Abstract
This study examined the phytochemical profile and the effects of Pleurotus fruiting bodies powder on cell-mediated immune response in both in vivo and in vitro assays. Although carbohydrates (55%, w/w) appear to be the most important immunomodulatory compound, secondary metabolites (terpenoids, phenols and flavonoids) would also enhance immunity. Pleurotus powder was administered orally during 7 days to Balb/c mice (1000 mg/kg) and cyclophosphamide (CY; 100 mg/kg) was inoculated intraperitoneally at the beginning of the experiment. The delayed-type hypersensitivity reaction measured at 48 and 72 h after antigen challenge was similar to that of control mice and it was associated with an increase in the mass index of popliteal lymph nodes (p< 0.05). An in vitro lymphoproliferative-stimulating response was also demonstrated with aqueous and ethanolic extracts obtained from Pleurotus powder. These effects suggest that Pleurotus supplement could potentiate the cellular immune response and should be promising for further immunotherapy studies.
Keywords:
1. Introduction
Mushrooms have been used for many years due to their nutritional and pharmacological properties (Costa, Cunha, & Carvalho, Citation2006; Wasser, Citation2002; Zhang, Cui, Cheung, & Wang, Citation2007). The edible mushrooms with medicinal or functional properties include species of Lentinula, Auricularia, Hericium, Grifola, Pleurotus, Ganoderma, Agaricus and Flammulina. These mushrooms particularly have long been suggested to possess immunomodulatory effects (Smiderle et al., Citation2008; Smith, Sullivan, & Rowan, Citation2003; Thekkuttuparambil & Kainoor, Citation2007).
In the last 5 years, the consumption of mushrooms, either as whole mushroom or extracted supplements, has increased (Wasser, Citation2010). Most mushroom-derived substances and preparations (extracts, powders and tablets) are usually included in the following categories of products: dietary supplements, functional foods, nutraceuticals, nutriceuticals, phytochemicals and design foods (Chang & Buswell, Citation1996, Citation2003; Wasser, Didukh, & Nevo, Citation2004) and immunoceuticals (Petrova, Wasser, Mahajna, & Denchev, Citation2005).
The bioactive compounds contained in mushrooms with immunomodulatory and antitumour activities are mainly polysaccharides, polysaccharopeptides and polysaccharide–protein complexes (Hu et al., Citation2006; Wasser & Weis, Citation1999). Other substances of therapeutic interest are low-molecular-weight secondary metabolites and mushroom trace elements (Liu et al, Citation2007; Zaidman, Yassin, Mahajna, & Wasser, Citation2005) which also play important roles in the immune function.
Immune system is a very complex homeostatic system consisting of a network of interacting cells, tissues and organs. It allows the organism to exist within itself and maintains a surveillance to recognise components considered nonself. The body's immunity has been shown to be suppressed in several diseases, like AIDS and cancer. The chemotherapy and radiotherapy in cancer treatment contribute to further depression of the immune system as a result of the destruction of lymphoid and bone marrow cells (Janeway, Travers, Walpert, & Shlomchik, Citation2005).
Use of immunomodulating therapeutic agents can solve these problems largely and efforts to find new immunomodulators are ongoing. Pleurotus species, like many edible and medicinal mushrooms, are a good source of immunomodulators and substances considered as ‘host defence potentiators’ (HDPs) as judged by their immunostimulating properties (Gregori, Švagel, & Pohleven, Citation2007; Sedaghat & Ghazanfari, Citation2011; Wasonga, Okoth, Mukuria, & Omwandho, Citation2008).
In previous papers, we reported the immunomodulating effects of a hot-water extract prepared from the mycelium of P. ostreatus on the immunosuppression caused by cyclophosphamide in mice (Morris et al., Citation2003) as well as the in vitro effects of five water-soluble fractions on macrophage activation (Morris, Lebeque, Fontaine, Bermúdez, Llauradó, & Marcos, Citation2007). Taking into account that Pleurotus fruiting bodies obtained under good manufacture practices can be used in the formulation of biologically active nutritional supplements such as, powders, capsules and tablets, the present study examined the phytochemical profile and the effects of Pleurotus fruiting bodies powder on cell-mediated immune response in both in vivo and in vitro assays.
2. Materials and methods
2.1 Mushroom material
Pleurotus sp. strain CCEBI-3024 (Pleurotaceae) is deposited at the Culture Collection of the Centre of Studies for Industrial Biotechnology (CEBI). For maintenance, slants with solid medium of agar–dextrose–potato (PDA) incubated at 37 °C for 7 days were used.
2.2 Preparation of Pleurotus fruiting bodies powder
Pleurotus sp. cultivation was performed by solid-state fermentation of mushroom spawn on pasteurised coffee pulp used as substrate in plastic bags of 2 kg (30×40 cm; Bermúdez, Garcia, Gross, & Serrano, Citation2001). The fruiting bodies were harvested, sliced into small pieces and dried at 45 °C for 24 h. The dried material was milled, and the resulting powder was preserved from light and humidity in plastic bags for further use.
2.3 Phytochemical profile of Pleurotus fruiting bodies powder
The sugar and protein contents in the powder were determined by the method of Dubois, Gilles, Hamilton, Rebers, & Smith (Citation1956) and by Lowry's method (Lowry, Rosebrough, Farr, & Randall, Citation1951) using glucose and bovine serum albumin (BSA) as standards, respectively. Total lipids were measured according to Association of Official Analytical Chemists (AOAC, Citation1995) approved method.
The powder was extracted with hot water and ethanol to obtain both aqueous and ethanolic extracts for assessing the phytochemical profile and the in vitro lymphoproliferative activity.
The phytochemicals contained in both aqueous and ethanolic extracts were estimated qualitatively according to Harbourne (Citation1984). Dragendorff's and Wagner reagents were used for alkaloid detection. Fehling and Benedict reagents were used for reducing sugars detection. The flavonoid content was determined according to the Rosemheim and concentrated sulphuric acid methods. Lieberman–Burchard and Solkowski assays were used for terpenoids identification. The presence of phenols and tannins was assessed by the reaction with FeCl3. Amino acids detection was performed by the ninhidrine assay. All reagents for the phytochemical tests were freshly prepared following standard procedures.
2.4 Animals and treatments
Pathogen-free male Balb/c mice were purchased from the National Centre for the Production of Laboratory Animals (CENPALAB, Havana, Cuba). The 20- to 25-g mice were fed a standard diet and acidified water ad libitum. Fifteen mice were divided into three groups (n=5). The two experimental groups were treated intraperitoneally (i.p) with cyclophosphamide (CY) USP 23 for injection obtained from JSLYP (China) at 100 mg/kg on day 0. The Pleurotus sp. fruiting bodies powder was administered by oral route (1000 mg/kg) for 7 days to the ‘CY-Pleurotus’ group, whereas physiological saline solution was administered to the ‘CY-Saline’ group in a similar schedule. Non-treated mice were used as controls in the experiment.
The research was approved by the institutional Ethical Committee (University of Oriente) and has been performed in accordance with Cuban legislation and the National Research Council Guidelines for the Care and Use of Laboratory Animals.
2.5 In vivo evaluation of Pleurotus powder effects on cell immunity
The effect of Pleurotus powder on cell-mediated immunity was determined by the delayed-type hypersensitivity (DTH) reaction (Kim et al., Citation1998). Mice were immunised by an intradermal (i.d) injection of 50 µl of 5 mg/ml BSA emulsified in Complete Freund Adjuvant (CFA; Sigma, St. Louis, MO, USA) at two sites on the abdomen. Eight days after immunisation, the mice were rechallenged by injection of 20 µl of 5 mg/ml BSA into one rear foot pad, while the other received a comparable volume of phosphate buffered saline (PBS). Measurements of foot pad swelling were taken at 24, 48 and 72 h after challenge by use of a micrometer (Mitutoyo, Tokyo, Japan). The magnitude of the DTH response was determined as the differences in foot pad thickness between the antigen and PBS-injected foot pads. A similar immunisation protocol was applied to control animals.
The popliteal lymph nodes (right and left) of the antigen sensitised and rechallenged animals of DTH experiment were removed and washed with PBS pH 7.4. Excess humidity was discarded with a filter paper, and the lymph nodes were immediately weighed separately in an electronic analytical balance (Sartorius). The mass index was expressed as the relation between the weight of the popliteal node belonging to BSA-injected foot pad with respect to that of PBS-injected pad (Descotes, Citation2006).
2.6 In vitro evaluation of nutritional supplement on cell immunity
The in vitro lymphoproliferative test was carried out according to a modification of the method described by Soto-Velazco, López, Vázquez, Valls, and Álvarez (Citation2002) using murine spleen lymphocytes instead of human lymphocytes. The suspension of splenocytes was obtained by the gentle teasing of spleens in RPMI-1640 (Sigma, St. Louis, MO, USA) containing 8% fetal calf serum and supplemented with antibiotics. Viable cells estimated by the Trypan Blue exclusion method were counted with a Neubauer chamber, and the cell concentration was adjusted to 2×106 cells/ml.
Briefly, phytohaemagglutinin (PHA; 200 µl) was added to conical tubes of 15 ml containing 5 ml of supplemented RPMI-1640 to a final concentration of 5 µg/tube, followed by the addition of 2×06 cells and 100 µl of powder extracts (aqueous or ethanolic). The tubes were incubated at 37 °C for 27 h and then 500 µl of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT; 5 mg/ml) was added. After the incubation of the resulting mixture at 37 °C for 4 h, the tubes were centrifuged 10 min at 1500 rpm. The supernatants were discarded and 1 ml of isopropanol was added to each tube. The absorbance of the mixture was measured at 570 nm in a Genesys 10 UV/VIS spectrophotometer. The stimulation index was calculated considering the absorbance of control cultures without PHA as the unit.
2.7 Statistical analysis
The results were expressed as the arithmetic means±standard deviation (SD). The Kruskal–Wallis rank test followed by the Student–Newman–Keuls test was applied to determine the significance of differences between treatments in DTH assay. The Mann–Whitney test was used to compare the two means in the experiment of popliteal lymph nodes mass index. Differences at p<0.05 were accepted as significant. The software Statgraphics Plus version 5.1 (Statistical Graphics Corporation, 1994–2001) was used in all the analysis.
3. Results and discussion
A wide variety of bioactive substances isolated from many species of mushrooms has been identified. Most of them are high-molecular-weight polysaccharides and proteoglycans or low-molecular-weight secondary metabolites such as terpenoids, steroids and phenols (Zaidman et al., Citation2005).
Dried Pleurotus mushroom would become an attractive alternative for the development of drugs and immunoceuticals preparations. The powder evaluated in this work contained 55% (w/w) carbohydrate, 20% (w/w) protein and 4% (w/w) lipids. Although, polysaccharides appear to be the most important bioactive component with respect to immunomodulation, the presence of different secondary metabolites in Pleurotus sp. fruiting bodies powder could lead to a synergy in the immune-enhancing activity.
Phytochemical analysis is commonly used to detect qualitatively secondary metabolites present in plants. The result of the preliminary phytochemical test shows that both aqueous and ethanolic extracts contain alkaloids, phenolic compounds like flavonoids and tannins, reducing sugars and amino acids (). These phytoconstituents were present in varying amounts. Remarkably, the ethanolic extract from Pleurotus powder exhibited a very strong terpenoid reaction. Fats and oil were generally absent in the extracts due to the polarity of the solvents used in their preparation.
Table 1. Phytochemical profile of Pleurotus sp. fruiting bodies powder.
Among the mentioned bioactive components identified in aqueous and ethanolic extracts, phenolic compounds have been studied for their antioxidant properties, and they also play an important role in cancer prevention (Dubost, Ou, & Beelman, Citation2007). An isoflavone (Genestein) isolated from Flammulina velutipes produced a growth inhibition of the human ovarian carcinoma SKOV-3 in a dose-dependent manner (Li & Mi, Citation2003).
Reducing sugars are structural constituents of beta-d-glucans, components of mycetes’ cell walls with a well-documented immunity-stimulating effect (Rop, Mlcek, & Jurikova, Citation2009). On the other hand, amino acids are the structural units of proteins that may be associated to polysaccharides to form immunomodulating complexes, like PSP isolated from the mycelium of Trametes versicolor (Hobbs, Citation2004). Moreover, fungal immunomodulatory proteins, purified from medicinal mushrooms comprise a group of novel proteins, possess immunomodulatory properties and have a strong potential of being applied to food or pharmaceutical products for commercial development (Ou, Hsiao, Wang, Ko, & Lin, Citation2009).
Terpenoids have been found in ethanolic extract and their ability to stimulate the immune system is known. For instance, the triterpene lanostenoide obtained from Ganoderma applanatus, inhibited the carcinogenesis induced by the Epstein Barr virus (Gao, Zhou, Huang, & Xu, Citation2003).
Recent studies in Pleurotus species have evidenced several therapeutic effects related with the immune system, such as antitumour, antimicrobial and antiviral activities. Most of these investigations, both in vivo and in vitro, have been carried out with extracts and isolated compounds (polysaccharides, proteins and DNA; Gregori et al., Citation2007). Therefore, the study of a dried pulverised preparation of Pleurotus fruiting bodies would be of interest in the development of new pharmaceutical formulations.
The immunomodulating properties of Pleurotus powder administered therapeutically to cyclophosphamide treated mice on cell-mediated immune response were assessed by the assay of induction of DTH response.
Mice supplemented with Pleurotus powder showed a higher DTH response as judged by the increase of foot pad swelling compared to saline control group, particularly at 48 and 72 h after antigen rechallenge (p <0,05; ). The DTH response mounted at these times by CY-Pleurotus group was similar to that of control mice. The reconstitution of DTH response reflected the induction of CD4+ Th1 cells and the activation of macrophages by cytokines: tumour necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ; Janeway et al., Citation2005; Kim et al., Citation1998).
Table 2. Effect of therapeutic administration of Pleurotus powder on the delayed-type hypersensitivity response (foot pad thickness) of cyclophosphamide treated Balb/c mice.
Macrophages have been suggested to play important roles in immunological surveillance. The ability to activate murine peritoneal macrophages in vitro by five water-soluble fractions (F-I to F-V) extracted from mycelium of Pleurotus sp. was demonstrated in a previous work, as judged by the increase found in glucose consumption and in lysosomal enzyme (acid phosphatase) activity (Morris et al., Citation2007).
The effects of two glucans obtained from Pleurotus ostreatus and yeast were assessed in different immunological functions of mice (Paulik, Svrcec, Mojzisova, Durove, Benisek, & Huska, Citation1996). Both glucans augmented the DTH response compared to control mice, but the induction was higher for Pleurotus glucan. A significant increase in the number of T cells (both CD4+ and CD8+) was found in mice administered with a water-soluble polysaccharide extracted from the fermentation broth of Pleurotus citrinopileatus (Wang, Hu, Liang, & Yeh, Citation2005).
These findings suggest that oral administration of edible mushroom-derived products with potential immunomodulating activities would stimulate the immune system after their absorption in the gastrointestinal tract and the activation of gut-associated lymphoid tissues, thus integrating different elements of the immune function.
However, the results of the administration of an aqueous extract of P. florida to female Balb/c mice showed that DTH responses were not affected by various doses of the product in different routes (i.p. or oral). With respect to viability of splenic cells and histological changes in secondary lymphoid tissues, this study indicated that the effects of P. florida on cellular responses depend on doses and routes of administration (Sedaghat & Ghazanfari, Citation2011).
In our study, the DTH reconstitution was associated with the increase observed in the mass index of popliteal lymph nodes of the Pleurotus supplemented animals (p<0.05; ). Antigens are concentrated in the secondary lymphoid organs, including lymph nodes, where they are presented by mature dendritic cells, the most efficient type of antigen-presenting cell for initiating responses of naive T cells (Janeway et al., Citation2005).
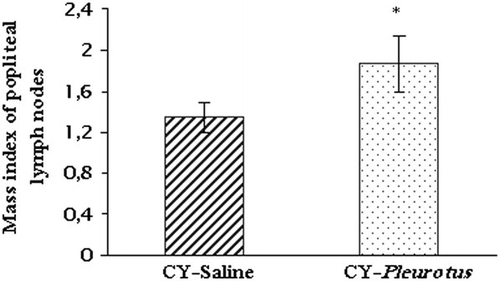
Considering the possible sex-based differences on cellular immune response, the use of both male and female animals is recommended in future preclinical evaluations of Pleurotus nutritional supplements.
Cellular immune response was also evaluated by the in vitro lymphoproliferative response of murine splenocytes. The incubation of splenocytes with aqueous and ethanolic extracts derived from Pleurotus powder for 72 h led to stimulation indexes of 1.90 and 1.28, respectively (). The higher index obtained for aqueous extract could be related with the presence in this fraction of immunomodulating glucans.
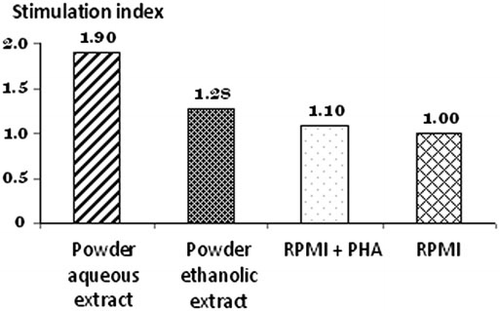
It has been reported that the incubation of human mononuclear cells with different extracts from Ganoderma lucidum led to the stimulation of lymphoproliferative response (Soto-Velazco et al., Citation2002). On the other hand, glucans isolated from P. florida fruiting bodies significantly induced the proliferative response as well as phagocytic activity of fish leukocytes (Catla catla) in vitro (Kamilya, Ghosh, Bandyopadhyay, Mal, & Maiti, Citation2006).
In sum, the nutritional supplement of Pleurotus sp. provides immunological benefits in terms of the stimulation of cell-mediated immune responses. Pleurotus powder could potentiate the host defence mechanisms, and further studies are needed to address effective phytochemicals and their mechanisms.
4. Conclusion
This study shows that Pleurotus fruiting bodies powder exhibited immunomodulating properties, as judged by the in vivo stimulation of cell immune response when administered orally to cyclophosphamide-treated Balb/c mice, and the in vitro lymphoproliferative-stimulating response. Although most of the phytoconstituents identified in Pleurotus powder have been implicated as immunomodulatory agents, we are unable, at present, to link the observed activity to any of the constituents. Moreover, phytochemical(s) can act alone or in combination to effect physiological changes. These effects on cell immunity are especially valuable in the prophylaxis of tumours, immunodeficiencies and as co-adjuvant in chemotherapy.
Acknowledgements
We would like to thank the Cuban Ministry of Science, Technology and Environment (Territorial Project 9072 of the Program for Development of Health Products and Services) and the University of Oriente's authorities that enabled us to carry out this research.
References
- Association of Official Analytical Chemists (AOAC). (1995). Official methods of analysis (16th ed.). Arlington: Author.
- Bermúdez, R.C., Garcia, N., Gross, P., & Serrano, M. (2001). Cultivation of Pleurotus on agricultural substrates in Cuba. Micologia Aplicada International, 3, 25–29.
- Chang, S.T., & Buswell, J.A. (1996). Mushroom nutriceuticals. World Journal of Microbiology and Biotechnology, 12, 473–476.
- Chang, S.T., & Buswell, J.A. (2003). Medicinal mushrooms – A prominent source of nutriceuticals for the 21st century. Current Topics in Nutraceutical Research, 1, 257–280.
- Costa, R., Cunha, V., & Carvalho, M.R. (2006). The immunomodulator role of β-D-glucans as co-adjuvant for cancer therapy. Revista Brasileira de Nutrição Clinica, 21, 163–168.
- Descotes, J. (2006). Methods of evaluating immunotoxicity. Expert Opinion on Drug Metabolism and Toxicology, 2, 249–259.
- Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
- Dubost, N.J., Ou, B., & Beelman, R.B. (2007). Quantification of polyphenols and ergothioneine in cultivated mushroom and correlation to total antioxidant capacity. Food Chemistry, 105, 727–735.
- Gao, Y., Zhou, S.H., Huang, M., & Xu, A. (2003). Antibacterial and antiviral value of the genus Ganoderma P. Karst. species (Aphyllophoromycetideae) – A review. International Journal of Medicinal Mushrooms, 5, 235–246.
- Gregori, A., Švagel, M., & Pohleven, J. (2007). Cultivation techniques and medicinal properties of Pleurotus spp. Food Technology and Biotechnology, 45, 238–249.
- Harbourne, H.B. (1984). Phytochemical methods: A guide to modern technique of plant analysis (2nd ed). London: Chapman and Hall.
- Hobbs, C. (2004). Medicinal value of Turkey tail fungus Trametes versicolor (L.: Fr) pilát (Aphyllophoromycetideae). A literature review. International Journal of Medicinal Mushrooms, 6, 195–218.
- Hu, S.H., Liang, Z.C., Chai, Y.C., Lien, J.L., Chen, K.S., & Lee, M.Y., et al. (2006). Antihyperlipidemic and antioxidant effects of extracts from Pleurotus citrinopileatus. Journal of Agricultural and Food Chemistry, 54, 2103–2110.
- Janeway, C.A., Travers, P., Walpert, M., & Shlomchik, M. (2005). Immunobiology (6th ed). London: Garlan Publishing.
- Kamilya, D., Ghosh, D., Bandyopadhyay, S., Mal, B.C., & Maiti, T.K. (2006). In vitro effects of bovine lactoferrin, mushroom glucan and Abrus agglutinin on Indian major carp, catla (Catla catla) head kidney leukocytes. Aquaculture, 253, 130–139.
- Kim, Y.S., Maslinski, W., Zheng, X.X., Stevens, A.C., Li, X.C., Tesch, G.H., et al. (1998). Targeting the IL-15 receptor with an antagonist IL-15 mutant/Fc gamma 2a protein blocks delayed-type hypersensitivity. Journal of Immunology, 160, 5742–5748.
- Li, Y., & Mi, C. (2003). Proliferation inhibition and apoptosis in human ovarian carcinoma cell line SKVO3 induced by genistein. Ai Zheng, 22, 586–591.
- Liu, D.Z., Liang, H.J., Chen, C.H., Su, C.H., Lee, T.H., Huang, C.T. et al. (2007). Comparative anti-inflammatory characterization of wild fruiting body, liquid-state fermentation, and solid-state culture of Taiwan fungus Camphoratus in microglia and the mechanism of its action. Journal of Ethnopharmacology, 113, 45–53.
- Lowry, H.O., Rosebrough, A., Farr, L., & Randall, J.R. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
- Morris, H.J., Lebeque, Y., Fontaine, R., Bermúdez, R.C., Llauradó, G., & Marcos, J. (2007). A note on the in vitro macrophage-stimulating activity of water-soluble extracts from mycelium of Pleurotus spp. Food and Agricultural Immunology, 18, 31–37.
- Morris, H.J., Marcos, J., Llauradó, G., Fontaine, R., Tamayo, V., & García, N., et al. (2003). Immunomodulating effects of hot-water extract from Pleurotus ostreatus mycelium on cyclophosphamide treated mice. Micologia Aplicada International, 15, 7–13.
- Ou, C.C., Hsiao, Y.M., Wang, W.H., Ko, J.L., & Lin, M.Y. (2009). Stability of fungal immunomodulatory protein, FIP-gts and FIP-fve, in IFN-γ production. Food and Agricultural Immunology, 20, 319–332.
- Paulik, S., Svrcec, K., Mojzisova, J., Durove, A., Benisek, Z., & Huska, M. (1996). The immunomodulatory effect of the soluble fungal glucan (Pleurotus ostreatus) on delayed-hypersensitivity and phagocytic ability of blood leukocytes in mice. Journal of Medicine Veterinary and Biology, 43, 129–135.
- Petrova, R., Wasser, S.P., Mahajna, J.A., & Denchev, C.M. (2005). Potential role of medicinal mushrooms in breast cancer treatment: Current knowledge and future perspectives. International Journal of Medicinal Mushrooms, 7, 141–155.
- Rop, O., Mlcek, J., & Jurikova, T. (2009). Beta-glucans in higher fungi and their health effects. Nutrition Reviews, 67, 624–631.
- Sedaghat, R., & Ghazanfari, T. (2011). The immunomodulating effects of Pleurotus florida on cell-mediated immunity and secondary lymphoid tissues in Balb/c mice. Immunopharmacology and Immunotoxicology, 33, 28–32.
- Smiderle, F.R., Olsen, L.M., Carbonero, E.R., Marcon, R., Baggio, C.H., & Freitas, C.S. (2008). A 3-O-methylated mannogalactan from Pleurotus pulmonarius: Structure and antinociceptive effect. Phytochemistry, 69, 2731–2736.
- Smith, J.E., Sullivan, R., & Rowan, N.J. (2003). The role of polysaccharides derived from medicinal mushrooms in cancer treatment programs. Current perspectives (review). International Journal of Medicinal Mushrooms, 5, 217–234.
- Soto-Velazco, C., López, M., Vázquez, A., Valls, E., & Álvarez, L. (2002). Cultivation of Ganoderma lucidum and its effects on the production of lymphocytes. In J.E. Sánchez, G. Huerta, & E. Montil (Eds.), 4th International Conference on Mushroom Biology and Mushroom Products (pp. 379–382). Cuernavaca: ECOSUR.
- Thekkuttuparambil, A.A., & Kainoor, K.J. (2007). Indian medicinal mushrooms as a source of antioxidant and antitumor agents. Journal of Clinical Biochemistry and Nutrition, 40, 157–162.
- Wang, J.C., Hu, S.H., Liang, Z.C., & Yeh, C.J. (2005). Optimization for the production of water-soluble polysaccharide from Pleurotus citrinopileatus in submerged culture and its antitumor effect. Applied Microbiology and Biotechnology, 67, 775–776.
- Wasonga, C.G., Okoth, S.A., Mukuria, J.C., & Omwandho, C.O. (2008). Mushroom polysaccharide extracts delay progression of carcinogenesis in mice. Journal of Experimental Therapeutics and Oncology, 7, 147–152.
- Wasser, S.P. (2002). Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology, 60, 258–274.
- Wasser, S.P. (2010). Medicinal mushroom science: History, current status, future trends, and unsolved problems. International Journal of Medicinal Mushrooms, 12, 1–16.
- Wasser, S.P., Didukh, M., & Nevo, E. (2004). Dietary supplements from culinary-medicinal mushrooms: A variety of regulations and safety concerns for the 21st century. International Journal of Medicinal Mushrooms, 6, 231–248.
- Wasser, S.P., & &Weis, A.L. (1999). Medicinal properties of substances occurring in higher Basidiomycetes mushrooms: Current perspectives. International Journal of Medicinal Mushrooms, 1, 31–62.
- Zaidman, B., Yassin, M., Mahajna, J., & Wasser, S.P. (2005). Medicinal mushrooms modulators of molecular targets as cancer therapeutics. Applied Microbiology and Biotechnology, 67, 453–468.
- Zhang, M., Cui, S.W., Cheung, P.C.K., & Wang, Q. (2007). Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends in Food Science and Technology, 18, 4–19.