs and TCs was appropriate. As can be expected, antibody R8, R12 and R13 recognise the 10 analytes simultaneously with CRs in the range of 9–100% (). Therefore, this paper first reported the production of the antibodies for simultaneously recognising PCs and TCs. The broad specificity of R8 also indicated the bi-hapten immunogen was successfully prepared. All the five antibodies showed no cross reactivity to other competitors (CE, CP and CL).
Table 1. Specificity and sensitivity of the five antibodies.
However, the IC50 values of R8 for PCs and TCs were higher than that of R2 or R4 (). This means for the six PCs, antibody R8 was less sensitive than R2 and for the four TCs, antibody R8 was less sensitive than R4. This is because antibody R8 was from the bi-hapten immunogen (two epitopes), and antibody R2 and R4 were from the respective single-hapten immunogen (one epitope); a ‘one-epitope’ immunogen can generate the antibody with higher recognition ability for the hapten than a ‘two-epitope’ immungen. Furthermore, an antibody from an immunogen with higher level of coupling ratio showed lower binding ability for the analytes (Franek et al., Citation2006). The coupling ratio in AOC-BSA-CTC (33) was higher than that of in AOC-BSA (21) and CTC-BSA (19), so the sensitivity of antibody R2 and R4 for PCs or TCs was higher than that of R8. Similarly, the performances of antibody R12 and R13, which were from the alternated immunogen or mixed immungens, were worse than R2 and R4.
3.3. Optimisation of the ELISA
The purpose of this study was to develop a multi-analyte ELISA for simultaneous detection of PCs and TCs drugs. Therefore, the antibodies of recognising both PCs and TCs (R8, R12 and R13) and two coating antigens (AOC-OA-CTC and the mixture of AOC-OA and CTC-OA) were incorporated into six combinations to optimise the ELISA.
As shown in , antibody R8, R12 and R13 all showed broad specificity to the six PCs and the four TCs. Among the three antibodies, the performances of antibody R8 (CRs≥29%, LODs≤4.5 ng/mL) were always better than that of antibody R12 and R13 (CR 8–100% and LODs 1.4–18.0 ng/mL) no matter with AOC-OA-CTC or the mixture of AOC-OA and CTC-OA as a coating antigen. This is because AOC-BSA-CTC, as a single immunogen, can stimulate the lymphocyte to produce the antibody more consistently than the alternated or the mixed immunogens. Furthermore, the total coupling ratio in the alternated and the mixed immunogens (AOC-BS, 21; CTC-BSA, 19) higher than that of in AOC-BSA-CTC (33) maybe is also a reason.
Table 2. Results of the optimised ELISA for the PCs and TCs.
The sensitivities and specificities of the three antibodies were variable depending on the used coating antigen. As shown in , when AOC-OA-CTC was as a coating antigen the CRs (> 19%) and LODs (0.4–11.8 ng/mL) were generally better than the CRs (> 9%) and LODs (0.8–18 ng/mL) when used as a mixed coating antigen. This is because the total coupling ratio in the mixed coating antigen (27) was higher than the coupling ratio in AOC-OA-CTC (21); just as Franek et al. (Citation2006) have reported the more substituted coating antigen may decrease the method sensitivity. There is another possibility that the reciprocal spatial interference of AOC and CTC in AOC-OA-CTC maybe influences the coating antigen's competitive binding to the antibody, thus, improving the antibody binding to the competitors (contributing to broad specificity).
Still, the LODs of the six combinations were all sensitive enough to detect the residues of these PCs and TCs below their MRL levels. Among the six combinations, AOC-OA-CTC combining R8 was the best combination with CRs higher than 31% and LODs in the range of 0.4–3.7 ng/mL (). Therefore, the ELISA incorporated the two reagents was used for the subsequent experiments. The inhibitory curves for these PCs and TCs are shown in and with concentrations of 0.2–100 ng/mL.
3.4. Sample extraction and ELISA determination
This is the first paper reporting an ELISA method to determine six PCs and four TCs in milk simultaneously. For extraction of these PCs and TCs from milk, a suitable extraction solvent is required. Acetonitrile has been reported to extract 10 PCs in milk with recoveries higher than 82.9% (Bailón-Pérez et al., Citation2009). Mcllvaine buffer has been reported to extract TCs in food samples with recoveries higher than 81% (Cinquina et al., Citation2003). In this study, the mixture of acetonitrile and Mcllvaine–EDTA solution (50:50, v/v) was used as extraction solvent. The use of EDTA here was to remove the possible present metal ions in milk and avoid the formation of TCs-metal ion chelates.
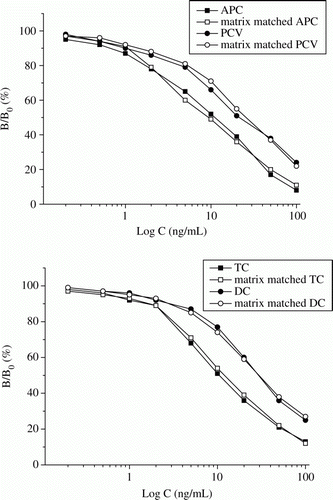
An important step to evaluate an analytical method is to assessment of the matrix effect. The matrix-matched solutions of PCs and TCs prepared with blank extracts were used to develop the matrix-matched competitive curves. As shown in , the matrix-matched competitive curves of APC, PCV, TC and DC were similar to that of their standards in PBS, revealing the matrix influence is minimal. Then, the LODs for these drugs in milk sample were according to their respective standards, that is, 0.4–3.7 ng/mL. Blank milk samples fortified each drug at different levels were analysed to evaluate the accuracy and precision of the ELISA method. As shown in , the intra-assay recoveries were in the range of 81.9–99.4% with CV lower than 9.2% and the inter-assay recoveries were in 80.8–99.2% with CV lower than 13%.
Table 3. Recoveries of the 10 PCs and TCs from blank milk (n=6).
3.5. Unknown samples
Three out of the 60 unknown milk samples were determined as positive, but the ELISA method cannot verify the specific analyte due to the antibody's broad specificity. The three samples were further determined with AOC-OA and CTC-OA replacement of AOC-OA-CTC, that is, by using single-hapten coating antigen. Results showed that one sample contained TCs residue (82 ng/mL, calculated as CTC) and two samples contained PCs residue (15 ng/mL and 37 ng/mL, calculated as AOC). This implies the proposed multi-analyte ELISA can detect an unknown sample as positive or negative, but the result needs to be confirmed with a confirmatory method, for example, HPLC or LC-MS. Such instrumental method of simultaneous determination of these PCs and TCs is remained to be studied.
4. Conclusion
This study first reported the preparation of a novel bi-hapten immunogen and the development of an ELISA for simultaneous detection of six PCs and four TCs. In general, the proposed ELISA method is better than the previously reported ELISA methods, though the results need to be confirmed with other instrumental method. Therefore, the present ELISA can be used as a rapid and simple screening tool for routine monitoring the residues of PCs and TCs in large numbers of milk samples.
Acknowledgements
The authors of this work are grateful for the financial support of Hebei Natural Science Foundation (C2011204021) and Shijiazhuang Scientific and Technological Project (11150252A).
Additional information
Notes on contributors
Jing Liu
Sai Nan Jiao and Jing Liu contributed equally to this workReferences
- Al-Mazeedi , H.M ., Abbas , A.B ., Alomirah , H.F ., Al-Jouhar , W.Y ., Al-Mufty , S.A ., Ezzelregal , M.M ., et al. 2010 . Screening for tetracycline residues in food products of animal origin in the State of Kuwait using Charm II radio-immunoassay and LC/MS/MS methods . Food Additives and Contaminants A , 27 , 291 – 301 . doi: 10.1080/19440040903331027
- Andersen , W.C ., Roybal , J.E ., Gonzales , S.A ., Turnipseed , S.B ., Pfenning , A.P ., & Kuck , L.R . 2005 . Determination of tetracycline residues in shrimp and whole milk using liquid chromatography with ultraviolet detection and residue confirmation by mass spectrometry . Analytica Chimica Acta , 529 , 145 – 150 . doi: 10.1016/j.aca.2004.08.012
- Bailón-Pérez , M.I ., García-Campaña , A.M ., del Olmo Iruela , M ., & Cruces-Blanco , C . 2009 . Trace determination of 10 β-lactam antibiotics in environmental and food samples by capillary liquid chromatography . Journal of Chromatography A , 1216 , 8355 – 8361 . doi: 10.1016/j.chroma.2009.09.042
- Benito-Pena , E ., Moreno-Bondi , M.C ., Orellana , G , Maquieira , A ., & Amerongen , A.V . 2005 . Development of a novel and automated fluorescent immunoassay for the analysis of beta-lactam antibiotics . Journal of Agricultural and Food Chemistry , 53 17 , 6635 – 6642 . doi: 10.1021/jf0511502
- Chopra , I . & Roberts , M . 2001 . Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance . Microbiology and Molecular Biology Reviews , 65 , 232 – 260 . doi: 10.1128/MMBR.65.2.232-260.2001
- Cinquina , A.L ., Longo , F ., Anastasi , G ., Giannetti , L ., & Cozzani , R . 2003 . Validation of a high-performance liquid chromatography method for the determination of oxytetracycline, tetracycline, chlortetracycline and doxycycline in bovine milk and muscle . Journal of Chromatography A , 987 , 227 – 233 . doi: 10.1016/S0021-9673(02)01446-2
- Franek , M ., Diblikova , I ., Cernoch , I ., Vass , M ., & Hruska , K . 2006 . Broad-specificity immunoassays for sulfonamide detection: Immunochemical strategy for generic antibodies and competitors . Analytical Chemistry , 78 5 , 1559 – 1567 . doi: 10.1021/ac0514422
- Franek , M ., Zeravik , J ., Eremin , S.A ., Yakovleva , J ., Badea , M ., Danet , A ., et al. . 2001 Antibody-based methods for surfactant screening . Fresenius Journal of Analytical Chemistry , 371 , 456 – 466 . doi: 10.1007/s002160101079
- Gaugain-Juhel , M ., Delepine , B ., Gautier , S ., Fourmond , M.P ., Gaudin , V ., Hurtaud-Pessel , D ., et al. 2009 . Validation of a liquid chromatography–tandem mass spectrometry screening method to monitor 58 antibiotics in milk: A qualitative approach . Food Additive & Contaminant A , 26 , 1459 – 1471 . doi: 10.1080/02652030903150575
- Gustavsson , E ., Bjurling , P ., & Ase , S.O . 2002 . Biosensor analysis of penicillin G in milk based on the inhibition of carboxy peptidase activity . Analytica Chimica Acta , 468 , 153 – 159 . doi: 10.1016/S0003-2670(02)00599-8
- Jacoby , G.A . 2006 . β-Lactamase nomenclature . Antimicrobial Agents and Chemotherapy , 50 , 1123 – 1129 . doi: 10.1128/AAC.50.4.1123-1129.2006
- Janine , L ., & Michael , P . 2007 . Development of a receptor-based microplate assay for the detection of beta-lactam antibiotics in different food matrices . Analytica Chimica Acta , 586 , 296 – 303 . doi: 10.1016/j.aca.2006.09.032
- Jeon , M . & Paeng , I.R . 2008 . Quantitative detection of tetracycline residues in honey by a simple sensitive immunoassay . Analytica Chimica Acta , 626 , 180 – 185 . doi: 10.1016/j.aca.2008.08.003
- Jing , T ., Gao , X.D ., Wang , P ., Wang , Y ., Lin , Y.F ., Hu , X.Z ., et al. 2009 . Determination of trace tetracycline antibiotics in foodstuffs by liquid chromatography tandem mass spectrometry coupled with selective molecular-imprinted solid-phase extraction . Analytical and Bioanalytical Chemistry , 393 , 2009 – 2018 . doi: 10.1007/s00216-009-2641-z
- Kukusamude , C ., Santalad , A ., Boonchiangma , S ., Burakham , R ., Srijaranai , S ., & Chailapakul , O . 2010 . Mixed micelle-cloud point extraction for the analysis of penicillin residues in bovine milk by high performance liquid chromatography . Talanta , 81 1–2 , 486 – 492 . doi: 10.1016/j.talanta.2009.12.029
- Miura , T. , Kouno , H. and Kitagawa , T. 1981 . Detection of residual penicillin in milk by sensitive enzyme immunoassay . Journal of Pharmacobiodyn , 4 ( 9 ) : 706 – 710 .
- Pastor-Navarro , N ., Morais , S ., Maquieira , A ., & Puchades , R . 2007 . Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues: Application to honey samples . Analytica Chimica Acta , 594 , 211 – 218 . doi: 10.1016/j.aca.2007.05.045
- Roesch , M ., Perreten , V ., Doherr , M.G ., Schaeren , W ., Schallibaum , M ., & Blum , J.W . 2006 . Comparison of antibiotic resistance of udder pathogens in dairy cows kept on organic and on conventional farms . Journal of Dairy Science , 89 , 989 – 997 . doi: 10.3168/jds.S0022-0302(06)72164-6
- Samanidou , V.F ., Nikolaidou K.I ., & Papadoyannis , I.N . 2007 . Development and validation of an HPLC confirmatory method for the determination of seven tetracycline antibiotics residues in milk according to the European Union regulation 2002/657/EC . Journal of Separation Science , 30 , 2430 – 2439 . doi: 10.1002/jssc.200700057
- Samsonova , Z ., Shchelokova , O ., Ivanova , N ., Rubtsova , M ., & Egorov , A . 2005 . Enzyme-linked immunosorbent assay of ampicillin in milk . Applied Biochemistry and Microbiology , 41 6 , 589 – 595 . doi: 10.1007/s10438-005-0107-4
- Sashidhar , R.B. , Capoor , A.K. and Ramana , D. 1994 . Quantitation of amino groups using amino acids as reference standards by trinitrobenzene sulfonic acid: A simple spectrophotometric method for the estimation of hapten to carrier protein ratio . Journal of Immunology Methods , 167 : 121 – 127 .
- Spisso , B.F ., de Oliveira e Jesus , A.L ., Gonçalves de Araújo Júnior , M.A ., & Monteiro , M.A . 2007 . Validation of a high-performance liquid chromatographic method with fluorescence detection for the simultaneous determination of tetracyclines residues in bovine milk . Analytica Chimica Acta , 581 , 108 – 117 . doi: 10.1016/j.aca.2006.08.004
- Strasser , A ., Usleber , E ., Schneider , E ., Dietrich , R ., Bürk , C ., & Märtlbauer , E . 2003 . Improved enzyme immunoassay for group-specific determination of penicillins in milk . Food and Agricultural Immunology , 15 2 , 135 – 143 . doi: 10.1080/09540100400003493
- Sullivan , T.J. , Wedner , H.J. , Shatz , G.S. , Yecies , L.D. and Parker , C.W. 1981 . Skin testing to detect penicillin allergy . Journal of Allergy and Clinical Immunology , 68 : 171 – 180 .
- Van Holthoon , F ., Mulder , P.P ., van Bennekom , E.O ., Heskamp , H ., Zuidema , T ., & van Rhijn , H.J . 2010 . Quantitative analysis of penicillins in porcine tissues, milk and animal feed using derivatisation with piperidine and stable isotope dilution liquid chromatography tandem mass spectrometry . Analytical and Bioanalytical Chemistry , 396 8 , 3027 – 3040 . doi: 10.1007/s00216-010-3523-0
- Yeh , L.C ., Lee , W.M ., Koh , B.W ., Chan , J.P ., Liu , C.H ., Kao , J.P ., et al. 2008 . Development of amoxicillin enzyme-linked immunosorbent assay and measurements of tissue amoxicillin concentrations in a pigeon microdialysis model . Poultry Science , 87 , 577 – 587 . doi: 10.3382/ps.2007-00167
- Zhang , Y ., Lu , S ., Liu , W ., Zhao , C ., & Xi , R . 2007 . Preparation of anti-tetracycline antibodies and development of an indirect heterologous competitive enzyme-linked immunosorbent assay to detect residues of tetracycline in milk . Journal of Agricultural and Food Chemistry , 55 , 211 – 218 . doi: 10.1021/jf062627s