Abstract
Gastric cancer (GC) is the fourth most common cancer and second leading cause of cancer-related death worldwide. Therefore, discovery of novel anti-cancer herbal drugs is of importance. Herbal medication is now being used for treatment of various diseases, including cancer in many countries. In this study, the cytotoxic effect of traditional herbal medicines (Aloe vera, Ginger, Ziziphora and Saffron extracts) was investigated on gastric AGS cell line. MTT assay was performed for cytotoxic effect of these herbal medicines. The most cytotoxic effects of Ginger, Ziziphora and Saffron extracts on AGS cell line were 47, 88 and 67%, respectively, compared to control group. According to our data, among these herbal extracts, Ziziphora has the highest cytotoxic effect on AGS cell line. Ziziphora extract seems to be a good candidate as an anti-cancer agent against GC. To clarify the effective molecules and their mechanisms, further studies are undertaken on animal models and humans.
1. Introduction
Gastric cancer (GC) is the fourth most common form of malignant diseases in the worldwide (Tepes, Citation2009; Tsugane & Sasazuki, Citation2007). Because GC prognosis is poor and it is often diagnosed in locally advanced or metastatic stages, its treatment remains a great challenge (Tetzlaff, Cen, & Ajani, Citation2008; Zaniboni & Meriggi, Citation2005).
Herbal medicines are rich sources of natural anti-cancer materials (Tan & Vanitha, Citation2004) and are thus good candidates for the development of anti-cancer drugs. In an attempt to identify novel herbal anti-cancer agents from traditional herbal medicines, we studied the in vitro cytotoxic effects of the Aloe vera, Ginger, Ziziphora and Saffron extracts on adenocarcinoma gastric cell line (AGS).
Aloe vera, a member of the family Liliaceae, is used as a natural treatment for various types of diseases such as cancer (El-Shemy et al., Citation2010; Vega, Uribe, Lemus, & Miranda, Citation2007).
Ginger, the rhizome of Zingiber officinale, is one of the most widely used species of the Ginger family (Zingiberaceae) which exhibits anti-oxidant, anti-inflammatory, anti-fungal and anti-carcinogenic proprieties (Surh, Citation2002; Surh, Lee, & Lee, Citation1998). According to our knowledge, there are no more studies regarding cytotoxic effect of Ginger extract on AGS cell line (Ishigura et al., 2007).
Since ancient times, Saffron (Crocus sativus L.) has been used in traditional medicine for various ailments (Abdullaev & Espinosa-Aguirre, Citation2004; Chryssanthi et al., Citation2007). Cytotoxic effect of Saffron extract has been studied in some cancer cell lines (Abdullaev, 2002, Abdullaev et al., 2003; Goel & Aggarwal, Citation2010), but it has not been investigated on AGS cell line, up to now.
Ziziphora (Z. clinopodioide Lam), a traditional Uygur medicinal plant is used for the treatment various diseases (Salehi, Sonboli, Eftekhar, Nejad-Ebrahimi, & Yousefzadi, Citation2005; Tian, Shi, Yu, & Upur, Citation2010). According to our knowledge, this is the first study of the effects of an aqueous extract of Ziziphora on cell viability of cancer cell line.
Herein, we investigated the cytotoxic effect of the extracts of these four herbal medicines on AGS gastric cancer cell line.
2. Materials and methods
2.1. Reagents
RPMI-1640 medium and foetal bovine serum (FBS) were obtained from Gibco. Penicillin–streptomycin was obtained from Sigma-Aldrich, USA. MTT [3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] powder and phosphate buffer saline (PBS) were obtained from Merck (Germany).
2.2. Plant materials
To prepare the extracts, the valuable part of each plant in traditional medicine was used. The gel of Aloe vera was supplied by Aloe vera Co. (Bushehr, Iran). Stigma of Saffron was collected from Mashhad (Iran), and aerial parts of Ziziphora and rhizomes of Ginger were supplied by local market (Tehran, Iran). These plants were then processed in Pharmacological Research Centre of Medicinal Plants, Shahed University.
2.3. Preparation of herbal extracts
In the present study, total aqueous herbal extracts were prepared by mixing with distilled and deionised water and centrifuged (5000 g/30 min), and the resultant supernatant was collected and stored in refrigerator until used. All extracts were used in different concentrations (5, 2, 1, 0.2. 0.1, 0.05, 0.02 and 0.01 mg/ml) based on their weight (mg) per water volume (ml).
2.4. Cell culture
Adenocarcinoma gastric cell line (AGS) was purchased from Pasteur Institute (Tehran, Iran) and maintained in RPMI with 10% FBS (both of them from Gibco) incubated at 37°C and 5% CO2. Exponentially growing AGS cells were digested by 2 ml trypsin 0.25% for 1–2 min. RPMI 1640 medium containing 10% FBS was subsequently added. Final cell suspensions were placed in 96-well plates (2 ×104/200 µl/well) in an incubator containing 5% CO2 and incubated at 37°C for 24 h. Then, 200 µL RPMI 1640 medium containing different concentrations (5–0.01 mg/ml) of each herbal extract (Aloe vera, Ginger, Saffron and Ziziphora) were added to each well of the plate. Cells were cultured for 24, 48 and 72 h.
2.5. Cell viability
We used MTT reduction assay for evaluating cells viability. Briefly, MTT powder (Merck, Germany) was dissolved in PBS (5 mg/ml). Cells were seeded at 20,000/well onto 96-well culture plates and allowed to grow. Then, the cells were treated with different concentrations of each herbal extract for 24, 48 and 72 hours. Four hours before reading of absorbance, 20 ml of MTT solution was added to each culture and cells were incubated for 4 h with MTT solution. Subsequently, MTT was converted by intact mitochondrial reductase and precipitated as blue crystals during a 4 h contact period. The supernatants were then gently removed, the formazan crystals were resolved in 100 µl acidic isopropanol (0.04 M HCl in isopropanol), and absorbance was read at 540 nm with a plate reader.
2.6. Statistical analysis
The results are presented as mean±SEM. Analysis of variation was done and comparisons between study groups were made with ANOVA and student's t-test. Differences were considered significant at p <0.05.
3. Results
3.1. Effect of Aloe vera extract on cell viability of AGS cell line
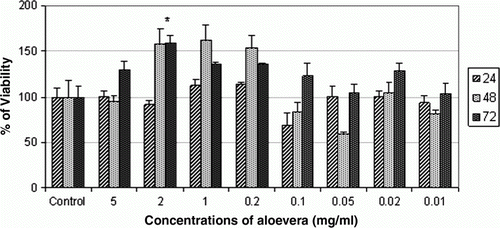
The results illustrated in show that there are no significant differences between test group and control group
Figure 1. Effect of aqueous extract of Aloe vera on cell viability of AGS cell line at 24, 48 and 72 h. The Aloe vera extract showed no significant cytotoxic activity against AGS gastric cancer cell line. * denotes significant differences compared to control group. *p<0.05, **p<0.01 and ***p<0.001 compared to control.
3.2. Effect of Ginger extract on cell viability of AGS cell line
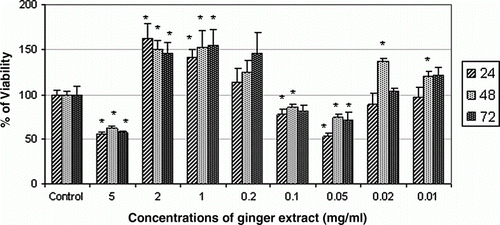
As our results show, cells treated with concentrations of 5, 0.1 and 0.01 mg/ml significantly decreased cell viability of AGS cells at 24, 48 and 72 h, whereas doses of 2 and 1 mg/ml significantly increased cell viability in comparison with control group at three times ().
Figure 2. Effect of aqueous extract of Ginger on cell viability of AGS cell line at 24, 48 and 72 h. The highest cytotoxic effect of Ginger extract on AGS cell line in 24 h was achieved at dose of 0.05 mg/ml. * denotes significant differences compared to control group. *p< 0.05, **p<0.01 and ***p<0.001 compared to control.
3.3. Effect of Ziziphora extract on cell viability of AGS cell line
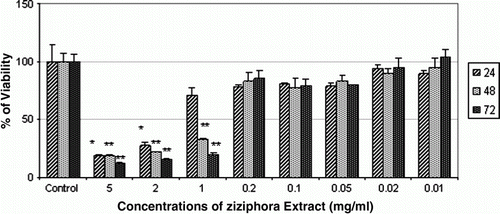
According to our results, cells treated with concentrations of 5, 2 and 1 mg/ml significantly (p<0.01) decreased cell viability of AGS cells at 48 and 72 h, and doses of 5 and 2 mg/ml significantly (p<0.05) decreased cell viability. However, no significant differences were observed compared to the control group ().
Figure 3. Effect of aqueous extract of Ziziphora on cell viability of AGS cell line at 24, 48 and 72 h. The highest cytotoxic effect of Ziziphora extract on AGS cell line in 72 h was achieved at dose of 5 mg/ml. * denotes significant differences compared to control group. *p< 0.05, **p<0.01 and ***p<0.001 compared to control.
3.4. Effect of Saffron extract on cell viability of AGS cell line
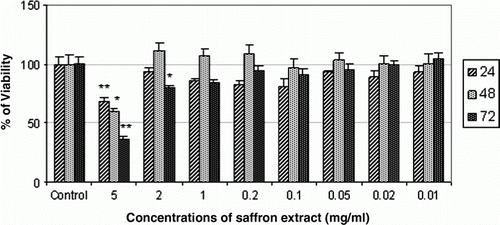
The results presented in show that cell viability of AGS cell was significantly decreased by high concentration (5 mg/ml) of Saffron extract at 24, 48 and 72 h, whereas other concentrations of Saffron extract showed no cytotoxic effects on AGS cell line in 24, 48 and 72 h.
Figure 4. Effect of aqueous extract of Saffron on cell viability of AGS cell line at 24, 48 and 72 h. The highest cytotoxic effect of Saffron extract on AGS cell line in 72 h was achieved at dose of 5 mg/ml. * denotes significant differences compared to control group. *p< 0.05, **p<0.01 and ***p<0.001 compared to control.
3.5. Comparison of cytotoxic effects of the four herbal extracts on AGS cell line
Our data suggest that Ziziphora extract is effective on AGS cells line higher than other extracts. Concentrations inducing 50% cell growth inhibition (IC50) on AGS cells are provided in .
Table 1. Concentrations inducing 50% cell growth inhibition (IC50) of Aloe vera, Ginger, Ziziphora and Saffron extracts against AGS cell line.
4. Discussion
GC remains one of the most common causes of cancer-related deaths worldwide (Milano et al., Citation2006). Poor prognosis (Zhao et al., Citation2008) and side effects of current anti-cancer drugs in the advanced GC are still the major problems for GC treatment.
In the recent years, many studies have focused on using herbal medicines. Main advantage of natural products is their mild side effects in comparison with chemical drugs. Therefore, discovery of novel anti-cancer herbal drugs is of importance. The aim of this study was to investigate the cytotoxic effects of Aloe vera, Saffron, Ginger and Ziziphora extracts on AGS gastric cell line.
4.1. Aloe vera extract
Our findings showed that, the Aloe vera extract did not show any significant cytotoxic activity on AGS gastric cancer cell line. In the previous studies, cytotoxic effect of Aloe vera extract has been evaluated on various GC cell lines. Chen, Lin, Chang, Fang and Lin (Citation2007) reported that aloe-emodin exhibits an anti-cancer effect against gastric AGS and NCI-N87 cell lines (Chen et al., Citation2007). Our finding is in disagreement with those of Chen, it seems that the effect of Aloe vera extract on human gastric cancer cell line (AGS) is different, depending on concentrations incubation and kind of Aloe vera extracts.
4.2. Ginger extract
Regarding the exposure of AGS cell line to Ginger, the highest cytotoxic effect of Ginger on AGS cell line in 24, 48 and 72 h was achieved at dose of 0.05 mg/ml (47%). Ishiguro et al. (Citation2007) reported that 1–4 µg/ml of 6-Shogaol (another ingredient of Ginger) reduced cell viability of AGS, HGC and KATO III gastric cancer cell lines at 24 h (Ishiguro et al., Citation2007). Although applied doses and materials used in Ishiguro's study are different from those in our study, that data confirmed the present work.
4.3. Ziziphora extract
In the present study, our results show that the most cytotoxic effect of Ziziphora on AGS cell line in 72 h was achieved at dose of 5 mg/ml (88%). To our knowledge, no previous report has been published on the anti-cancer effect of Ziziphora on cancer cell lines, up to now.
4.4. Saffron extract
In the last few years, Saffron has been known for its anti-cancer and anti-tumour properties, and its cytotoxic effects have been studied on some cancer cell lines (Abdullaev, Citation2002; Abdullaev et al., Citation2003). So far, there has been no report concerning cytotoxic effect of Saffron extract on various gastric cell lines. In the present study, dose of 5 mg/ml of Saffron extract had the highest cytotoxic effect on AGS cell line at 72 h (64%). Regarding our data, this study shows that AGS cells are more sensitive to Ziziphora than the other three herbal extracts ().
These results suggest that Ziziphora extract is a chemotherapeutic drug candidate for treatment of GC. Further studies in animal models are needed to clarify other mechanisms for cytotoxic and anti-tumour actions of Ziziphora on AGS cell line and on GC in humans.
Acknowledgements
The work of this study was performed by the Immunoregulation Research Centre of Shahed University and supported by the Iran National Science Foundation.
References
- Abdullaev , F.I. 2002 . Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) . Experimental Biology and Medicine (Maywood) , 227 : 20 – 25 .
- Abdullaev , F.I. and Espinosa-Aguirre , J.J. 2004 . Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials . Cancer Detection and Prevention , 28 : 426 – 432 .
- Abdullaev , F.I. , Riverón-Negrete , L. , Caballero-Ortega , H. , Manuel Hernández , J. , Pérez-López , I. Pereda-Miranda , R. 2003 . Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.) . Toxicol In Vitro , 17 : 731 – 736 .
- Chen , S.H. , Lin , K.Y. , Chang , C.C. , Fang , C.L. and Lin , C.P. 2007 . Aloe-emodin-induced apoptosis in human gastric carcinoma cells . Food and Chemical Toxicology , 45 : 2296 – 2303 .
- Chryssanthi , D.G. , Lamari , F.N. , Iatrou , G. , Pylara , A. , Karamanos , N.K. and Cordopatis , P. 2007 . Inhibition of breast cancer cell proliferation by style constituents of different Crocus species . Anticancer Research , 27 : 357 – 362 .
- El-Shemy , H.A. , Aboul-Soud , M.A. , Nassr-Allah , A.A. , Aboul-Enein , K.M. , Kabash , A. and Yagi , A. 2010 . Antitumor properties and modulation of antioxidant enzymes’ activity by Aloe vera leaf active principles isolated via supercritical carbon dioxide extraction . Current Medicinal Chemistry , 17 : 129 – 138 .
- Goel , A. and Aggarwal , B.B. 2010 . Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs . Nutrition and Cancer , 62 : 919 – 930 .
- Ishiguro , K. , Ando , T. , Maeda , O. , Ohmiya , N. , Niwa , Y. Kadomatsu , K. 2007 . Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms . Biochemical and Biophysical Research Communications , 362 : 218 – 223 .
- Milano , M.T. , Garofalo , M.C. , Chmura , S.J. , Farrey , K. , Rash , C. Heimann , R. 2006 . Intensity-modulated radiation therapy in the treatment of gastric cancer: Early clinical outcome and dosimetric comparison with conventional techniques . British Journal of Radiology , 79 : 497 – 503 .
- Salehi , P. , Sonboli , A. , Eftekhar , F. , Nejad-Ebrahimi , S. and Yousefzadi , M. 2005 . Essential oil composition, antibacterial and antioxidant activity of the oil and various extracts of Ziziphora clinopodioides subsp. rigida (BOISS.) RECH. f. from Iran . Biological and Pharmaceutical Bulletin , 28 : 1892 – 1896 .
- Surh , Y.J. 2002 . Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review . Food and Chemical Toxicology , 40 : 1091 – 1097 .
- Surh , Y.J. , Lee , E. and Lee , J.M. 1998 . Chemoprotective properties of some pungent ingredients present in red pepper and ginger . Mutation Research , 402 : 259 – 267 .
- Tan , B.K. and Vanitha , J. 2004 . Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: A review . Current Medicinal Chemistry , 11 : 1423 – 1430 .
- Tepes , B. 2009 . Can gastric cancer be prevented? . Journal of Physiology and Pharmacology , 7 : 71 – 77 .
- Tetzlaff , E.D. , Cen , P. and Ajani , J.A. 2008 . Emerging drugs in the treatment of advanced gastric cancer . Expert Opinion on Emerging Drugs , 13 : 135 – 144 .
- Tian , S. , Shi , Y. , Yu , Q. and Upur , H. 2010 . Determination of oleanolic acid and ursolic acid contents in Ziziphora clinopodioides Lam. by HPLC method . Pharmacognosy Magazine , 6 : 116 – 119 .
- Tsugane , S. and Sasazuki , S. 2007 . Diet and the risk of gastric cancer: Review of epidemiological evidence . Gastric Cancer , 10 : 75 – 83 .
- Vega , A. , Uribe , E. , Lemus , R. and Miranda , M. 2007 . Hot-air drying characteristics of Aloe vera (Aloe barbadensis Miller) and influence of temperature on kinetic parameters . LWT – Food Science and Technology , 40 : 1698 – 1707 .
- Zaniboni , A. and Meriggi , F. 2005 . The emerging role of oxaliplatin in the treatment of gastric cancer . Journal of Chemotheraphy , 17 : 656 – 662 .
- Zhao , A.G. , Li , T. , You , S.F. , Zhao , H.L. , Gu , Y. Tang , L.D. 2008 . Effects of Wei Chang An on expression of multiple genes in human gastric cancer grafted onto nude mice . World Journal of Gastroenterology , 14 : 693 – 700 .