Abstract
Response surface methodology was employed to study the effects of Maillard reaction conditions on the antigenicity of β-lactoglobulin (β-LG) in whey protein isolate (WPI) and to optimise the Maillard reaction conditions of WPI conjugate with oligoisomaltose under which the antigenicity of β-LG reduced to the minimum value. The antigenicity of β-LG and α-lactalbumin (α-LA) in natural and glycated WPI during simulated gastric digestion were investigated. The antigenicity of β-LG was reduced from 272.4 µg mL−1 to 30.99 µg mL−1 under the optimal Maillard reaction conditions. After 120 min simulated gastric digestion, the antigenicity of β-LG in natural and glycated WPI were 42.83 µg mL−1 and 15.66 µg mL−1, respectively. And the antigenicity of α-LA in natural and glycated WPI were 0.78 µg mL−1 and 0.03 µg mL−1, respectively.
1. Introduction
Food allergy, which affects 6% of young children and 3–4% of adults, is of great concern (Sicherer & Sampson, Citation2006). Cow milk allergy is at the top of all lists of epidemiologic data (Sampson, Citation2004). A higher incidence of milk allergy is usually in neonates and small children. The main whey proteins are β-lactoglobulin (β-LG) and α-lactalbumin (α-LA) which have nutritional and functional properties. β-LG is the most prevalent protein in whey protein and it is the major allergen of bovine milk. Approximately 10% of the total milk protein and 58% of the whey protein is β-LG. It contains 162 amino acids and its molecular weight is approximately 18.4 kDa (Taheri-Kafrani, Asgari-Mobarakeh, Bordbar, & Haertlé, Citation2010). β-LG consists of two genetic isoforms A and B which are known to possess allergenic potential. β-LG is very stabile against peptic digestion under acidic conditions, and this phenomenon is considered to correlate with its high allergenicity (Breiteneder & Mills, Citation2005; Maier, Okun, Pittner, & Lindner, Citation2006; Schmidt, Meijer, Slangen, & Van Beresteijn, Citation1995).
In previous studies, some processing methods have been reported that they can change the allergenic potential of cow milk protein, such as heating treatment (Bu, Luo, & Zheng, Citation2009a), high-pressure (Bonomi et al., Citation2003), microwave (Grar et al., Citation2009; Izquierdo, Peñas, Baeza, & Gomez, Citation2008; Zellal et al., Citation2011), enzymatic hydrolysis (Liu, Luo, & Li, Citation2012; Zheng, Shen, Bu, & Luo, Citation2008), fermentation (Bu, Luo, Zhang, & Chen, Citation2010a; Ehn, Allmere, Telemo, Bengtsson, & Ekstrand, Citation2005) and glycosylation (Li, Luo, & Feng, Citation2011). These processing methods can alter the allergenic properties of protein by hiding, destroying or disclosing allergenic epitopes through conformational changes or by changing digestibility of protein. Chicón, Belloque, Alonso, & López-Fandiño (Citation2008, Citation2009) reported that high-pressure treatment (400 MPa) on β-LG can increase IgG-binding and promote the hydrolysis of β-LG by pepsin. Kananen et al. (Citation2000) modified the whey protein by sulfitolysis and they pointed out that the antigenicity of β-LG decreased markedly during pepsin hydrolysis. Maier et al. (Citation2006) reported that fermentation of milk products increases the susceptibility of β-LG towards peptic digestion and the immunoreactive β-LG content of fermented products is reduced. Some studies had been reported that glycosylation was an effective way to change the antigenicity of some potentially allergenic proteins. It had been found that conjugation with sugars can change the antigenicity of ovalbumin (Slütter et al., Citation2010), soy protein (van de Lagemaat, Manuel Silván, Javier Moreno, Olano, & Dolores del Castillo, Citation2007), peanut (Gruber, Becker, & Hofmann, Citation2005) and wheat protein (András et al., Citation2009). In the case of milk, glucose (Bu, Lu, Zheng, & Luo, Citation2009b; Bu, Luo, Lu, & Zhang, Citation2010b), maltopentaose (Enomot et al., Citation2009), lactose (Taheri-Kafrani et al., Citation2009), chitosan (Aoki, Iskandar, Yoshida, Takahashi, & Hattori, Citation2006), carboxymethyl dextran (Kobayashi et al., Citation2001), acidic oligosaccharides (Hattori et al., Citation2004) and other sugars had been reported can change the antigenicity of whey protein.
The impacts of conjugation with different sugars on the antigenicity were different. Investigations into the effect of oligoisomaltose on the antigenicity of β-LG during Maillard reaction were scarce. Oligoisomaltose can effectively promote the growth and reproduction of bifidobacterium in body. Moreover, studies about the antigenicity changes of whey protein isolate (WPI) and glycated WPI during simulated gastric digestion were also lacking. β-LG is the main allergen of cow milk. The aim of this study was to investigate the effects of Maillard reaction conditions on the antigenicity of β-LG, and use response surface methodology to optimise Maillard reaction conditions in order to obtain the lowest antigenicity of β-LG. This study also performed on the simulated gastric digestion of natural and glycated whey protein and explored the antigenicity changes of α-LA and β-LG during simulated gastric digestion.
2. Materials and methods
2.1. Materials
α-Lactalbumin (L5385, purity>85%) and β-LG (L3908, purity>90%) used in enzyme-linked immunosorbent assay (ELISA) were purchased from Sigma Chemical Company (St. Louis, MO, USA). WPI (9410) was obtained from Hilmar (USA). Oligoisomaltose was purchased from Baolingbao Biotechnology Co., Ltd (China). Pepsin was purchased from Amresco (USA). Other reagents were analytical grade.
2.2. Sample preparation: Maillard reaction between WPI and oligoisomaltose
WPI and oligoisomaltose were mixed in demineralised water in order to evenly mix them, and then the mixture was freeze-dried to powder. The weight ratios of oligoisomaltose and WPI (oligoisomaltose/WPI weight ratio) were different. The powder was incubated in a desiccator which was exposed to a saturated KBr solution (relative humidity of 79%) at different reaction conditions according to the experimental design. At last samples were carried out from the desiccators and dissolved and then freeze-dried before analysis.
2.3. Indirect competitive enzyme-linked immunosorbent assay
Indirect competitive ELISA was carried out as described by Bu et al. (Citation2010b) to measure the antigenicity of α-LA and β-LG in the samples. After preliminary experiments, the determined ELISA test conditions were as follows: coating concentration of α-LA and β-LG were 0.5 µg mL−1 and 1 µg mL−1, respectively, rabbit anti-α-LA serum diluted 2,56,000 times, rabbit anti-β-LG serum diluted 1,28,000 times, sample was dissolved in PBS at a protein concentration of 0.1 mg mL−1. The antigenicity was calculated from standard curve. For α-LA, a linear logarithmic correlation was observed in the range: 2–256 µg mL−1. For β-LG, a linear logarithmic correlation was observed in the range: 0.5–256 µg mL−1.
2.4. Experimental design and statistical analysis
Oligoisomaltose/WPI weight ratio (X1), temperature (X2) and time (X3) were chosen as independent variables (k=3) in the experimental design. The dependent variable was the antigenicity of β-LG (Y) in the conjugates of WPI with oligoisomaltose. The independent variables were optimised using central composite rotatable design which contains five levels coded as −1.682, −1, 0, 1 and 1.682 for each independent variable. showed the coded values and the corresponding actual values of the three independent variables. The complete central composite design consisted of 23 experiments (). And the 23 experiments included a full factorial design plus 2×3 star experiments and nine centre experiments. The centre experiments were to measure the accuracy and to verify changes in the estimation procedure. In addition, all of the 23 experiments were run in identical environment. Experimental data were analysed by SAS 8.2 (SAS Institute Inc, Cary, NC, USA) and carried out as described by Li et al. (Citation2011).
Table 1. Coded and uncoded settings of independent variables for Maillard reaction conditions according to central composite rotatable design.
Table 2. Full factorial central composite design matrix and the antigenicity of β-LG in WPI-oligoisomaltose conjugates.
2.5. Simulated gastric digestion
Simulated gastric digestion was carried out as Moreno, Mellon, Wickham, Bottrill, and Mills (Citation2005) described. The simulated gastric fluid (SGF) was 0.15 M NaCl solution whose pH was adjusted to 2.5 with 1 M HCl. WPI and WPI-oligoisomaltose were dissolved in SGF (3 mg mL−1). After incubation at 37 °C for 15 min, pepsin (Amresco, activity: 3000 U/mg) was dissolved in SGF (0.32%) and then added to SGF at an approximately physiological ratio of enzyme/substrate (1:20, w/w). The digestion was performed at 37 °C for 2 h. Samples were taken at 0, 2.5, 5, 10, 20, 30, 60 and 120 min for further analysis.
2.6. Free amino acids analysis
Free amino groups were measured using trinitrobenzene sulfonic acid (TNBS) method (Adler-Nissen, Citation1979). The value transformed into µmol of Leu mL−1 using a calibration curve within the range 0.25–3.5 µmol.
3. Results and discussion
3.1. Assessment on models of antigenicity of β-LG for three independent variables
WPI and oligoisomaltose occurred Maillard reaction under the conditions as the experiment design (). The antigenicity of β-LG in WPI-oligoisomaltose was shown in . From regression analysis on 23 experiments (, full model), the results revealed that several terms were not significant (P>0.05). The non-significant terms were eliminated step by step from the regression model in the procedure of fit the full second-order model. After this procedure, there were six regression terms in the second-order model for β-LG (, fitted model). The model of predicting the antigenicity of β-LG under different Maillard reaction conditions was as follow:
Table 3. Regression coefficients for the regression prediction model of the antigenicity of β-LG.
3.2. Effect of Maillard reaction conditions on antigenicity of β-LG in WPI-oligoisomaltose conjugates
The regression coefficients were shown in . The highly significant (P<0.01) effects on the antigenicity were temperature and time of linear effects. The significance (P<0.05) effect on the antigenicity was temperature2 of quadratic effects. Temperature was the most important effect on the antigenicity because of its highest regression coefficient among the three independent variables. The optimal values of three independent variables were as follows: oligoisomaltose/WPI weight ratio=4.7, reaction temperature=68.4 °C, reaction time=29.0 h. In theory, on the optimal reaction conditions, the antigenicity of β-LG should be the lowest. The predicted lowest value was 30.99 µg mL−1. The antigenicity of β-LG in WPI was 272.4 µg mL−1. So it could be proved that glycated WPI with oligoisomaltose was an effectively method to reduce the antigenicity of β-LG.
The effects of Maillard reaction conditions on the antigenicity of β-LG were shown by the response surface plots in . Within the scope of this study, the antigenicity of β-LG decreased at first and then increased with the oligoisomaltose/WPI weight ratio increasing. The impact of weight ratio on the antigenicity was weaker than the other two reaction conditions. As the reaction temperature changed in the range showed in a and , the higher the temperature, the lower the antigenicity. When the temperature was high enough, increasing the temperature did not significantly reduce the antigenicity. The antigenicity of β-LG first decreased and then changed little as time expanded in the range showed in b and . van de Lagemaat et al. (Citation2007) reported that heating for longer than 1h of soy protein isolate with fructooligosaccharides did not significantly reduce the antigenic response. It was similar to the results of this study.
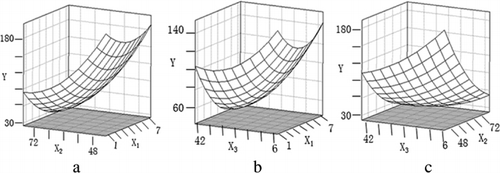
During the glycosylation process, some factors could cause changes in antigenic epitopes, thus affected the antigenicity of the protein. Glycation of proteins occurred by reducing sugars with free amino groups in proteins to form the Schiff's base linkage (Singh, Barden, Mori, & Beilin, Citation2001) and this chemical reaction could cause reduction of antigenic epitopes. Heat treatment could affect the structure of the protein, such as exposure of hidden SH-groups, polymerisation and cross-linking (Oldfield, Singh, Taylor, & Pearce, Citation1998), which resulted in changes of antigenic epitopes. Thereby increasing the reaction time and temperature might promote binding of WPI and oligoisomaltose, but it might also promote changes in protein structure. By analysing the effects of reaction conditions on the antigenicity of β-LG, it could be concluded that with the increment of oligoisomaltose/WPI weight ratio, reaction temperature and time, the antigenicity would not reduced infinitely. Therefore, optimisation of reaction conditions was necessary.
3.3. The free amino acid in natural and glycated WPI during simulated gastric digestion
showed the free amino acid changes of WPI and WPI-oligoisomaltose which obtained under the optimal Maillared reaction conditions during simulated gastric digestion. The free amino acid increased within 20 min in the beginning and then changed little from 20 min to 120 min. After 120 min of pepsin hydrolysis, the free amino acid of WPI increased from 1.568 mmol L−1 to 1.943 mmol L−1, with an increment of 0.375 mmol L−1, and the free amino acid of WPI-oligoisomaltose increased from 0.573 mmol L−1 to 0.996 mmol L−1, with an increment of 0.423 mmol L−1. Compared with natural WPI, WPI-oligoisomaltose was more susceptible to pepsin hydrolysis. But Chevalier, Chobert, Dalgalarrondo, Choiset, and Haertlé (Citation2002) stated that β-LG became more resistant to digestive enzymes as a result of modifications evoked by Maillard reaction and it was contrast to the conclusions of this study. Perhaps the reason was that the sugars used in Maillard reaction were different. Marciniak-Darmochwal and Kostyra (Citation2009) reported that glycosylation of pea extract changed the susceptibility of it towards peptic digestion. The degree of hydrolysis of raw pea extract was lower than pea extract glycated by fructose and pea extract glycated by glucosamine, but it was higher than pea extract glycated by glucose and pea extract glycated by lactose.
Table 4. The free amino acid and the antigenicity of β-LG and α-LA in WPI and WPI-oligoisomaltose during simulated gastric digestion.
3.4. The antigenicity of α-LA and β-LG in natural and glycated WPI during simulated gastric digestion
The antigenicity of α-LA and β-LG in the hydrolysates during simulated gastric digestion () were determined. The antigenicity of α-LA in digested natural WPI was decreased from 26.73 to 0.78 µg mL−1. Moreover, the antigenicity of α-LA in digested WPI-oligoisomaltose almost decreased to zero. Although the antigenicity of β-LG in digested natural WPI was significantly lower than that of undigested, the former still exhibited a clear antigenicity of 42.83 µg mL−1. After the 120 min pepsin hydrolysis, the antigenicity of β-LG in digested WPI-oligoisomaltose was 15.66 µg mL−1. Therefore, highly allergic patients would still react to WPI-oligoisomaltose, but glycosylation might be a primary strategy for reducing the antigenicity of β-LG. The antigenicity of α-LA and β-LG in digested WPI and WPI-oligoisomaltose were all decreased fast in 20 min and then changed little from 20 min to 120 min. This was consistent with the trend of the changes of free amino acid during digestion. It might be because the epitopes were destroyed during pepsin hydrolysis, which could result in reduction of antigenicity.
4. Conclusion
Under the optimal Maillard reaction conditions (oligoisomaltose/WPI weight ratio=4.7, temperature=68.4°C, time=29.0 h), the antigenicity of β-LG was efficiently reduced about 88.6% by glycosylation with oligoisomaltose. Compared with natural WPI, WPI-oligoisomaltose was more susceptible to pepsin hydrolysis. After 120 min simulated gastric digestion, the antigenicity of α-LA and β-LG in glycated WPI were reduced to 0.03 µg mL−1 and 15.66 µg mL−1, respectively.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (award nr 30471224, 30871817 and 31171715) and National Science and Technology Ministry of China (award nr 2011BAD09B03) and Chinese Universities Scientific Fund (award nr 2012YJ078).
References
- Adler-Nissen, J. (1979). Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. Journal of Agricultural and Food Chemistry, 27, 1256–1262.
- András, N., Marciniak-Darmochwał, K., Krawczuk, S., Mierzejewska, D., Kostyra, H., & Gelencsér, E. (2009). Influence of glycation and pepsin hydrolysis on immunoreactivity of Albumin/globulin fraction of herbicide resistant wheat line. Czech Journal of Food Science, 27, 320–329.
- Aoki, T., Iskandar, S., Yoshida, T., Takahashi, K., & Hattori, M. (2006). Reduced immunogenicity of β-lactoglobulin by conjugating with chitosan. Bioscience, Biotechnology and Biochemistry, 70, 2349–2356.
- Bonomi, F., Fiocchi, A., Frokieer, H., Gaiaschi, A., Larnetti, S., Poiesi, C., et al. (2003). Reduction of immunoreactivity of bovine β-lactoglobulin upon combined physical and proteolytic treatment. Journal of Dairy Research, 70, 51–59.
- Breiteneder, H., & Mills, E.N.C. (2005). Molecular properties of food allergens. Journal of Allergy and Clinical Immunology, 115, 14–24.
- Bu, G., Luo, Y., & Zheng, Z. (2009a). Effect of heat treatment on the antigenicity of bovine α-lactolbumin and β-lactoglobulin in whey protein isolate. Food and Agricultural Immunology, 20, 195–206.
- Bu, G., Lu, J., Zheng, Z., & Luo, Y. (2009b). Influence of Maillard reaction conditions on the antigenicity of bovine α-lactalbumin using response surface methodology. Journal of the Science of Food and Agriculture, 89, 2428–2434.
- Bu, G., Luo, Y., Zhang, Y., & Chen, F. (2010a). Effects of fermentation by lactic acid bacteria on the antigenicity of bovine whey proteins. Journal of the Science of Food and Agriculture, 90, 2015–2020.
- Bu, G., Luo, Y., Lu, J., & Zhang, Y. (2010b). Reduced antigenicity of β-lactoglobulin by conjugation with glucose through controlled Maillard reaction conditions. Food and Agricultural Immunology, 21, 143–156.
- Chevalier, F., Chobert, J.M., Dalgalarrondo, M., Choiset, Y., & Haertlé, T. (2002). Maillard glycation of beta-lactoglobulin induces conformation changes. Die Nahrung, 46, 58–63.
- Chicón, R., Belloque, J., Alonso, E., & López-Fandiño, R. (2008). Immunoreactivity and digestibility of high-pressure-treated whey proteins. International Dairy Journal, 18, 367–376.
- Chicón, R., Belloque, J., Alonso, E., & López-Fandiño, R. (2009). Antibody binding and functional properties of whey protein hydrolysates obtained under high pressure. Food Hydrocolloids, 23, 593–599.
- Ehn, B.M., Allmere, T., Telemo, E., Bengtsson, U., & Ekstrand, B.O. (2005). Modification of IgE binding to β-Lactoglobulin by fermentation and Proteolysis of cow's milk. Journal of Agricultural and Food Chemistry, 53, 3743–3748.
- Enomot, H., Hayashi, Y., Li, C.P., Ohki, S., Ohtomo, H., Shiokawa, M., et al. (2009). Glycation and phosphorylation of α-lactalbumin by dry heating: Effect on protein structure and physiological functions. Journal of Dairy Science, 92, 3057–3068.
- Grar, H., Kaddouri, H., Gourine, H., Negaoui, H., Kheroua, O., & Saïdi, D. (2009). Microwave irradiation under different pH conditions induced a decrease in β-lactoglobulin antigenicity. European Food Research and Technology, 229, 779–783.
- Gruber, P., Becker, W.M., & Hofmann, T. (2005). Influence of the maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin. Journal of Agricultural and Food chemistry, 53, 2289–2296.
- Hattori, M., Miyakawa, S., Ohama, Y., Kawamura, H., Yoshida, T., To-O, K., et al. (2004). Reduced immunogenicity of β-lactoglobulin by conjugation with acidic oligosaccharides. Journal of Agricultural and Food Chemistry, 52, 4546–4553.
- Izquierdo, F.J., Peñas, E., Baeza, M.L., & Gomez, R. (2008). Effects of combined microwave and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins. International Dairy journal, 18, 918–922.
- Kananen, A., Savolainen, J., Mäkinen, J., Perttilä, U., Myllykoski, L., & Pihlanto-Leppälä, A. (2000). Influence of chemical modification of whey protein conformation on hydrolysis with pepsin and trypsin. International Dairy Journal, 10, 691–697.
- Kobayashi, K., Hirano, A., Ohta, A., Yoshida, T., Takahashi, K., & Hattori, M. (2001). Reduced immunogenicity of β-lactoglobulin by conjugation with carboxymethyl dextran differing in molecular weight. Journal of Agricultural and Food Chemistry, 49, 823–831.
- Li, Z., Luo, Y., & Feng, L. (2011). Effects of Maillard reaction conditions on the antigenicity of α-lactalbumin and β-lactoglobulin in whey protein conjugated with maltose. European Food Research and Technology, 233, 387–394.
- Liu, X., Luo, Y., & Li, Z. (2012). Effects of pH, temperature, enzyme-to-substrate ratio and reaction time on the antigenicity of casein hydrolysates prepared by papain. Food and Agricultural Immunology, 23, 69–82.
- Maier, I., Okun, V.M., Pittner, F., & Lindner, W. (2006). Changes in peptic digestibility of bovine β-lactoglobulin as a result of food processing studied by capillary electrophoresis and immunochemical methods. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Science, 841, 160–167.
- Marciniak-Darmochwal, K., & Kostyra, H. (2009). Influence of nonenzymatic glycosylation (glycation) of pea proteins (Pisum sativum) on their susceptibility to enzymatic hydrolysis. Journal of Food Biochemistry, 33, 506–521.
- Moreno, F.J., Mellon, F.A., Wickham, M.S.J., Bottrill, A.R., & Mills, E.N.C. (2005). Stability of the major allergen Brazil nut 2S albumin (Ber e 1) to physiologically relevant in vitro gastrointestinal digestion. FEBS Journal, 272, 341–352.
- Oldfield, D.J., Singh, H., Taylor, M.W., & Pearce, K.N. (1998). Kinetics of denaturation and aggregation of whey proteins in skim milk heated in an ultra-high temperature (UHT) pilot plant. International Dairy Journal, 8, 311–318.
- Sampson, H.A. (2004). Update on food allergy. Journal of Allergy and Clinical immunology, 113, 805–819.
- Schmidt, D.G., Meijer, R.J.G.M., Slangen, C.J., & Van Beresteijn, E.C.H. (1995). Raising the pH of the pepsin-catalysed hydrolysis of bovine whey proteins increases the antigenicity of the hydrolysates. Clinical and Experimental Allergy, 25, 1007–1017.
- Sicherer, S.H., & Sampson, H.A. (2006). Food allergy. Journal of Allergy and Clinical Immunology, 117, S470 ––S475. S489.
- Singh, R., Barden, A., Mori, T., & Beilin, L. (2001). Advanced glycation end-products: A review. Diabetologia, 44, 129–146.
- Slütter, B., Soema, P.C., Ding, Z., Verheul, R., Hennink, W., & Jiskoot, W. (2010). Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. Journal of Controlled Release, 143, 207–214.
- Taheri-Kafrani, A., Gaudin, J.C., Rabesona, H., Nioi, C., Agarwal, D., & Drouet, M., et al. (2009). Effcets of heating and glycation of β-Lactoglobulin on its recognition by IgE of sera from cow milk allergy patients. Journal of Agricultural and Food Chemistry, 57, 4974–4982.
- Taheri-Kafrani, A., Asgari-Mobarakeh, E., Bordbar, A.K., & Haertlé, T. (2010). Structure-function relationship of β-lactoglobulin in the presence of dodecyltrimethyl ammonium bromide. Colloid and Surfaces B:Biointerfaces, 75, 268–274.
- van de Lagemaat, J., Manuel Silván, J., Javier Moreno, F., Olano, A., & Dolores del Castillo, M. (2007). In vitro glycation and antigenicity of soy protein. Food Research International, 40, 153–160.
- Zellal, D., Kaddouri, H., Grar, H., Belarbi, H., Kheroua, O., & Saidi, D. (2011). Allergenic changes in β-lactoglobulin induced by microwave irradiation under different pH conditions. Food and Agricultural Immunology, 22, 355–363.
- Zheng, H., Shen, X., Bu, G., & Luo, Y. (2008). Effects of pH, temperature and enzyme-to-substrate ratio on the antigenicity of whey protein hydrolysates prepared by Alcalase. International Dairy journal, 18, 1028–1033.