Abstract
Butyl benzyl phthalate (BBP) is widely used as a plasticizer in carrier of pesticide, food packing and many daily products. It could be hydrolysed to mono-butyl phthalate (MBP) inside the body of organisms, which is highly toxic to humans and other organisms, affecting the reproductive and developmental function. Immune spleen cells were collected from mice immunised intraperitoneally (i.p.) and multiple subcutaneously (s.c.). A hybridoma cell line named 2F7 was obtained via colony hybridisation of immune spleen cells and myeloma cells SP2/0. The mAb against MBP secreted by 2F7 was an isotype of IgG1, and showed 1.93×108 of an affinity and the CR values of the mAb with other analogues of MBP were not more than 5%, except for BBP (11.5%). The high affinity and IgG characteristics of the mAb against MBP could be used for MBP detection in the future.
Introduction
Phthalate esters (PAEs) are widely used as plasticizers in polyvinyl chloride (PVC) resins to improve flexibility and resilience, as well as stabilizers in the manufacture of consumer products such as food packing, medical equipment, children's toys and cosmetics (Adibi, Perera, Jedrychowski, & Camann, Citation2003; Sadowski, Needham, & Calafat, Citation2003). For low water solubility, high octanol-water partition coefficient and relatively stable in the natural environment, PAEs have become ubiquitous environmental pollutants in natural water, soil, air and food. Moreover, the metabolites of PAEs also can be detected in human serum and plasma (Becker, Seiwert, & Angerer, Citation2004). PAEs are classified as priority pollutants and endocrine disrupting compounds which interfere with reproductive and behavioral action in humans (Ursel, Volker, & Jurgen, Citation2007).
Butyl benzyl phthalate (BBP), as a kind of PAEs, is one of the most widespread phthalate plasticizer usually found in food packing, preservative film, carrier of pesticide pipes, vinyl floor tiles, adhesives and many daily products. Several studies reported BBP as a reproductive and developmental toxicity in human and animal body (Liu, Lin, & Pan, Citation2003; Raquel, Richard, & Irma, Citation2007). Humans are exposed to BBP through ingestion, inhalation and dermal contact. BBP can be hydrolysed to mono-butyl phthalate (MBP) after entering the human body (Calafat, Brock, & Silva, Citation2006). MBP was considered to be the active metabolite responsible for adverse effects and possessed more reproductive and developmental toxicity than BBP. It has an inverse dose-response relationship with sperm motility and concentration (Mutsuko & Makoto, Citation2008). In addition, MBP also can alter the levels of inhibin B and follicle-stimulating hormone (FSH) in humans (Susan & Antonia, Citation2005). Furthermore, urinary concentrations of MBP in pregnant mothers were inversely correlated to anogenital distance and other malformations of the external genitalia among male infants (Marsee, Woodruff, & Swan, Citation2006). Because of the ubiquitous use and contamination of BBP, the metabolites of BBP can be detected in urine samples of the general population (Susan, Rodney, Jason, & Christina, Citation2009; Wormuth, Scheringer, Vollenweider, & Hungerbuhler, Citation2006). We can estimate the exposure level of humans to BBP by detecting the metabolites of BBP in urinary and assess the toxic potential on human health, accordingly (Corton & Lapinskas, Citation2005; Huang et al., Citation2007).
Recently, the event of elasticizer that BBP can be detected in many drinks was caused by PAEs in Taiwan. The adverse effects of BBP and MBP have been a matter of public concern after this event. BBP and MBP are classified as the fourth of the most toxic chemicals in Taiwan and are not permitted to add in food.
At present, the mostly used method to detect MBP is high-performance liquid chromatography (HPLC). Although HPLC method can give accurate results, it has some disadvantages, such as complicated sample pre-treatment processes and expensive instrumentation dependence. Immunoassay could be used to make up for these deficiencies. The key step of immunoassay is to obtain a monoclonal antibody (mAb) against MBP. Because MBP is a kind of hapten, it cannot stimulate the organism to produce the specific antibody-antigen response. It must be coupled with carrier protein to produce the mAb against MBP. Up until the present day, we have not found any reports on MBP mAb detection method. In this study, we aimed to prepare the complete antigen of MBP and obtain a high affinity mAb against MBP, which could provide a probe for development of an immunoassay for MBP.
Materials and methods
Materials
Mono-butyl phthalate (MBP) was obtained from Dr. Ehrenstorfer Company (Germany). Bovine Serum Albumin (BSA), Ovalbumin (OVA), complete Freund's adjuvant (CFA) and incomplete Freund's adjuvant (IFA), horseradish peroxidase-conjugated goat anti-mice IgG (HRP-IgG), polyethylene glycol-1000 (PEG) and hypoxanthine aminopterin thymidine (HAT) were purchased from Sigma (Changchun, China). Microtiter plates, microculture plates and cell culture bottles were obtained from Costar Group, Inc. (Bethesda, MD, USA). All other reagents were of analytical grade. Myeloma cells SP2/0 were reserved by Key Laboratory of Zoonosis Research, Ministry of Education, China. Female Balb/C mice (eight- and ten-week old) were obtained from Changchun Institute of Biological Products (China). The absorbencies were read by a MK3 microplate reader from Thermo (Shanghai, China).
Preparation of complete antigen
The immunogens were prepared by conjugating MBP to BSA based on mixed anhydride method according to literature (Kim & Kim, Citation1997; Maurice, Denis, Christopher, & William, Citation1993) with some modifications as follows: MBP (3 mg) was dissolved in 75 µL of DMF (N, N-Dimethylformamide) with 16 µL of 3-butylamine. After stirring for 10 min at 4°C, 9 µL of isobutylchloroformate was added to the mixture, and then the solution was stirred at 4°C for 40 min. The mixture of 5, 10 and 15 µL were added dropwise to 2.5 mL of 1 mg/mL BSA in phosphate buffer (PBS pH 7.4), respectively, and then the solution was stirred at 4°C for 4 h. The reagent-treated BSA was prepared by the same method only without MBP in solution. The coating antigen was prepared by conjugating the same hapten to OVA using the same method. The complete antigens were dialysed in 1000 mL of 0.01 M PBS (pH 7.4) at 4°C for 72 h with four-time changes of PBS to remove residual free MBP, and then analysed by nondenaturing agarose gel electrophoresis and UV-vis spectrometry (Zhou, Zhang, & Shen, Citation2009).
Immunisation
Mono-butyl phthalate-Bovine Serum Albumin (MBP-BSA) with three different conjugate ratios was used to immunise the Balb/C mice using two immunisation methods, respectively.
Intraperitoneally (i.p.) and multiple subcutaneously (s.c.) immunisation
The procedures of i.p. and s.c. immunisation were referenced to literature (Shen & Zhou, Citation1998). A mixture of complete antigen MBP-BSA (100 µL, 1 mg/mL) and Freunds complete adjuvant (100 µL) were stirred to prepare a water-in-oil emulsion. Eight-week-old female Balb/C mice were immunised with the emulsion at the i.p. and s.c. sites as the first time. Seventy-five micro litres of MBP-BSA with the same volume of Freunds incomplete adjuvant were injected at the third, fifth and seventh week, respectively. Seven days after the fourth immunisation, the mice were tail-bled and the sera were tested for antibody titers by indirect ELISA. The procedures were as follows: The microtiter plate was coated with MBP-OVA (1 µg/mL) 100 µL per well and incubated overnight at 4°C. The plate was washed three times with PBS containing 0.05% Tween 20 (PBS-T). The sera were diluted to 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:6400, 1:12,800 and 1:25,600 and dropped into the plate, respectively. Following 1 h of incubation at 37°C, the plate was washed again. One hundred micro litre of HRP-IgG (1:4000 diluted in PBS) was dropped into each well. The plate was incubated for another hour at 37°C and then was washed again. One hundred micro litre of O-phenylenediamine (OPD) solution was dropped into each well and the plate was incubated about 15 min at 37°C. The colour development was stopped by adding 2 M H2SO4 (50 µL per well). The absorbance was measured at 492 nm and recorded in a spectrophotometer. Instead of the sera collected from the immunised mice, 0.1 M PBS or the sera of mice without any immunisation were respectively added into wells following the indirect ELISA protocol as blank or negative control. The mice whose serum titers were the highest would be chosen for hybridoma production and received intravenous injections (i.v.) boosts of 100 µL of MBP-BSA without Freund adjuvant. Four days after the final boost, spleen cells were obtained from the immunised mice.
Intrasplenic direct immunisation
The procedures of intrasplenic direct immunisation were referenced to literature (Regina, Citation1989). Thirty micro litres of MBP-BSA was injected in spleen, directly. Seven days later, the sera were tested by indirect ELISA. The procedure was the same as described earlier.
Preparation of Hybridoma
Spleen cell suspensions were mixed with murine myeloma cells SP2/0 at a ratio of 5–10:1 under the action of PEG to control cell aggregation and fusion. The hybridoma cells were selected with HAT medium and cultured at 37°C in an atmosphere of 5% CO2. The positive hybridoma cells which were probably to secrete anti-MBP mAb were screened by indirect competitive ELISA and further cloned by limiting dilution. The positive hybridoma cells (1×105 cells per mice) were injected into abdomens of 10-week-old Balb/C mice after 0.5 mL of liquid paraffin was injected. The ascites were collected about 10 days later and purified by saturated ammonium sulfate method (Zhao, Sun, & Zhang, Citation2003) and stored at −80°C.
Specificity and sensitivity of the mAb
The isotyping of the mAb was identified by an ELISA commercial kit ‘mouse monoclonal antibody isotyping reagents (Sigma)’. The affinity of mAb was assessed by an indirect ELISA method: the coating antigen of 0.25, 0.5, 1 and 2 µg/mL was added into the microtitre plate (100 µL per well), respectively, and incubated overnight at 4°C. The plate was washed three times with PBS-T. The mAb was diluted to 1:10,000, 1:20,000, 1:40,000 and 1:80,000 and dropped into the plate, respectively. The rest steps were the same as described in section: Intraperitoneally (i.p.) and multiple subcutaneously (s.c.) immunisation. The affinity of mAb was measured using the following formula (Zhou, Li, & Pan, Citation2010): Ka=(n−1)/2(n [Ab/]t−[Ab]t). ‘n’ is the ratio of two different coating concentration, [Ab/]t and [Ab]t are the concentrations (mol/L) of mAb corresponding to 50% of maximum absorbance values of two different coating concentrations.
The cross-reactivity (CR) were assessed by an indirect competition ELISA method: The microtiter plate was coated with MBP-OVA (1 µg/mL) 100 µL per well and incubated overnight at 4°C. The plate was washed three times with PBS-T, and then 50 µL of MBP and other analogues in 0.01 M PBS at different dilutions together with the mAb (1:60,000 with the dilution buffer solution, 50 µL per well) were added to the plate, respectively, and incubated 1 h at 37°C. The other steps were the same as described in section: Intraperitoneally (i.p.) and multiple subcutaneously (s.c.) immunisation.
The CRs% were determined using the following equation:
CR (%)=[IC50 (MBP)/IC50 (analogue)]×100%. IC50 means the concentration of MBP reducing the ELISA maximum response to 50% and the CR (%) of MBP was defined as 100%. IC50 were readout from the calibration curves of MBP and the analogues (Zhang, Cong, & Sheng, Citation2010).
Results and discussion
Analysis of the complete antigen
The synthesis of the appropriate complete antigen is the critical step for obtaining the mAb, which can affect the specificity of antibody against the target analyte (Kuang, Xu, Cui, Ma, & Xu, Citation2010). The complete antigen can be analysed by many methods, such as nondenaturing agarose gel electrophoresis, UV-vis spectrometry, labelled antigen tracer method and nuclear magnetic resonance; however, each method has its own advantages. In this study, we chose nondenaturing agarose gel electrophoresis and UV-vis spectrometry to analyse the complete antigen, which showed us a clear result about binding or non binding antigen.
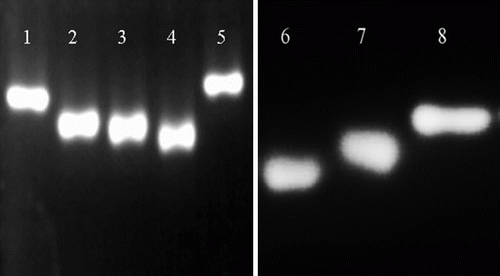
The complete antigen was analysed by nondenaturing agarose gel electrophoresis. The complete antigen has more negative charge than the carrier protein alone. Hence, it will migrate further than carrier protein in the gel towards the anode (+). As MBP has a carboxyl group with negative charge, the more MBP were connected with carrier protein, the further the antigens would migrate. shows the results of nondenaturing agarose gel electrophoresis for the complete antigen. The net charge of MBP-BSA with three different conjugate ratios in lane 2, 3 and 4 became more negative than that of reagent-treated BSA and BSA alone in lanes 1 and 5, hence they migrate further than reagent-treated protein and carrier protein alone. Moreover, three different conjugate ratios of MBP-BSA (in lane 4, 3 and 2, respectively) migrated differently. The results indicated that MBP was coupled with BSA with different conjugate ratios successfully. Because of MBP-OVA with more negative charge than OVA and reagent-treated OVA, MBP-OVA (in lane 6) migrated further than those of reagent-treated OVA (in lane 7) and OVA alone (in lane 8), which indicated that MBP was coupled with OVA successfully.
Figure 1. Analysis of complete antigen by nondenaturing agarose gel electrophoresis.Lane 1: reagent-treated BSA, lane 2-4: MBP-BSA (conjugate ratios are 9, 14.7 and 27, respectively), lane 5: BSA, lane 6: MBP-OVA, lane 7: reagent-treated OVA, lane 8: OVA.
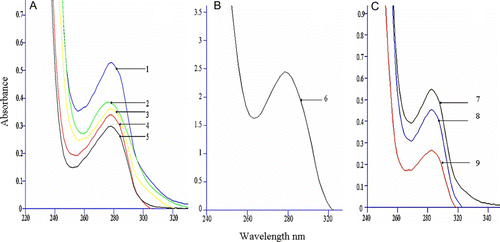
The complete antigen was analysed by UV-vis spectrometry. Different substance has its own ultraviolet absorption. When the structure of the substance is changed, the ultraviolet absorption will be changed accordingly. The absorbance of mixed solution equals the summation of all substances. Hence, the value of absorbance will be changed when MBP was coupled with carrier protein. The more MBP was coupled with carrier protein, the higher value of absorption could be observed. As shown in A and B, the absorbance values of BSA, reagent-treated BSA, MBP-BSA (three different conjugate ratios) and MBP were 0.28, 0.32, 0.36, 0.4, 0.5 and 2.5, respectively. These substances were at the same concentration, so after MBP was coupled with BSA successfully, the absorbance values of MBP-BSA would be increased. According to the molar absorbancy index and the absorbance values, we can calculate the conjugate ratios. The real conjugate ratios of MBP-BSA were 9.8, 14.7 and 27, respectively (Erlanger et al., Citation1957; Yang, Wei, & Guo, Citation1997). From the results of nondenaturing agarose gel electrophoresis and UV-vis spectrometry, we can have a conclusion that MBP-BSA of different conjugate ratios was synthesised. As shown in C, the absorbance values of OVA, reagent-treated OVA and MBP-OVA were 0.25, 0.45 and 0.55, respectively. The result indicated that the MBP-OVA were synthesised successfully.
Figure 2. UV-visible spectra of the complete antigen. ‘A’ is the UV-visible spectra of MBP-BSA with different conjugate ratios. 1-3: MBP-BSA (conjugate ratios are 27, 14.7 and 9, respectively), 4: reagent-treated BSA, 5: BSA. ‘B’ is the UV-visible spectra of MBP. 6: MBP. ‘C’ is the UV-visible spectra of MBP-OVA. 7: MBP-OVA, 8: reagent-treated OVA, 9: OVA.
Mono-butyl phthalate (MBP), as a kind of hapten, cannot stimulate the animal to produce the specific antibody-antigen response. Preparation of a complete antigen must be coupled with carrier protein. It is very important to choose the suitable carrier proteins, which must be stable at different pH, ionic strength and convenient conjugation (Liu, Luo, & Li, Citation2012). In this study, BSA and OVA were chosen as carrier proteins considering their stability of physico-chemical property and high solubility. As carrier proteins, the pro-source relations between BSA and OVA are distant, so it is easy to remove the antibodies against the carrier protein BSA (Che et al., Citation2009). MBP has a carboxylic acid, it can be used as a functional group for conjugation to the amino-group of protein. Both the activated ester method and mixed acid anhydride method can be used to prepare the complete antigen. In this study, the mixed acid anhydride method is better than the activated ester method via comparing the results of the two methods, because it can decrease the protein polymerisation (date not shown).
Bovine Serum Albumin (BSA) has 61 amino-groups and about half of them can be used for coupling with hapten. The theoretical conjugate ratio (hapten/protein) is 61, but only 8-31 conjugate ratios can be obtained actually (Shen & Zhou, Citation1998). Different immune result could be stimulated by the complete antigen with different conjugate ratios. It does not mean that the more haptens were coupled with the carrier protein, the better complete antigen could be gotten. In order to obtain a higher affinity antibody for MBP, the optimum conjugate ratio of hapten to carrier protein was very important. In this study, the complete antigen MBP-BSA with three different conjugate ratios (9.8, 14.7 and 27) of MBP to BSA were designed and synthesised. The result showed best immunogen at 14.7:1 of MBP to BSA in .
Table 1. Comparison of the sera titers of the mice immunised with MBP-BSA of different conjugate ratios by i.p. and s.c. immunisation and intrasplenic direct immunisation.
Antisera titers of immunised mice
The result of i.p. and s.c. immunisation
The i.p. and s.c. immunisation is a standard method for immune spleen obtaining and it usually needs at least three times of injection during 1–2 months and considerable quantities of immunogen (200–475 µg per mouse). Although the immunisation period of the i.p. and s.c. immunisation is longer, it could obtain satisfactory serum titers. As shown in , serum titers of all the mice immunised by this method were higher enough for hybridoma production.
Higher serum titers were the foundation to obtain high titers of mAb. The different immune results have been obtained using MBP-BSA with different conjugate ratios of hapten and carrier protein (). The serum titer of No. 2 mouse, which was immunised by MBP-BSA with conjugate ratio of 14.7, was the highest (reached 1:51,200). Hence, the No. 2 mouse (immunised by MBP-BSA of conjugate ratio 14.7) was chosen for hybridoma production.
The result of intrasplenic direct immunisation
The immunogen was directly injected into the intraspleen without adjuvant (30–50 µg of immunogen per mouse). The spleen cells can be used for cell fusion 5–7 days later. The advantages of the method are a short immunisation period and a little amount of antigen. As shown in , only the serum titer of No. 6 mouse (immunised by MBP-BSA with the conjugate ratio of 14.7) was high enough for hybridoma production. Different conjugate ratios of the complete antigen could induce different immune results. The titers of the mice immunised by MBP-BSA with the conjugate ratio of 14.7 were higher than that of MBP-BSA with conjugate ratios of 9.8 and 27, and moreover the result obtained was coincident with that of i.p. and s.c. immunisation. As usual, if the other immunisation methods (i.p and s.c.) does not produce any satisfactory results and/or the antigen is very limited, the intrasplenic method will be the best choice. However, in the present study, we had enough complete antigen and the results showed that the serum titers of mice i.p. and s.c. were higher than that of intrasplenic direct immunisation. Since the affinity, sensitivity and specificity of the mAb play an important role in estimating the quality of the mAb it is necessary to obtain the high serum titers of the mice. Hence, we compared the different immune methods and the different conjugate ratios of complete antigen. The results indicated that the optimal immunisation method was i.p. and s.c. protocol and the optimal conjugate ratio of MBP to BSA was 14.7:1.
Screening of hybridoma and characteristics of mAb
A hybridoma cell line (2F7) was screened by an indirect competitive ELISA and established after being subcloned for three cycles by ‘limiting dilution’. The positive cell line was expanded and injected into the abdomens of 10-week-old Balb/C mice seven days after liquid olefin was injected. The ascites collected from Balb/c mice 10 days after injecting cells were purified by mean of the caprylic acid-ammonium sulfate method. The titers of ascites and purified ascites were 7.2×105 and 6.4×105, respectively. The isotyping of the mAb was IgG1 and the average affinity of mAb was 1.93×108. The affinity constant and the isotyping of the mAb are the significant indicator of antibody stability.
The CRs of the mAb with other analogues of MBP were analysed by an indirect competitive ELISA. The results of the CR were listed in . All CR values of the mAb with mono-benzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), mono-methly phthalate (MMP), dibutyl phthalate (DBP), diethylhexyl phthalate (DEHP), di(2-Methoxyethyl) phthalate (DMEP), diethyl phthalate (DEP), di-n-octyl phthalate (DNOP), diisooctyl phthalate (DIOP) and dimethyl phthalate (DMP) were lower than 5%, except for BBP (11.5%). As the structure of the PAEs are very similar, it is very important to obtain a mAb with high specificity. The lower the CR was, the higher the specificity of the mAb would be. The results indicated that the mAb against MBP could be used as a probe for the detection of MBP in urine. It can be the foundation to learn the relationship between the level of MBP and human health.
Table 2. Cross-reactivity of the mAb with the analogues of MBP.
Conclusion
In this study by comparing the immune effect with different methods, the complete antigen MBP-BSA was prepared and chosen based on the optional conjugate ratio MBP to BSA (14.7:1) to immunise the mice via i.p. and s.c. immunisation. The mAb secreted by the hybridoma cell line named 2F7, showed a high affinity parameter about 1.93×108 and low CR with other analogues of MBP. For the first time, the mAb against MBP was prepared and thus could be used for MBP detection in urinary to assess the toxic potential on human health in the future.
Acknowledgements
The authors are thankful to the financial support of ‘the key project of Jilin province’ and ‘the National Nature Science Foundation of China’ (NSFC, No. 31071539, 60971011, 30771657 and 61171022).
Additional information
Notes on contributors
Yu Zhou
Le Li and Yu Zhou contributed equally to the preparation of this studyReferences
- Adibi , J.J. , Perera , F.P. , Jedrychowski , W. and Camann , D.E. 2003 . Prenatal exposures to phthalates among women in New York City and Krakow, Poland . Environmental Health Perspectives , 111 : 1719
- Becker , K. , Seiwert , M. and Angerer , J. 2004 . DEHP metabolites in urine of children and DEHP in house dust . International Journal of Hygiene and Environmental Health , 207 : 409
- Calafat , A.M. , Brock , J.W. and Silva , M.J. 2006 . Urinary and amniotic fluid levels of phthalate monoesters in rats after the oral administration of di(2-ethylhexyl) phthalate and di-n-butyl phthalate . Toxicology , 217 : 22
- Che , H.L. , Shi , B.X. and Chen , J.L. 2009 . Preparation and characterization of olaquindox polyclonal antibody . Chinese Journal of Chemistry , 27 : 999
- Corton , J.C. and Lapinskas , P. 2005 . Peroxisome proliferator-activated receptors: Mediators of phthalate ester-induced effects in the male reproductive tract . Journal of Toxicology , 83 : 4
- Erlanger , B.F. , Borek , F. and Beiser , S.M. 1957 . Preparation and characterization of conjugates of bovine serum albumin with testosterone and with cortisone . Journal of Biological Chemistry , 228 : 713
- Huang , P.C. , Ku , P.L.O. and Guo , Y.L. 2007 . Associations between urinary phthalate monoestersand thyroid hormones in pregnant women . Journal of Human Reproduction , 22 : 2715
- Kuang , H. , Xu , L.G. , Cui , G. , Ma , W. and Xu , C.L. 2010 . Development of determination of di-n-octyl phthalate (DOP) residue by an indirect enzyme-linked immunosorbent assay . Food and Agricultural Immunology , 3 : 265
- Kim , Y.H. and Kim , Y.S. 1997 . Effect of active immunization against clenbuterol on the growth-promoting effect of clenbuterol in rats . Journal of Animal science , 75 : 446
- Liu , P.S. , Lin , C.M. and Pan , C.Y. 2003 . Butyl benzyl phthalate blocks Ca2+ signaling and catecholamine secretion coupled with nicotinic acetylcholine receptors in Bovine . NeuroToxicology , 24 : 97
- Liu , X.Y. , Luo , Y.K. and Li , Z. 2012 . Effects of pH, temperature, enzyme-to-substrate ratio and reaction time on the antigenicity of casein hydrolysates prepared by papain . Food and Agricultural Immunology , 23 : 69
- Marsee , K. , Woodruff , T.J. and Swan , S.H. 2006 . Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance . Environmental Health Perspectives , 114 : 805
- Maurice , J. , Denis , R. , Christopher , O. and William , T. 1993 . Production of a monoclonal antibody with specificity for deoxynivalenol, 3-acetyldeoxynivalenol and 15-acetyldeoxynivalenol . Food and Agricultural Immunology , 4 : 199
- Mutsuko , H.K. and Makoto , E. 2008 . Potential adverse effects of phthalic acid esters on human health: A review of recent studies on reproduction . Regulatory Toxicology and Pharmacology , 50 : 37
- Raquel , M. , Richard , W. and Irma , H.R. 2007 . The plasticizer butyl benzyl phthalate induces genomic changes in rat mammary gland after neonatal/prepubertal exposure BMC Genomics . Toxicology , 8 : 453
- Regina , M.D. 1989 . Procedure for intrasplenic immunization of mice . Journal of Tissue Culture Methods , 12 : 3
- Sadowski , M. , Needham , L.L. and Calafat , A.M. 2003 . Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers . Environmental Health Perspectives , 111 : 1148
- Shen , G.X. and Zhou , R.L. 1998 . Experimental techniques of modern immunology , WuHan , , China : Hubei Science and Technology Press .
- Susan , M.D. and Antonia , M.C. 2005 . Phthalate exposure and reproductive hormones in adult men . Journal of Human Reproduction , 50 : 37
- Susan , S. , Rodney , S. , Jason , B. and Christina , M. 2009 . Metabolomics in the assessment of chemical-induced reproductive and developmental outcomes using non-invasive biologicaluids: Application to the study of butylbenzyl phthalate . Journal of Applied Toxicology , 29 : 703
- Ursel , H. , Volker , M.S. and Jurgen , A. 2007 . Phthalates: Toxicology and exposure . International Journal of Hygiene and Environmental Health , 210 : 623
- Wormuth , M. , Scheringer , M. , Vollenweider , M. and Hungerbuhler , K. 2006 . What are the sources of exposure to eight frequently used phthalic acid esters in Europeans . Risk Analysis , 26 : 803
- Yang , L.G. , Wei , P.H. , & Guo , A.Z. 1997 . The techniques of Enzyme immunoassay . NanJing , , China : NanJing University Press , 242 , 288 .
- Zhang , M.G. , Cong , Y. and Sheng , L.Y. 2010 . A direct competitive enzyme-linked immunosorbent assay by antibody coated for diethyl phthalate analysis . Analytical Biochemistry , 406 : 24
- Zhao , S.Q. , Sun , Y.M. and Zhang , C.Y. 2003 . Studies on purification of methamidophos monoclonal antibodies and comparative immunoactivity of purified antibodies . Biomedical and Environmental Sciences , 16 : 119
- Zhou , Y. , Li , Y.S. and Pan , F.G. 2010 . Development of a new monoclonal antibody based direct competitive enzyme-linked immunosorbent assay for detection of brevetoxins in food samples . Food Chemistry , 118 : 467
- Zhou , Y. , Zhang , Y.Y. and Shen , Q.F. 2009 . Development of a novel antibody probe useful for domoic acid detection . Biosensors and Bioelectronics , 24 : 3159