Abstract
This study presents the generation of monoclonal antibodies (mAbs) through cell fusion procedure and the development of mAb-based indirect competitive ELISA (icELISA) for detecting ciprofloxacin (CIP) residue in poultry samples. Under optimal experimental conditions, this assay exhibited a working range of 0.05–83.2 ng/mL, with the half maximal inhibitory concentration (IC50) and the limit of detection values of 0.56 and 0.03 ng/mL, respectively. Cross-reactivity results showed a class-specificity immunoassay and 20-fold dilution in muscle extracts gave an inhibition curve almost the same as that in PBS buffer. It also indicated that the optimised concentrations of pH and NaCl in dilute solution were 7.4 and 0.1 M, respectively, and the icELISA can tolerate higher concentrations of methanol (30%) than acetonitrile (10%) tested. When applied to poultry muscles, the correlation coefficients (R2) between the icELISA and liquid chromatography–mass spectrometry data were 0.9411 in chicken, 0.953 in duck and 0.894 in geese. Therefore, this assay has the potential to be incorporated into a quantitative monitoring programme for the rapid screening of CIP residue in food.
Introduction
Fluoroquinolones (FQs) are derived from quinolone nalidixic acid by the introduction of the piperazine moiety at position 7 and a fluorine atom at position 6 (), which act as broad-spectrum antibiotics through the inhibition of an essential bacterial enzyme, DNA gyrase, and therefore are more effective against Gram-negative bacteria and some Gram-positive bacteria than quinolone antibiotics (Wang et al., Citation2007). Therefore, FQs have found widespread application in the treatment and prevention of veterinary diseases in food-producing animals, and they have even been used as growth-promoting agents (Zhang, Jiang, Wang, et al., Citation2011). They are also alternative agents for the treatment of many sexually transmitted diseases, as well as osteomyelitis, some cases of wound infection and selected respiratory infections (Rishton, Citation2005). Ciprofloxacin (CIP) is a major metabolite of enrofloxacin in animals, and is also one of the most widely used FQs for the treatment of urinary tract infections, respiratory tract infections and gastrointestinal tract, as well as skin and soft tissue infections (Ahmad, Parveen, & Khan, Citation2006). However, these FQ residues may persist in animal body, resulting in the development of drug-resistant bacterial strains or allergies (Yan, Wang, Qin, Liu, & Du, Citation2011). Further research demonstrates that these antibiotics and their metabolites may directly cause oxidative damages to cell membranes. Some FQs have also been suspected to affect the central nervous systems. For example, in human, they have been associated in some cases of severe disorders such as headaches, dizziness or convulsions (Zhang, Jiang, Li, et al., Citation2011).
Because of concerns about drug residues entering the food chain and contributing to bacterial resistance, more and more countries have set the maximum residue levels (MRLs) and withdrawal periods for FQs. According to different food resources and different drugs, the MRLs of FQs are set in the range of 30–1500 µg/kg (Huet et al., Citation2006; Lu et al., Citation2006). For example, the European Commission has established the MRLs for the sum of enrofloxacin and its major metabolite CIP in chicken tissues as follows: muscle, 100 µg/kg; skin, 100 µg/kg; liver, 200 µg/kg and kidney, 300 µg/kg (Anonymous, Citation1999). In China, the species of animal, usage, dosage and withdrawal period of FQs have been established by the Ministry of Agriculture of the People's Republic of China (No. 278, 22 May 2003). Therefore, monitoring of CIP residue in the poultry industry is crucial for safeguarding public health, preventing illicit use and facilitating government regulation and surveillance, and there is also a growing demand for easy, rapid and economical methods to monitor FQ residues in foodstuffs.
Up to now, a variety of methods for detecting FQ residues in biological matrices have been developed, such as high-performance liquid chromatography (Christodoulou, Samanidou, & Papadoyannis, Citation2008; Hassouan, Ballesteros, Zafra, Vílchez, & Navalón, Citation2007), liquid chromatography–mass spectrometry (LC–MS/MS; Dufresne, Fouquet, Forsyth, & Tittlemier, Citation2007; Hermo, Nemutlu, Kir, Barrón, & Barbosa, Citation2008; Volmer, Mansoori, & Locke, Citation1997) and enzyme-linked immunosorbent assays (ELISAs; Huet et al., Citation2006; Lu et al., Citation2006; Wang et al., Citation2007; Zhang, Jiang, Li, et al., Citation2011). Chromatographic analysis provides sensitive and accurate techniques; however, they rely on highly trained individuals to operate the sophisticated instruments and interpret complicated chromatograms or spectral results. Moreover, these procedures require a large quantity of time and money and use of harmful organic solvents that cause health hazards to analytical chemists and load to the environment. Hence, these methods may be unsuitable for on-site screening because quickness and simplicity are needed. ELISAs are analytical methods based on the specific interaction between antibody and its corresponding antigen. Owing to the advantages of high sensitivity and rapidity, portability and high throughput, simple sample preparation and low cost, ELISAs have become popular and are increasingly considered as alternative or complementary methods for residue analysis.
Therefore, the aim of this study was to produce a monoclonal antibody (mAb) against CIP and develop a sensitive, indirect, competitive ELISA (icELISA) for the detection of CIP residue in poultry muscles. The influence of several physicochemical factors such as ionic strength, pH value and organic solvent tolerance were carefully evaluated for their effects on the icELISA performance. The correlation between the ELISA and LC–MS/MS analysis on detecting authentic poultry samples was also investigated. This work potentially optimises the pre-treatment procedures for physicochemical spectrometry techniques, lays a solid foundation for ELISA kits and tests strip development.
Materials and methods
Chemicals and materials
Ciprofloxacin, enrofloxacin, sarafloxacin, ofloxacin, norfloxacin and danofloxacin were provided by Sigma (St. Louis, MO, USA), while the other FQs were purchased from the China Institute of Veterinary Drug Control (Beijing, China). 1-Ethyl-3-(3-dimethylaminopropy) carbodiimide (EDC), Freund's complete adjuvant (FCA) and Freund's incomplete adjuvant (FIA) were obtained from Pierce. GaMIgG-HRP was purchased from Sino-American Biotechnology Company (Shanghai, China). Transparent 96-well polystyrene microtitre plates (Boyang Experimental Equipment Factory, Jiangsu, China) were used for the colorimetric measurement. N-hydroxysuccinimide (NHS), hypoxanthine/thymidine/aminopterin (HAT) and hypoxanthine/thymidine were obtained from Sigma-Aldrich (USA). RPMI-1640 with L-glutamine was obtained from Gibco. Polyethylene glycol 1500 (PEG 1500, 50%) was from Roche Diagnostics Corporation (Indianapolis, USA). Foetal bovine serum (FBS) was from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou, China). 3,3′,5,5′-Tetramethylbenzidine (TMB), phenacetin and urea peroxide were obtained from Sigma Company. All other solvents and reagents were of analytical grade or higher, unless otherwise stated.
Instruments
A spectrophotometric microtitre reader (Multiskan MK3, Thermo Company, USA), provided with a 450-nm filter, was used for absorbance measurements. UV–visible spectra were obtained by using a DU800 Ultraviolet–visible Spectrophotometer (Beckman-Coulter Company, USA). A GS15R high-speed refrigerated centrifuge were supplied by Thermo Company (USA). CO2 incubator from RS-Biotech (Galaxy S+, UK) was used for cell cultivation. Deionised water was prepared using an Ultra class UV plus water purification system (SG Company, Germany).
Preparation of cationised BSA and OVA
In this procedure, carboxylic acid groups of the carrier proteins of bovine serum albumin (BSA) and ovoalbumin (OVA) were converted into primary amine groups with an excess of ethylenediamine (EDA) (Lu et al., Citation2006). A solution of 1 g of BSA (15 µmol) and 56 mg of EDC (300 µmol) in 20 mL of PBS (0.01 M, pH 7.4) was added slowly into a solution of 18 mg of EDA (300 µmol) in 20 mL of PBS under stirring. The mixture solution was incubated continuously for 2 h at room temperature and then dialysed under stirring against PBS to remove the free EDA. The solution was lyophilised, and the cationised BSA (cBSA) obtained was stored at −20°C before use in the next reaction. The cationised OVA (cOVA) was prepared in a similar method.
Artificial antigen synthesis for CIP
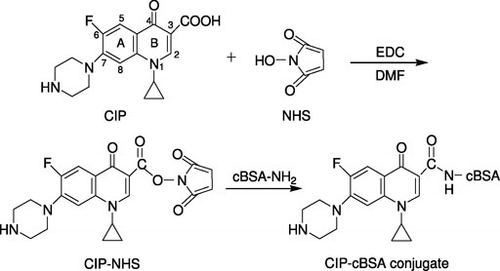
A modified active ester method was employed to synthesise the artificial antigen of CIP–cBSA, and the procedure is shown in . Briefly, 33.2 mg (0.1 mmol) of CIP was suspended in 3-mL N,N-Dimethylformamide (DMF) and then 12 mg (0.1 mmol) of NHS and 38 mg (0.2 mmol) of EDC were added. During the following 24-h incubation in dark chamber, the mixture was stirred with an HY-4 Reciprocal Shaker at 37°C. To this solution, 66 mg (0.001 mmol) of cBSA dissolved in 20 mL of PBS was added dropwise under stirring. The resulting mixture was stirred by rotor in dark chamber at 37°C for 1 h, and then incubated with a reciprocal shaker for 3 h. After being centrifuged at 3000 rpm for 10 min, the obtained supernatant was dialysed against distilled water and followed by PBS for 4 days. When the absorption peak of the dialysed solution disappeared, it was checked by UV–visible spectra, stored the artificial immunogen of CIP–cBSA in ampoule and kept at −20°C. The coating antigen of CIP–cOVA conjugate was prepared in a similar method. UV–visible spectra were recorded to calculate the conjugation ratio.
Production of monoclonal antibodies
Immunisation schedule
Five female BALB/c mice (8–10 weeks old) were injected subcutaneously at multiple points with CIP–cBSA immunogen (60 µg in 0.1 mL of PBS, mixed with an equal volume of FCA to form an emulsion). For subsequent boosters, FIA was substituted for the FCA as an emulsifier every 3 weeks. After the third booster immunisation, blood was obtained from the coccygeal vein section, and the sera were checked for their titre and ability to compete with CIP. The mouse whose antiserum showed strongest competition towards CIP was selected for the fusion experiment. Four days prior to splenocyte harvest, the mouse was injected with 100 µg of immunogen in PBS, divided equally for intravenous and intraperitoneal (i.p.) injections.
Cell fusion
Portions of the cell fusion procedures and subcloning conditions were detailed previously (Jiang, Zhang, et al., Citation2011; Ren, Zhang, Chen, & Yang, Citation2009), and are only briefly described here. NS0 myeloma cells were maintained in an exponential growth stage in RPMI-1640 supplemented with 10% FBS. The spleen from the mouse with the strongest competition towards CIP and highest titre was aseptically harvested and fused with myeloma cells at a 10:1 ratio using PEG 1500 as the fusing agent. The fused cells were then distributed into 96-well culture plates, in which mouse peritoneal macrophages were prepared on the day before the fusion and were grown with the selective HAT medium. Well cultures showing significant CIP recognition activity were expanded, and subcloned three times by limiting dilution. Colonies of interest were frozen in culture medium containing 10% dimethyl sulphoxide (DMSO) and cryopreserved in liquid nitrogen.
Production and characterisation of mAbs
A mature female BALB/c mouse was injected i.p. with 0.5-mL paraffin 10 days before receiving an i.p. injection of the positive hybridoma cells suspended in RPMI-1640 medium. Ascites fluid was collected 14 days after the injection and then stored at −20°C until use. Purification of mAb was achieved by saturated ammonium sulphate precipitation. The protein content of the antibody was determined according to the following formula: protein concentration (mg/mL) =1.45OD280nm−1.74OD260nm, where OD value is the optical density. Measurement of mAb affinity (K a) was carried out according to the procedure described by Wang Guo, Li, and Chang (Citation2002). The class and subclass of the isotypes of the purified antibody were determined by using a mouse mAb isotyping kit (Pierce Biotechnology, Inc., USA).
Indirect ELISA and icELISA procedures
Indirect ELISA was performed using a procedure described before (Le et al., Citation2009). Briefly, 96-well microplates were coated with coating antigen in 100 µL of coating buffer (0.05 mol/L carbonate buffers, pH 9.6) overnight at 4°C. Plates were washed three times with PBS containing 0.05% Tween-20 (PBST) and unbound active sites were blocked with 250 µL/well of blocking buffer, followed by incubation for 2 h at 37°C. The plates were then washed with PBST, followed by the addition of 100 µL/well of antiserum or mAb. After incubation for 15 min at 37°C and another washing, GaMIgG-HRP (100 µL/well) was added, followed by incubation for 25 min at 37°C. The final washing procedure was followed by colour development, which was initiated by adding 100 µL/well of freshly prepared TMB substrate solution. The samples were incubated for 15 min at room temperature and then 2-M sulphuric acid (100 µL/well) was added to stop the enzymatic reaction. Absorbance was measured at a wavelength of 450 nm using a Multiskan MK3 microplate reader and the antibody titre was defined as the reciprocal of the dilution that resulted in an absorbance value that was twice higher than that of the background.
The icELISA was employed to determine sensitivity and specificity, and the procedure was the same as that of the indirect ELISA except that after blocking, a competition step was introduced by adding 100 µL/well of analyte, followed by 100 µL/well of appropriate concentration of antibody. With the icELISA format, analytes that do not react with the antibody would produce absorbance near 100%; conversely, analytes that do react with the antibody would decrease in percentage of absorbance. The inhibition rate was expressed as %B/B 0, where B is the absorbance of the well containing competitor and B 0 is the absorbance of the well without competitor. Standard curves were calculated by mathematically fitting experimental points to a four-parameter logistic equation. Specificity is defined as the ability of structurally related chemicals to bind to the anti-CIP mAb, and the cross-reactivity (CR) was calculated as (IC50 of CIP)/(IC50 of competitors)×100.
Physicochemical effects on assay performance
It is commonly acknowledged that immunoassay performance is often affected by physicochemical parameters such as ionic strength, pH values, organic solvent concentration and other substances in the sample matrix (Sheng et al., Citation2009). The effects of these parameters were estimated by running standard curves under various conditions. The maximum absorbance (A max, the absorbance value at zero concentration of CIP) and half-maximum inhibition concentration (IC50, the value represents the concentration of CIP that produce 50% inhibition of antibody binding to the hapten conjugate) were calculated, and the maximal A max/IC50 ratio was chosen (Hao et al., Citation2009). To determine the effects of pH in assay buffer, CIP was diluted in 0.01-M PBS with pH values of 5.0, 6.0, 7.4, 8.0 and 9.0. At the optimum pH value, the effect of ionic strength was evaluated using different levels of NaCl ranging from 0.05 to 1.0 M. In addition, the effects of acetonitrile and methanol were studied because both solvents are water-miscible and are often employed in sample extraction.
Sample preparation for icELISA and LC–MS/MS analysis
Authentic poultry muscle samples (chicken, duck and geese) were purchased in retail outlets in Xinxiang, China. After fat and connective tissues were removed by dissection, the samples were homogenised with a high-speed triturator and collected in a 50-mL round-bottom plastic flask. For ELISA analysis, 2-mL aliquot of sample was mixed with 2 mL of extraction solvent consisting of a 1:1 (v/v) mixture of methanol and PBS adjusted to pH 7.4 with 6-M HCl. The homogenate was mixed on a vortex mixer for 30 s, vigorously shaken for 5 min and then centrifuged at 4°C with a speed of 8000 rpm for 10 min. The supernatant layer was transferred into a calibrated flask, and 20-fold diluted in assay buffer before they were applied to the microtitre plate.
For LC–MS/MS analysis, the supernatants were submitted to solid-phase for clean-up process. The SPE C18 cartridges (Dalian Sipore Co., Ltd., China) were consecutively conditioned with 5 mL of methanol and then 5 mL of deionised water at a flow rate of 0.3 mL/min. After loading with the aqueous extract solution, the cartridge was washed with 10 mL of the elution solution (n-hexane–ether [70:30, v/v]) at a flow rate of less than 0.5 mL/min. After centrifugation, the organic layer was removed under a stream of nitrogen in a water bath at 45°C, and the extracts were redissolved using a mobile phase solution of 1% formic acid in water/acetonitrile/methanol (60:20:20, pH 2.5) for further LC–MS/MS analysis. The detection conditions were almost the same as that described by Pearce, Burns, van de Riet, Casey, and Potter (Citation2009). Positive chemical ionisation mode was used and the relative collision energy was optimised to 36%. A pseudo-molecular ion [M + H]+ was selected as the parent ion, and selected ion monitoring (SIM) was used for screening of individual analyte in this study.
Results and discussion
Artificial antigen synthesis
Ciprofloxacin with a molecular weight of 331.35 is too small to be immunogenic and must be conjugated to a carrier protein before immunisation to elicit an immune response. Among carrier proteins, BSA and OVA are the two of the most often used ones, and they usually give satisfying results. But, under concentrated concentrations, the drawbacks of poor solubility and protein cross-linking are presented. Therefore, BSA and OVA were modified with the diamine EDA as described previously, to convert carboxylic acid groups on the carrier proteins to primary amine groups. The use of cationised carriers has the advantage that more amino groups on the carrier become available for coupling and that protein cross-linking is minimised. Furthermore, cationised proteins treated with EDA to increase their pI are known to generate an increased immune response compared with their native forms (Lu et al., Citation2006).
UV–vis spectrogram
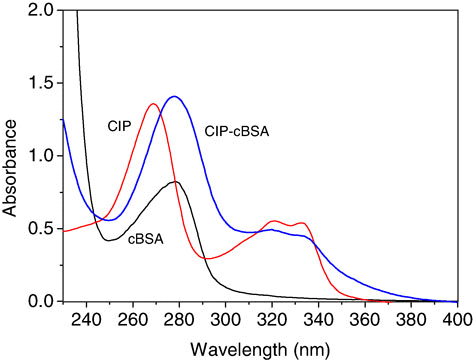
UV–vis spectrum for CIP–cBSA, CIP and cBSA is shown in . The absorbance for CIP–cBSA (279 and 321 nm) gave a significant shifted peak at 279 nm compared with the 269 nm peak for CIP (269, 321 and 333 nm), while the maximum absorbance of cBSA was at 276 nm, which indicated that CIP was successfully conjugated with cBSA. The coating antigen of CIP–cOVA gave a UV pattern similar to that of immunogen. Calculated from the formula (Huang et al., Citation2010), molar ratio of 18.2:1 for CIP–cBSA conjugate was obtained, and the conjugation ratio for CIP–cOVA was 9.5:1.
Monoclonal antibodies (mAbs) production and characterisation
Fourteen days following the fusion, growing hybridoma cell clones could be observed in many wells of the seeded 96-well plates. The fusion rate of the mouse spleen cells with myeloma cells was about 76%. Supernatants of all wells were screened by simultaneous noncompetitive and competitive assays, and the positive well rate was 22%. Selection of clones from these positive cultures by limiting dilution led to six stable hybridoma cell lines. These monoclonal cultures and their corresponding cell lines were named C1G2B3, C3D2F1, C3E2C6, C4D5H3, C5E2F6 and C7A1D8, respectively. Using a mouse mAb isotyping kit, five antibodies were of the IgG1 isotype, and one of them was IgG2a isotype; all immunoglobulins contained the k light chain, and the protein concentrations of all mAbs were between 3.6 and 6.4 mg/mL. The affinity of an antibody for its corresponding antigen is crucial with parameters affecting the performance of an immunoassay, and high-affinity antibodies can lower the dissociation tendency of the antigen/antibody complex and produce sensitive IC50 values (Wang, Zhang, Gao, Duan, & Wang, Citation2010). In our study, the affinity constants (K a) of the six selected hybridomas could be measured by ELISA using serial dilutions of both coating antigen and CIP mAbs (Wang et al., Citation2002), and the values were determined to be 3.2×109, 4.5×109, 1.2×1010, 3.6×1010, 5.2×109 and 1.8×1010 L/mol, respectively. From the inhibition curves, mAb C4D5H3 afforded the most sensitive assay (results not shown), which was selected for further evaluation and subsequent immunoassay development.
Establishment of the icELISA standard curve
It is well known that immunoassay performance may be affected by many physicochemical features of the media and by a variety of experimental conditions, of which the working concentrations of antibody and coating antigen are crucial factors for the sensitivity of competitive ELISA methods (Li et al., Citation2008). For this reason, checkerboard titrations were performed, taking into account the optimal dilutions. The optimal reagent concentrations were determined when the A max was between 1.5 and 2.0, and the dose–response curve of inhibition ratio versus the CIP concentration pursued the lowest IC50 values. From the checkerboard assays, the optimum concentration of coating antigen was chosen as 0.6 µg/mL and mAb was 0.2 µg/mL (1:30,000 dilutions). On the basis of the results of the checkerboard titration, a representative standard curve with icELISA format is shown in .
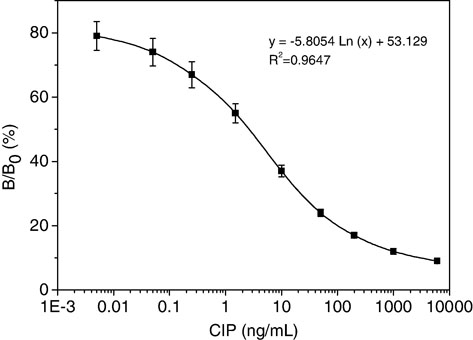
Sensitivity was evaluated according to the inhibition rate, and the data were calculated using the IC50 values, which represented the concentration of CIP that produced 50% inhibition. The limit of detection (LOD) was defined as the lowest concentration that exhibits a signal of 15% inhibition (Jiang, Wang, et al., Citation2011). The dynamic range for the icELISA was calculated as the concentration of the analyte providing a 20–80% inhibition rate (IC20–IC80 values) of the maximum signal. Therefore, this assay allowed the detection of CIP (20–80% inhibition) from 0.05 to 83.2 ng/mL, with an IC50 value of 0.56 ng/mL. The LOD of the assay was determined to be 0.03 ng/mL.
Specificity of the developed immunoassay
Specificity is a phenomenon inherent to all immunoassays, which can affect analytical results by either false positives or by elevating the predicted concentration of the target compound when both the target and one or more structurally similar compounds are present. Therefore, investigations on CR of the obtained antibody are crucial for an assessment of the results. In this work, the study was undertaken by adding various competitors to compete with binding of the antibody to the coating antigen. Analytes that do not react with the antibody would produce absorbance near 100%; conversely, analytes that do react with the antibody would decrease in percentage of absorbance. The IC50 value and CR rate for each compound are shown in .
Table 1 Cross-reactivities of related FQ analogues in the CIP immunoassay.
Of all the cross-reacting analogues, this assay exhibited a high CR to norfloxacin (76.7%), enrofloxacin (75.6%) and pefloxacin (61.5%). In our research, the immunogen was synthesised by the linkage of carboxylic acid group of CIP with the amino group of cBSA (). In this linkage, the furthest group of CIP from the linking point is the piperazinyl moiety. Therefore, our results are consistent with the well-accepted rule in immunology that antibodies elicited to haptenic conjugates show a preferential recognition to the part of molecule that is furthest from the attachment site of the hapten (Bucknall, Silverlight, Coldham, Thorne, & Jackman, Citation2003). The second group consists of five drugs, although they all contain the piperazinyl ring at position 7 of ring A, some heteroatom structures exist in these molecules, such as the nitrogen at position 8 in ring A of enoxacin; the fluorophenyl group at position 1 in ring B of sarafloxacin. Therefore, moderate CR to enoxacin (29.7%), sarafloxacin (24.6%), ofloxacin (17.3%), lomefloxacin (11.5%) and marbofloxacin (9.8%) are not surprised. The other two FQs such as danofloxacin and flumequine own fully different structures, which demonstrated no detectable CR (). It proves that this immunoassay has the potential to be incorpated into a multiresidue programme for detecting veterinary FQ residues in animal-producing foodstuffs.
Effects of pH and ionic strength in assay buffer
To study the influence of pH on the assay characteristics, competitive curves were prepared using standards in PBS. shows the effects of pH of the assay solution on the icELISA. Using the methods described previously, these parameters of Amax and IC50 were considered, and the ratio of Amax/IC50 was used to estimate the optimum pH value. Although Amax values were lower at pH less than 5.0 and greater than 8.0, there were no significant fluctuations in the IC50 values between pH 6.0 and 8.0. This indicated that acidic and alkaline solutions likely promote the denaturation of the antibody and/or enzyme conjugate, causing changes in their spatial structures with adverse effects on the reactions between the antibody and the analyte. Consequently, neutral assay buffer provides the best conditions for the binding of antibody and hapten, and the physiological pH 7.4 was selected for the assay buffer of the immunoassay.
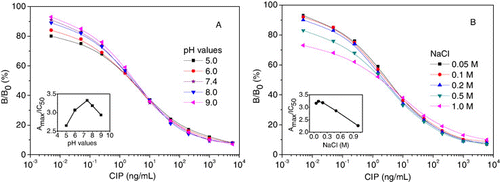
The salt effects on immunoassay were dramatic. Although the B0 and IC50 values remained essentially constant at low NaCl concentrations 0.05, 0.1 and 0.2 M, the IC50 increased twofold and the Amax decreased more than 30% when the NaCl concentration increased from 0.2 to 1.0 M (B). Some authors have reported that a high-salt concentration is desirable in the extraction solution as it causes the separation of proteins in samples, from sugars and lipids, which often bind to proteins and cause purification problems (Hao et al., Citation2009). However, the results obtained showed that the use of NaCl as the only component of the extraction solution did not provide enough sensitivity.
Organic solvents tolerance
Acetonitrile and methanol were commonly used to extract CIP from samples and increase the solubility of analytes that were tested for their effects on the icELISA. shows the normalised dose–response curves at various solvent concentrations. First, the influence of acetonitrile from 2 to 30% (v/v) in PBS on the immunosorbent assay was studied (). Increasing the concentration of acetonitrile generally decreased and then increased the IC50 value, but a continuous increase in the time for colour development. This is because acetonitrile may affect the reaction between antigen and antibody by decreasing the bioactivity of antibody and hindering the enzyme activity (Jin et al., Citation2008). But, as the contents of acetonitrile increased from 2 to 10%, the absorbance gradually approached to that of the PBS buffer, indicating that 10% of acetonitrile still allowed a significant gain in the detectability of this analyte. Taking account of these results, acetonitrile concentration in the assay should not be higher than 10%.
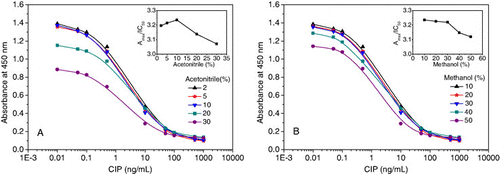
B shows the normalised dose–response curves at different methanol compositions. It was observed that methanol concentrations from 10 to 20% had no significant effects on the sensitivity, and the IC50 value just changed from 0.56 to 0.57 ng/mL. This sensitivity increase may be due to the dispersion and weakening of the nonspecific binding derived from mAbs. Consequently, concentrations of methanol higher than 30% resulted in lower absorbance and sensitivity drop as the higher methanol may weaken the antibody–hapten interaction. Therefore, it was necessary to control the methanol concentration below 30% in the sample extract solution before detection by icELISA in plates. In this study, 20% of methanol in PBS was recommended as the preferred assay buffer, and was employed in the remainder of experiments.
Correlation studies between ELISA and LC–MS/MS analysis
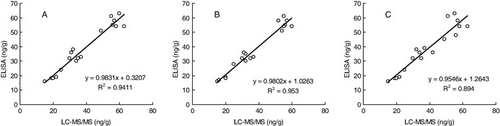
To determine the performance of the icELISA method in the real world, authentic poultry muscle samples (chicken, duck and geese) purchased in retail outlets in Xinxiang were analysed by using the icELISA test kit, and the results were confirmed by LC–MS/MS. shows the correlation of dual results of icELISA and LC–MS/MS method, and the linear regression between the two group data. From we can find that the data spots are nearly distributed on both sides of the standard line, that is to say, the data obtained from these two methods are very similar and with no significant differences, although there is a slight tendency for the ELISA values to be slightly higher than the LC–MS/MS values. We believe that the chromatographic purification procedure used in the sample preparation is responsible for the loss of CIP contents in LC–MS/MS. The correlation coefficients (R2) between the icELISA and LC–MS/MS data were 0.9411 in chicken, 0.953 in duck and 0.894 in geese, respectively.
Conclusion
In summary, we have prepared high-quality mAbs and established a sensitive icELISA method to detect CIP residue in poultry muscles for the first time. The results demonstrate that icELISA can be used as a screening method for detecting CIP residues in foodstuffs, and it provides a noticeable practical advantage over methods requiring a tedious sample cleanup procedure. As the percentage of negative samples is usually high, this rapid, inexpensive and sensitive icELISA programme may be performed on farms or in abattoirs and allow the detection of CIP in other matrices from meat producing animals.
Acknowledgements
This research was supported by the Eleventh Five-Year Plan for National Science and Technology of China (grant No. 2006BAK02A21/1) and the Key Scientific & Technological Project of Education Department in Henan Province of China (grant No. 2011A230003).
References
- Ahmad, B., Parveen, S., & Khan, R.H. (2006). Effect of albumin conformation on the binding of ciprofloxacin to human serum albumin: A novel approach directly assigning binding site. Biomacromolecules, 7, 1350–1356.
- Anonymous. (1999). EC. Commission Directive 1999/508/EC. Amending annexes I to IV to council regulation 2377/90 laying down a community procedure for the established of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Official Journal of European Communities, L60, 16–52.
- Bucknall, S., Silverlight, J., Coldham, N., Thorne, L., & Jackman, R. (2003). Antibodies to the quinolones and fluoroquinolones for the development of generic and specific immunoassays for detection of these residues in animal products. Food Additives and Contaminants, 20, 221–228.
- Christodoulou, E.A., Samanidou, V.F., & Papadoyannis, I.N. (2008). Development of an HPLC multi-residue method for the determination of ten quinolones in bovine liver and porcine kidney according to the European Union Decision 2002/657/EC. Journal of Separation Science, 31, 119–127.
- Dufresne, G., Fouquet, A., Forsyth, D., & Tittlemier, S.A. (2007). Multiresidue determination of quinolone and fluoroquinolone antibiotics in fish and shrimp by liquid chromatography/tandem mass spectrometry. Journal of AOAC International, 90, 604–612.
- Hao, X.L., Kuang, H., Li, Y.L., Yuan, Y., Peng, C.F., Chen, W., et al. (2009). Development of an enzyme-linked immunosorbent assay for the alpha-cyano pyrethroids multiresidue in Tai lake water. Journal of Agricultural and Food Chemistry, 57, 3033–3039.
- Hassouan, M.K., Ballesteros, O., Zafra, A., Vílchez, J.L., & Navalón, A. (2007). Multiresidue method for simultaneous determination of quinolone antibacterials in pig kidney samples by liquid chromatography with fluorescence detection. Journal of Chromatography, B, 859, 282–288.
- Hermo, M.P., Nemutlu, E., Kir, S., Barrón, D., & Barbosa, J. (2008). Improved determination of quinolones in milk at their MRL levels using LC-UV, LC-FD, LC-MS and LC-MS/MS and validation in line with regulation 2002/657/EC. Analytica Chimica Acta, 613, 98–107.
- Huang, B., Yin, Y., Lu, L., Ding, H., Wang, L., Yu, T., et al. (2010). Preparation of high-affinity rabbit monoclonal antibodies for ciprofloxacin and development of an indirect competitive ELISA for residues in milk. Journal of Zhejiang University Science B, 11, 812–818.
- Huet, A.C., Charlier, C., Tittlemier, S.A., Singh, G., Benrejeb, S., & Delahaut, P. (2006). Simultaneous determination of (fluoro)quinolone antibiotics in kidney, marine products, eggs, and muscle by enzyme-linked immunosorbent assay (ELISA). Journal of Agricultural and Food Chemistry, 54, 2822–2827.
- Jiang, J., Wang, Z., Zhang, H., Zhang, X., Liu, X., & Wang, S. (2011). Monoclonal antibody-based ELISA and colloidal gold immunoassay for detecting 19-nortestosterone residue in animal tissues. Journal of Agricultural and Food Chemistry, 59, 9763–9769.
- Jiang, J.Q., Zhang, H.T., Fan, G.Y., Ma, J.Y., Wang, Z.L., & Wang, J.H. (2011). Preparation of monoclonal antibody based indirect competitive ELISA for detecting 19-nortestosterone residue. Chinese Science Bulletin, 56, 2698–2705.
- Jin, R.Y., Gui, W.J., Guo, Y.R., Wang, C.M., Wu, J.X., & Zhu, G.N. (2008). Comparison of monoclonal antibody-based ELISA for triazophos between the indirect and direct formats. Food and Agricultural Immunology, 19, 49–60.
- Le, T., Yu, H., Guo, Y.C., Ngom, B., Shen, Y.A., & Bi, D. (2009). Development of an indirect competitive ELISA for the detection of doxycycline residue in animal edible tissues. Food and Agricultural Immunology, 20, 111–124.
- Li, Y.L., Ji, B.Q., Chen, W., Liu, L.Q., Xu, C.L., Peng, C.F., et al. (2008). Production of new class-specific polyclonal antibody for determination of fluoroquinolone antibiotics by indirect competitive ELISA. Food and Agricultural Immunology, 19, 251–264.
- Lu, S., Zhang, Y., Liu, J., Zhao, C., Liu, W., & Xi, R. (2006). Preparation of anti-pefloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of pefloxacin residue in chicken liver. Journal of Agricultural and Food Chemistry, 54, 6995–7000.
- Pearce, J.N., Burns, B.G., van de Riet, J.M., Casey, M.D., & Potter, R.A. (2009). Determination of fluoroquinolones in aquaculture products by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Food Additives and Contaminants, 26, 39–46.
- Ren, X.F., Zhang, F.M., Chen, F.J., & Yang, T.B. (2009). Development of a sensitive monoclonal antibody-based ELISA for the detection of clenbuterol in animal tissues. Food and Agricultural Immunology, 20, 333–344.
- Rishton, G.M. (2005). Failure and success in modern drug discovery: Guiding principles in the establishment of high probability of success drug discovery organizations. Medicinal Chemistry, 1, 519–527.
- Sheng, W., Xu, T., Ma, H.X., Wang, X.T., Li, Q.X., & Li, J. (2009). Development of an indirect competitive enzyme-linked immunosorbent assay for detection of danofloxacin residues in beef, chicken and pork meats. Food and Agricultural Immunology, 20, 35–47.
- Volmer, D.A., Mansoori, B., & Locke, S.J. (1997). Study of 4-quinolone antibiotics in biological samples by short-column liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Analytical Chemistry, 69, 4143–4155.
- Wang, Y.C., Guo, Z.Q., Li, Y.Z., & Chang, W.B. (2002). Production and characterization of anti-estrone monoclonal antibody. Biomedical and Environmental Sciences, 15, 103–112.
- Wang, L., Zhang, Y., Gao, X., Duan, Z., & Wang, S. (2010). Determination of chloramphenicol residues in milk by enzyme-linked immunosorbent assay: Improvement by biotin-streptavidin-amplified system. Journal of Agricultural and Food Chemistry, 58, 3265–3270.
- Wang, Z., Zhu, Y., Ding, S., He, F., Beier, R.C., Li, J., et al. (2007). Development of a monoclonal antibody-based broad-specificity ELISA for fluoroquinolone antibiotics in foods and molecular modeling studies of cross-reactive compounds. Analytical Chemistry, 79, 4471–4483.
- Yan, H., Wang, H., Qin, X., Liu, B., & Du, J. (2011). Ultrasound-assisted dispersive liquid–liquid microextraction for determination of fluoroquinolones in pharmaceutical wastewater. Journal of Pharmaceutical and Biomedical Analysis, 54, 53–57.
- Zhang, L., Jiang, J.Q., Li, G.L., Wang, Z.L., Yang, X.F., Ma, J.Y., et al. (2011). Development and optimization of a monoclonal antibody based indirect competitive ELISA for detecting fluoroquinolone residue in milk. Journal of Food Agriculture and Environment, 9, 113–120.
- Zhang, H.T., Jiang, J.Q., Wang, Z.L., Chang, X.Y., Liu, X.Y., Wang, S.H., et al. (2011). Development of an indirect competitive ELISA for simultaneous detection of enrofloxacin and ciprofloxacin. Journal of Zhejiang University Science B, 12, 884–891.