Abstract
Cross-reactivity related to birch pollynosis and ingestion of certain food poses a severe clinical and diagnostic problem. Sera were taken from 21 adult patients allergic to birch pollen with symptoms after ingestion of apples, carrots and celeries, and seven control subjects allergic to birch pollen only. Concentrations of allergen-specific IgE against allergens of birch pollen, apple, carrot and celery were measured with enzyme immunoassay, fluorimetric enzyme-linked immunoassay and immunoblotting. Immunoblotting technique may serve as a valuable diagnostic tool for birch pollen associated cross-reactivity.
Introduction
Recent studies caused a significant progress in understanding cross-allergies among plants pollens, certain vegetables and fruits, house-dust mite and seafood. Complex immune and biochemical mechanisms are responsible in development of these reactions. Allergic cross-reactivity was most often described in patients allergic to birch pollen, who upon contact with pollen allergens developed immune response with a character of hypersensitivity reaction. Symptoms can be also manifested after eating certain food such as fruits, vegetables and/or nuts. It was shown that in over half of adults with symptoms of birch pollen allergy, reactions involving IgE immunoglobulins were described as most often occurring. Cross-reactions may also concern receptors on T lymphocytes that identify allergens epitopes derived from different allergen sources. It has been demonstrated that these allergens reveal significant structural homology (Bohle, Citation2007; Bohle, Radakovics, & Jahn-Schmid, Citation2003; Ferreira, Hawranek, Gruber, Wopfner, & Mari, Citation2004). Therefore, it is believed that the presence of the common epitopes may be responsible for various cross-reactivity reactions. Noteworthy, simultaneous presence of both types of reactions is not excluded. Pathological syndromes connected with allergic cross-reactions have been termed as pollen-food syndromes.
Several protein allergens have been well characterised both on molecular and immunologic levels, including birch pollen ~17-kDa Bet v1 allergen, which is responsible for most of cross-reactivity responses (Rodriguez, Crespo, & Lopez-Rubio, Citation2000; Vieths, Citation1997). Its structure is similar to allergens found in apples, carrots, celeries, apricots, pears, cherries, mango fruits, poppy seeds and other. Bet v1 belongs to PR-10 family of proteins involved in pathogenesis (pathogenesis-related proteins) and is expressed in over 20 isoforms. Its synthesis is stimulated by severe mechanic damage, infections and unfavourable conditions such as drought, low temperatures and/or UVB light. Other members of PR-10 family are present in other plants, and include apple Mal d1, celery Api g1 and carrot Dau c1 (Radauer, Bublin, Wagner, Mari, & Breiteneder, Citation2008). These proteins reveal significant similarity in their primary and tertiary structures, therefore antibodies against Bet v1 can easily detect these other allergens.
Allergy evoked by ingestion food causing cross-reactivity with pollen also poses a difficult diagnostic problem. Its assessment should be comprehensive and requires employment of specific tests, also those using recombinant antigens. One of them is immunoblotting technique, widely used for studying specific protein–protein interactions.
In this study, we provide data confirming that immunoblotting may be used as one of the procedures to detect cross-reactions in patients allergic to birch pollen. To our knowledge this is a first study using three allergenic sources (apple, carrot and celery), most often causing cross-reactivity in adult persons allergic to birch pollen.
Materials and methods
Studies were performed with consent of the University Bioethical Committee of yhr Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicholaus Copernicus University [consent number KB 683/2009].
Patient and control groups
A group of 21 adults, 12 women and 9 men, was qualified for the study (mean age 41.7±5.2). They have not only developed sensitivity to birch pollen but also exhibited symptoms after ingestion of sensitising food and developed positive answer to native food allergens during skin prick tests (SPT) and/or prick-by-prick skin tests, indicative of possible cross-allergy. Cross-reactivity concerned vegetables from Apiaceae family (carrots, celeries) and fruits from Rosaceae family (apples). SPT test was performed with the use of allergen extracts of birch, carrot, apple and celery from Allergopharma (Germany) and prick-by-prick test with the use of fresh plants.
The control group consisted of seven adults: three women and four men (mean age 39.8±4.7) allergic to birch pollen and who did not reveal symptoms after ingestion of apples, carrots and celery.
Concentration of antibodies in the sera
Concentrations of total IgE (tIgE) as well as of allergen-specific IgE (asIgE) class antibodies produced against allergens of birch pollen, apple, carrot or celery were measured in sera of patients and control subjects with enzyme immunoassay method using HYCOR™ kits (HYCOR, UK). Concentration of asIgE antibodies raised against recombinant allergens of birch pollen Bet v1 and Bet v2, and celery Api g1 was determined with a fluorimetric enzyme-linked immunoassay (FEIA) using UniCAP 100 kit from Phadia (Sweden).
The concentrations of asIgE antibodies were classified into classes 0–6, based on the generally accepted recommendations. Concentrations <0.35 kU/l constituted class 0, concentrations 0.35–0.7 kU/l – class 1, concentrations 0.7–3.5 kU/l – class 2, concentration 3.5–17.5 kU/l – class 3, concentrations 17.5–50 kU/l – class 4, concentrations 50–100 kU/l – class 5 and concentrations >100 kU/l – class 6. Only patients with concentration classified as class ≥ 2 of asIgE against birch pollen as well as against at least one of the above mentioned food allergens were qualified for the study. Allergic rhinitis, atopic dermatitis and symptoms of oral allergy syndrome (OAS) were most often developed clinical manifestations.
Allergen extracts
For immunoblot analysis extracts prepared from fresh apples (a mixture of Paulared and Golden Delicious apple cultivars, separately peel and pulp), and vegetables (carrots and celeries) were used except of birch extracts, which were from Allergopharma. Extracts were prepared from 8 g of liquid nitrogen-frozen carrots and celeries as well as of pulp and peel of apples. Tissues were grinded in a mortar and transferred to 50-ml conical tubes and supplemented with 40 ml of isolating solution (0.25 M Tris-HCl buffer pH 6.8; 7.5% glycerol, 2% sodium dodecyl sulfate (SDS), 5% β-mercaptoethanol), which prior to use was diluted 1:1 (v/v) with distilled water. The samples were incubated for 12 hours at room temperature and then centrifuged for 20 min at 11,000 g at 4°C. Next, 300 µl of supernatant was transferred to 1.5-ml Eppendorf tubes and 100 µl of the standard Laemmli denaturing solution was added, and the samples were stored at –20°C.
Electrophoresis and immunoblotting
The samples were denatured in thermal block for 10 min at 95°C and then subjected to electrophoretic separation in 15% polyacrylamide gel slabs in the standard separation buffer (25 mM Tris, 192 mM glicyne and 0.1% SDS) according to Laemmli (Citation1970), followed by a transfer to a nitrocellulose membrane (0.45 µm, BioRad). Usually 30 µl of the sample were loaded on to a gel. Transfer and subsequent immunoblot analysis were performed essentially according to Towbin, Staehlin, and Gordon (Citation1979). Briefly, samples were transferred at room temperature either overnight at 80 mA or for 3 hours at 320 mA. After transfer, the membrane was blocked for 1 h at room temperature in Tris-buffered saline (150 mM NaCl, 25 mM Tris-HCl buffer pH 7.4) containing 5% non-fat milk powder and 0.2% Triton X-100 followed by 1-hour incubation with primary antibodies, which were the sera, obtained from patients and control subjects, diluted 1:5 (v/v) with the blocking solution. Primary antibodies were detected using 1:5000 dilutions of anti-human antibodies conjugated with horse radish peroxidase and the reaction was developed using the enhanced chemiluminescence (ECL) method with Super Signed West Femto Maximum Sensitivity Substrate (Pierce, USA). Molecular weight standards were from BioRad (USA) or Fermentas (Canada).
Results
Results of studies assessing concentration of tIgE antibodies and asIgE antibodies in sera obtained from 21 patients and seven control subjects were shown in . For all the sera, the concentration of tIgE was measured as well as the specific IgE antibodies against either extracts from birch, apples, carrot and celery or recombinant allergens from birch (rBet v1, rBet v2) and celery (rApi g1). Noteworthy, no major difference in asIgE concentration was observed in sera of control subjects allergic to birch pollen but not revealing allergic reaction to apples, carrot and celery.
Table 1. Concentration of total (tIgE) and allergen-specific IgE (asIgE) in the analysed sera.
The sera were next subjected for immunoblot analysis to detect the presence of IgE antibodies reacting with antigens of extracts from fresh apples, carrots and celeries ( and , ). Proteins with molecular weights of ~17 kDa and ~10 kDa were detected, corresponding most probably to the known antigens of the examined extracts. Proteins homologous to birch pollen Bet v1 (~17 kDa; a major birch pollen allergen) are: apple Mal d1, celery Api g1 and carrot Dau c1. Noteworthy, a protein with molecular mass of about 10 kDa (Mal d3 or lipid transfer protein (LTP), expressed predominantly under the peel) is expressed in apples that is believed to be a major cause of clinical symptoms manifested after apple ingestion (Sancho et al., Citation2008). The detection levels of allergen proteins of individual extracts varied, and the most intensive one was for birch pollen extracts ().
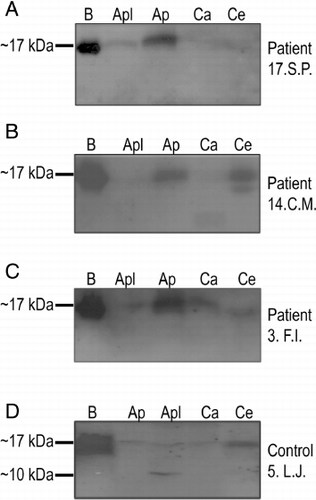
Table 2. Manifestation of clinical symptoms and the presence of specific IgE antibodies revealing cross-reactivity assessed with the immunoblotting method.
Table 3. Quantification of manifestation of clinical symptoms and the presence of specific IgE antibodies revealing cross-reactivity of the patient and control groups.
Occurrence of clinical manifestations and the presence of antibodies against protein allergens for individual patients and control subjects are presented in and the overall summary in . The presence of antibodies against ~17-kDa allergens of birch extract was detected in 20 of 21 patients, but the detection was weak in four of them, and in 6 of 7 control subjects ( and ), which is in agreement with the data obtained with FEIA method ().
The presence of IgE against Mal d1 in the apple pulp was shown in 14 patients (~67%), in three of them it was a very weak reaction, and there was no detection in four cases (~19%). Antibodies against Mal d3 of apple peel extract were detected in sera of six patients (~29%). Simultaneous presence of specific IgE against Mal d1 and Mal d3 proteins was detected in six patients (28.6%). In the control group, there were positive reactions for Mal d1 and Mal d3 in three persons, there was no simultaneousness, however, in four cases.
The presence of IgE for Dau c1 was observed only in one patient (4.75%, patient F.I.), who interestingly developed antibodies against all other studied ~17-kDa antigens (Bet v1, Mal d1 and Api g1) and manifested clinical symptoms after contact with birch pollen and ingestion of apples, carrots and celeries (). Detection of antibodies against Dau c1 was also observed for one control subject (, control subject 5.L.J.).
The presence of asIgE for Api g1 was detected in 11 patients and 2 persons from the control group.
Interestingly, despite the fact that none of the subjects in the control group developed clinical symptoms after eating carrots, apples and celeries, in few of them antibodies against the studied allergens were found, including the one against Mal d3 (see , control subject 5.L.J., ). Noticeable, there was nobody in the patient group who exhibited such the response pattern.
Discussion
Results obtained herein with immunoblotting demonstrate that specific IgE antibodies against proteins with molecular weights corresponding to Bet v1 from birch and its homologues from apples, celeries and carrots were detected in the sera of adult subjects with the symptoms of allergy to birch pollen after probing with extracts of birch pollen, apples, carrots and celeries. Also, the data suggest that the presence of the antibodies against these major allergens may confirm the already clinically manifested cross-reactivity and/or be indicative of future development of symptoms of cross-reactivity.
Immunoblot analysis allows for both the detection of protein allergens in allergen-containing extracts as well as the presence of specific antibodies in the studied samples. Therefore, it has been recently considered as a valuable tool for confirmation of the information gathered with the currently used techniques, and a valuable addendum do the diagnostics.
The most often observed symptoms of cross-reaction within the patients concerned birch-apple link and were confirmed by the presence in the sera of 14 patients of the antibodies against a ~17-kDa protein from apple pulp extract, most probably corresponding to Mal d1 allergen. Interestingly, the presence of anti-Mal d1 antibodies was found in three control subjects not revealing symptoms of cross-reactivity. This may be due to the fact that there is over 65% identity in the amino-acid sequence between Mal d1 and Bet v1 proteins (Vanek-Krebitz et al., Citation1995). It should be emphasised that the severity of the symptoms is determined by the number and conformability of the epitopes detectable by the antibodies. Also, several studies revealed that the expression level of Mal 1d varied in different apple types; there was more Mal 1d synthesised in green apples than in the red ones (Asero, Marzan, & Martinelli Citation2006). Moreover, there are different Mal d1 isoforms produced in different apple cultivars, which may exhibit different allergenic potential, especially after heat treatment (Son, Scheurer, Hoffmann, Haustein, & Vieths, Citation1999). This may explain why the commercially available apple extracts were not applicable for our studies. Also, 6 out of the 14 patients, who produced antibody against Mal d1 exhibited the presence of antibodies against Mal d3, ~10-kDa apple allergen. These patients revealed clinical manifestation upon ingestion of apples. Mal d3, also known as LTP belongs to panallergens and is responsible for life threatening severe systemic allergic reactions (Asero, Mistrello, & Amato, Citation2003; Sancho et al., Citation2008). None of the patients revealed such a traumatic response, however, few of them were also allergic to carrots and/or celery, and had increased concentration of asIgE against allergens from these vegetables. Cudowska, Kaczmarski, and Restani (Citation2005, Citation2008) also reported the presence of the antibodies against Mal d1 and Mal d3 in the sera from children with birch pollynosis, and Mal d3 antibodies were found in all the subjects with concomitant cross-allergy to apples and birch pollen.
A protein with molecular mass of ~17 kDa of celery extract, corresponding to the major celery antigen Api g1 was recognised by the sera of 11 patients. The positive response was also observed for two control subjects not revealing symptoms of cross-reactivity. Api g1 can exist as two isoforms, Api g1.0101 and Api g1.0201 that differ in their homology to Bet v1 and binding to asIgE (Hoffmann-Sommergruber, Ferris, & Pec, Citation2000). In the Api g1-Bet v1 cross-reactivity not only IgE-dependent reactions may be involved but also reactions engaging specific T lymphocytes, which may affect the occurrence of clinical symptoms. In fact, four of 11 patients exhibited allergy symptoms, which may be explained by involvement of Bet v1 specific T lymphocytes (Bohle et al., Citation2003; Bohle, Citation2007). Also, three of them had the elevated concentrations of asIgE (estimated by FEIA method) against Bet v1 and Api g1, and the presence of antibodies against birch pollen, carrot and apples.
A protein with molecular mass of ~17 kDa of carrot extracts, corresponding to the major carrot antigen Dau c1 was recognised by the sera from only one patient and one control subject. The elevated concentrations of asIgE (estimated by FEIA method) against Bet v1 and carrot extracts, and the presence of antibodies against birch pollen, celery and apples were also observed for this patient, who exhibited allergy symptoms upon ingestion of these plants. There are three isoforms of Dau c1, which differ only by few amino acids and there is almost 40% identity of Dau c1 to Bet v1 and Mal d1, and 81% identity of Dau C1 to Api g1 from celery, most closely related to carrot (Hoffmann-Sommergruber et al., Citation1999; Hoffmann-Sommergruber, Ferris, & Pec, Citation2000). Moreover, crystal structure of Dau c1 is similar to Bet v1 and Api g1. Modelling of the surface topology revealed that these allergens may have some, but not all, common epitopes, which was in agreement with the observations that the majority of carrot-allergic patients have Bet v1 cross-reactive IgE antibodies, whereas other produce Dau c1-specific IgE antibodies not recognising Bet v1 (Markovic-Housley, Basle, & Padavarhan, Citation2009). Our and other's data indicate separateness of epitope groups within the major allergen structure (Bollen, Garcia, & Cordewener, Citation2007).
Surprisingly, a positive response of the sera of control subjects to the examined plant extracts was observed. It might be due to the fact that these persons have already developed allergic reaction to birch pollen therefore their immune system was already stimulated. Second, because they do not avoid apples, carrots and celeries, known to evoke cross-reactivity, so the antibodies have been produced. Therefore, it is not excluded that the cross-reaction process has already begun and clinical symptoms might develop in a future. There is a study showing that some of the patients with allergic rhinitis (ANN) caused by birch pollens, with positive prick-by-prick tests and for about 60 months lacking OAS symptoms developed, however, in the end typical OAS symptoms after about a year (Asero, Citation1997). On the other hand, the fact that we did not observe the signal for allergens in all the studied patients might be due to avoidance of the food containing the plants of interest leading to low of IgE production, below the detection level for immunoblot technique. Moreover, IgE antibodies are cytophilic with a short half live in circulating blood, and quickly translocate and deposit to the effector cells. Therefore, while taking a blood sample it is crucial to ask a patient during anamnesis for the time passed from his/her exposition to an allergen.
Also, the procedure of preparing the extracts, the amount of a given allergen in the plant material are very important. Besides, as it was mentioned earlier, certain allergens are heat-sensitive and their interaction can be weaken by the treatment, due to denaturation of the epitopes.
Our study confirmed that immunoblot analysis may be considered as a valuable diagnostic tool for confirmation of the information gathered with the currently used techniques, and a valuable addendum to the already existing diagnostic procedures for testing birch-pollen evoked cross-reactivity after ingestion apples, carrots and celeries.
Acknowledgements
Dr. Iwona Fares is greatly acknowledged for her invaluable help in gathering the sera from the patients. The work was supported by statutory grants from the Ministry of Science and Higher Education Health to Collegium Medicum in Bydgoszcz of Nicolaus Copernicus University and the Nencki Institute of Experimental Biology.
References
- Asero, R. (1997). Relevance of pollen-specific IgE levels to the development of Apiaceae hypersensitivity in patients with birch pollen allergy. Allergy, 52, 560–564.
- Asero, R., Marzan, G., & Martinelli, A. (2006). Search for low – allergenic apple cultivars for birch-pollen allergic patients: Is there a correlation between in vitro assays and patient response? European Annals of Allergy and Clinical Immunology, 38, 94–98.
- Asero, R., Mistrello, G., & Amato, S. (2003). Analysis of the heat stability of lipid transfer protein from apple. Journal of Allergy and Clinical Immunology, 112, 1009–1010.
- Bohle, B. (2007). The impact of pollen – related food allergens on pollen allergy. Allergy, 62, 3–10.
- Bohle, B., Radakovics, A., & Jahn-Schmid, B. (2003). Bet V1, the major birch pollen allergen initiates sensitization the T cell level. European Journal of Immunology, 33, 3303–3310.
- Bollen, M.A., Garcia, A., & Cordewener, J.H.G. (2007). Purification and characterization of natural Bet v1 from birch pollen and related allergens from carrot and celery. Molecular Nutrition & Food Research, 51, 1527–1536.
- Cudowska, B., Kaczmarski, M., & Restani, P. (2005). Immunoblotting in the diagnosis of cross-reactivity in children allergic to birch. Roczniki Akademii Medycznej W Bialymstoku, 50, 268–273.
- Cudowska, B., Kaczmarski, M., & Restani, P. (2008). Lipid transfer protein in diagnosis of birch – apple syndrome in children. Immunobiology, 213, 89–96.
- Ferreira, F., Hawranek, T., Gruber, P., Wopfner, N., & Mari, A. (2004). Allergic cross-reactivity: From gene to the clinic. Allergy, 59, 243–267.
- Hoffmann-Sommergruber, K., Ferris, R., & Pec, M. (2000). Characterization of Api g1.0201, a new member of the Api g1 family of celery allergens. International Archives of Allergy and Immunology, 122, 115–123.
- Hoffmann-Sommergruber, K., O'Riordain, G., Ahorn, H., Ebner, C., Laimer Da Camara Machado, M., Pühringer, H., et al. (1999). Molecular characterization of Dau c 1, the Bet v 1 homologous protein from carrot and its cross-reactivity with Bet v 1 and Api g 1. Clinical & Experimental Allergy, 29, 840–847.
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
- Markovic-Housley, Z., Basle, A., & Padavarhan, S. (2009). Structure of the major carrot allergen Dau c1. Acta Crystallographica, 65, 1206–1212.
- Radauer, C., Bublin, M., Wagner, S., Mari, A., & Breiteneder, H. (2008). Allergens are distributed into few protein families and possess a restricted number of biochemical functions. Journal of Allergy and Clinical Immunology, 121, 847–852.
- Rodriguez, J., Crespo, J.F., & Lopez-Rubio, A. (2000). Clinical cross-reactivity among foods of the Rosacea family. Journal of Allergy and Clinical Immunology, 106, 183–189.
- Sancho, A.I., van Ree, R., van Leeuwen, A., Meulenbroek, B.J., van de Weg, E.W., Gilissen, L.J., et al. (2008). Measurement of lipid transfer protein in 88 apple cultivars. International Archives of Allergy and Immunology, 146, 19–26.
- Son, D.Y., Scheurer, S., Hoffmann, A., Haustein, D., & Vieths, S. (1999). Pollen-related food allergy: Cloning and immunological analysis of isoforms and mutants of Mal d 1, the major apple allergen, and Bet v 1, the major birch pollen allergen. European Journal of Nutrition, 38, 201–215.
- Towbin, H., Staehlin, T., & Gordon, J. (1979). Electrophoretic transfer of proteins from polyactylamide gels to nitrocellulose. Procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America, 76, 4350–4354.
- Vanek-Krebitz, M., Hoffmann-Sommergruber, K., Laimer da Camara Machado, M., Susani, M., Ebner, C., Kraft, D., et al. (1995). Cloning and sequencing of Mal d 1, the major allergen from apple (Malus domestica), and its immunological relationship to Bet v 1, the major birch pollen allergen. Biochemical and Biophysical Research Communications, 214, 538–551.
- Vieths, S. (1997). Allergenic cross-reactivity, food allergy and pollen. Environmental Toxicology and Pharmacology, 4, 61–70.