Abstract
In this study, an enzyme-linked immunosorbent assay (ELISA) was developed for the simultaneous quantitative determination of five quinoxaline-1,4-dioxides (olaquindox, carbadox, mequindox, quinocetone and cyadox) in porcine and chicken feeds. Polyclonal antibodies (PAbs) with group-specificity to quinoxaline-1,4-dioxides were raised in rabbits after immunization with quinoaxline-2-carboxylic acid-para-aminobenzoic-bovine serum albumin conjugate (QCA-PABA-BSA). The specificity of anti-sera was determined by competitive indirect ELISA (ciELISA), and the 50% inhibitions (IC50) of quinocetone (QCT), cyadox (CYA), carbadox (CBX), mequindox (MEQ) and olaquindox (OLA) were 8.6, 13.1, 17.3, 24.5 and 26.4 ng ml−1, respectively. The limit of detection (LOD) for the QCT, CYA, CBX, MEQ and OLA were as low as 0.41, 0.59, 0.67, 0.80 and 0.86 ng g−1, respectively. On the basis of PAb's strong cross-reactivity to QCT (100%), CYA (49.9%), CBX (66.2%), MEQ (35.3%) and OLA (32.8%), no cross-reactivity was found with other antibiotics, indicating that the assay displayed not only high-sensitivity but also high-specificity. When used to analyse animal feed samples, acceptable recovery rates of 71.6–92.9% and coefficients of variation of 4.1–10.8% were obtained. The ciELISA for 10 spiked samples was confirmed by high-performance liquid chromatography (HPLC) with a high correlation coefficient of 0.9959 (n=10). Therefore, the optimised ciELISA may be a potential analytical tool for monitoring quinoxaline-1,4-dioxide residues in animal feed.
Introduction
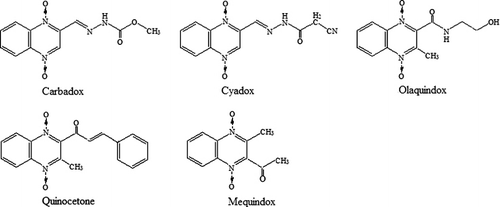
Quinoxaline-1,4-dioxides belong to a group of synthetic antibacterial agents which are widely used as medicinal feed additives because of their growth-promoting effects and for the prevention of swine dysentery and bacterial enteritis in young swine (Wu et al., Citation2009). Carbadox (CBX), mequindox (MEQ), olaquindox (OLA), quinocetone (QCT) and cyadox (CYA) are members of the quinoxaline-1,4-dioxides family, and all have the quinoxaline ring as a common structure, with different side chains, as shown in . CBX and OLA are well-known members of the quinoxaline family, but they have been banned by the European Union (EU) since 1998 (Commission regulation No. 2788/98, Citation1998) for the use in food-producing animals due to their toxicity. MEQ, QCT and CYA, the new antibiotic derivatives in the quinoxaline-1,4-dioxide family, can effectively improve growth and prevent disease of animals while exhibiting lower toxicity. Therefore, they are widely used in China as feed additives. Nevertheless, recent studies show that MEQ and QCT also have the potential of cytotoxicity and genotoxicity (Chen et al., Citation2009). Therefore, MEQ and QCT had been permitted to be used only as medicinal feed additives in China. To prevent illegal utilization of the quinoaxlines, it is necessary to establish a method to detect the amount of quinoaxlines in animal feed and monitor the compliance of the ban of CBX and OLA.
The published methods for their detection were based on liquid chromatography coupled with ultraviolet light, fluorescence detection or mass spectrometry (Bories, Citation1979; Fuh, Chan, Wang, & Lin, Citation2000; Graaf & Spierenburg, Citation1985; Hutchinson, Young, & Kennedy, Citation2005; Wu et al., Citation2006) as means of detection. However, only a few methods have been developed for the determination of either one or two quinoxaline-1,4-dioxides in feeds (Graaf & Spierenburg, Citation1985; Hutchinson et al., Citation2005; Lin & Jeng, Citation2001; Mcgary, Citation1986; Ramos & Silveira, Citation1991; Roybal, Munns, & Shimoda, Citation1985; Thorpe, Citation1988). Nevertheless, these instrumental methods cannot fulfil the demand of rapid screening as they are time consuming and require complicated sample preparation, and expensive equipment. Therefore, the development of simple and time-saving screening techniques is essential. Immunoassays are considered to be the most sensitive technique for the detection of chemicals of low-molecular weight among the commonly used immunological screening methods. Until now, only one immunoassay method for the detection of OLA in feed has been developed (Situ & Elliott, Citation2005). Prior to our work, there was no method that could simultaneously detect the five quinoaxlines mentioned above in animal feeds. This paper reports a ciELISA method used to simultaneously detect five quinoaxlines in animal feeds. As confirmed by high-performance liquid chromatography (HPLC), the method is reliable and satis?es the EU requirements for evaluating these banned veterinary drugs. To our knowledge, this is the first description of a ciELISA method using PAbs for the simultaneous determination of five quinoaxlines in animal feeds.
Experimental
Reagents
CBX, MEQ, OLA, QCT and CYA were from National Institute for the Control of Pharmaceutical and Biological Products (NICPBP, China). Quinoxaline-2-carboxylic acid, N,N-dicyclohexylcarbodiimide (DCC), goat anti-rabbit IgG, ovalbumin (OVA) and bovine serum albumin (BSA) were purchased from Sigma–Aldrich (Shanghai, China). Ultrapure water was made using the Milli-Q Ultrapure System (Millipore, Bedford, MA). Acetonitrile and methanol of HPLC grade were from Fisher Chemical Company (New Jersey). Buffers were prepared in our laboratory, and all other chemicals used were of analytical grade or higher.
Instrumentation
The following equipments were used: 2802-style UV–vis scanner (Shanghai Unico Analytical Instrument Co.); HJ-3 magnetic stirrer, high-speed centrifuge, 303AS electron box (Shanghai, Tianda Apparatus Co. Ltd.); polystyrene 96-well microtitre plates were purchased from Costar (Corning Costar, Cambridge, MA) and ELISA plate washer from Nunc Maxisorp (Nunc MaxiSorp, Roskilde, Denmark). ELX800 Universal Microplate Reader (Bio-Tek Instruments Inc., Winooski, VT) was used to measure the optical density of the microplates.
Synthesis of artificial antigens
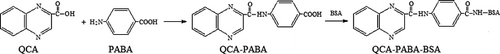
The synthetic routes for the two haptens used in this study are shown in . In brief, an amount of 50-mg QCA (metabolite of QCT and CBX) was dissolved in 10-ml N,N-dimethylformamide and followed by addition of 5 ml of para-aminobenzoic acid (PABA). After reaction for 15 h at room temperature, the mixture was centrifuged and the supernatant was obtained. One hundred milligams of BSA dissolved in 20 ml water, together with 14 mg of NHS and 23 mg DCC, was added to the supernatant. The mixture was reacted overnight, the supernatant was collected and dialysed against 0.01 mol l−1 phosphate buffer saline (PBS) for 3 d, and the PBS was replaced with a fresh solution every 4 h. Then QCA-PABA-BSA was obtained and lyophilised and stored at −20 °C. Similarly, QCA-PABA-OVA used for the plate coating antigen was prepared by a similar procedure.
Immunization schedule and antiserum preparation
Eight female New Zealand white rabbits (2.0–2.5 kg) were purchased from the Chongqing Center for Disease Control and Prevention (Chongqing, China). Routinely, 500 µg of the conjugate (QCA-PABA-BSA) was dissolved in 500 µl of PBS and emulsified with Freund's complete adjuvant (1:1, v/v). The immunogen emulsion was injected subcutaneously into multiple sites on the back of each rabbit. Animals were boosted at 21-day intervals with the same immunogen (500 µg of the conjugate emulsified with Freund's incomplete adjuvant). A booster was given to each rabbit at 2-week intervals. On the 8th day after each booster, a 5-ml blood sample was taken from an ear vein of each rabbit to check the titre of the antiserum using a non-competitive ELISA protocol. When the booster schedule was finished, blood was obtained from the rabbit's heart, with the highest titre, and allowed to coagulate overnight at 4 °C. Then, the antisera were separated by centrifugation, and sodium azide was added as a preservative. The antisera were then aliquoted and stored at −70 °C.
ELISA development
The method of ciELISA was as previously described (Le et al., Citation2009), and the ciELISA format was adopted for analysing quinoaxline compounds. The ELISA procedures were as follows. QCA-PABA-OVA (1 µg ml−1) diluted with carbonate buffer, pH 9.8 was added to the wells of a microtitre plate (200 µl per well). The plate was incubated overnight at 4 °C and then washed with PBS-T (PBS with 0.05% Tween-20), using an automated plate washer. The binding sites not occupied by the coating antigen were then blocked by the blocking buffer (200 µl per well) for 1 h at 37 °C. After the plate was washed as before, standard solutions or samples (50 µl per well) and diluted PAb (50 µl per well) were added and incubated for 1 h at 37 °C. After washing, goat anti-rabbit IgG-HRP conjugate was added (200 µl per well) and the plate was incubated for 45 min at 37 °C. Then, the plate was washed and the substrate solution (100 µl per well) added. After incubation at 37 °C for 15 min, the colour development was stopped by adding 50 µl per well of H2SO4. Finally the optical density (OD) of each well was measured at 450 nm. Calibration curves were constructed in the form of (B/B0)×100% vs. log C, where B and B0 were the absorbance of the analyte at the standard point and at zero concentration of the analyte, respectively.
Standard inhibition curve
The standard inhibition curve was established by using QCA-PABA-OVA as coating antigen for the ciELISA described above. After incubation and development, the average OD of the wells without quinoaxline-1,4-dioxides was normalised to 100%. The half-maximal inhibition concentration (IC50) was determined as a measure of the sensitivity of the ciELISA.
Cross-reactivity
The specificity of the produced PAb was investigated by cross-reactivity (CR) experiments. Nine compounds were selected for testing CR (). Standard solutions for testing the compounds were prepared in the concentration range of 0.01–1000 ng ml−1 with PBS and applied according to the ELISA procedures. The values of CR were calculated as follows: CR%=(IC50 of QCT/IC50 of competitor)×100% (Le et al., Citation2009).
Table 1. Cross-reactivity of the PAb to quinoxaline-1,4-dioxides and related compounds tested by ciELISA.
Sample pretreatment
The pretreatment procedure of feed samples reported by Wu et al. (Citation2006) was modified slightly and adopted in this study. One gram of feed was weighed into a 10-ml plastic tube, 5 ml of extract solution comprised of methanol:acetonitrile:water (35/35/30, v/v/v) was added and the mixture was votexed for 1 min in an ultrasonic bath and centrifugation at 8,000 g for 5 min. The supernatant (1 ml) was transferred to a glass tube followed by removing the organic phase by a gentle stream of nitrogen at 40 °C and supplementing the volume to 2 ml with PBS, and then 2 ml of hexane was added and the mixture was vortexed for 1 min. Finally, the hexane layer was removed, and 50 µl of the PBS layer was analysed by the developed ciELISA.
Recovery and precision studies
To study the recovery, feed samples were spiked with different concentrations of OLA, MEQ, CBX, CYA and QCT at the levels of 50, 100 and 200 ng g−1. Reproducibility and precision of the assay were determined by ciELISA. The intra-variations between five replicates and inter-variations from five independent experiments were determined for the sample recovery, according to the standard curve, by ciELISA.
Comparison of ciELISA with HPLC
To validate the applicability of the present method determination, the five quinoaxlines were detected both by ciELISA and HPLC in feed samples spiked with quinoaxlines. The ciELISA test protocol was as described above. The methods were confirmed by HPLC method reported by Wu et al. (Citation2006). In brief, HPLC analyses were carried out on a Varian HPLC system (CA, USA) with a 230 pump, 310 UV detector and model 400 autosampler. The chromatographic separation was accomplished with gradient elution on an Eclipse XDB C18 column (4.6 mm×250 mm, 5 µm) coupled with a 2-mm C18 guard-column at 30 °C in a column oven. The sample volume injected was 20 µl. The mobile phase consisted of methanol and water. The flow rate was 1 ml per min. The gradient elution performed was: 0–3 min: methanol/water (10/90); 6 min: methanol/water (20/80); 6.01 min: methanol/water (25/75); 22 min: methanol/water (70/30); 22.01–30 min: methanol/water (75/25). The UV detector was set at a wavelength of 380 nm for all the compounds.
Results and discussion
Synthesis and identification of mimic antigens
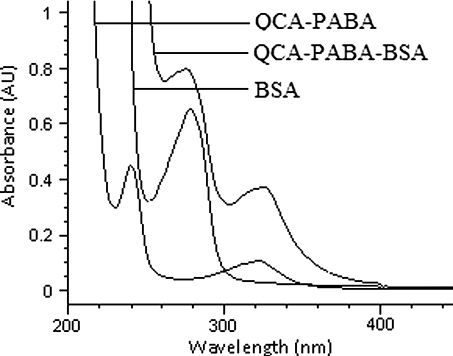
The UV–VIS spectrum of QCA-PABA-BSA was shown in . The spectrum of QCA-PABA-BSA conjugate showed both maximum absorbance wavelength of BSA and that of QCA-PABA at 280 and 330 nm, respectively. Similarly, conjugates of QCA-PABA-OVA were identified successfully by UV–VIS spectrum (data not shown). The results demonstrated that the synthesis of mimic antigens was successful.
Screening of antisera by checkerboard titration
The antisera of terminal bleeds from three rabbits were screened against QCA-PABA-OVA coating antigen using a checkerboard titration method with the coated antigen format (Le et al., Citation2009). The heterologous assay, in which different concentrations of the hapten-conjugates were used as the coating antigen and immunogen, showed that the titre value of PAb-1 was 1/64,000, titre value of PAb-2 was 1/32,000 and titre value of PAb-3 was 1/128,000. So the antiserum of PAb-3 with higher affinity was selected for further development.
Standard curves and specificity of ciELISA
The standard calibration curves plotted by ciELISA analysis () showed that the working concentrations ranged from 0.01 to 1000 ng ml−1. Good linearity was obtained from range 1 to 100 ng ml−1 (based on IC80 to IC20) with acceptable correlation coefficients (r2=96.9–98.8%). The IC50 values were calculated to be 8.6, 13.1, 17.3, 24.5 and 26.4 ng ml−1 for QCT, CYA, CBX, MEQ and OLA, respectively. Moreover, the IC50 for QCT is the smallest, and the IC50s for OLA and MEX are the largest of the five compounds. The reason is probably that the structure of the QCA derivative is more consistent with QCT for a benzene ring than that with OLA and MEX. The limits of detection (90% of B/Bo) were 0.41, 0.59, 0.67, 0.80 and 0.86 ng ml−1 for QCT, CYA, CBX, MEQ and OLA, respectively ().
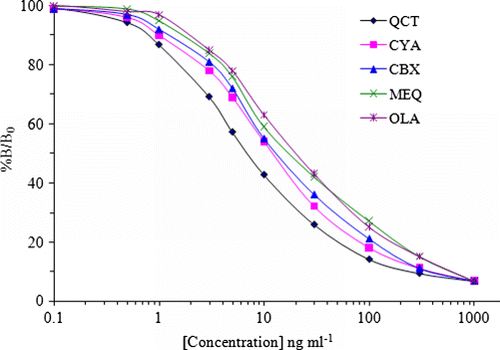
To determine the specificity of the optimised ciELISA, five quinoxalines and several other antibiotics were tested for cross-reactivity. The detailed cross-reactivity data were summarised in on the basis of the PAb with strong cross-reactivity to QCT (100%), CYA (49.9%), CBX (66.2%), MEQ (35.3%) and OLA (32.8%). The high cross-reactivity of PAb with four quinoxalines can be explained by the fact that quinoxaline-1,4-dioxides exhibited the highest structural homology among all quinoxalines. Low cross-reactivity was observed in the other five antibiotics (<0.02). While the analysis indicated that the PAb showed high affinity with quinoxaline-1,4-dioxides and low affinity with other antibiotics, and proved that this ciELISA assay was highly specific.
Recovery and precision studies
Importantly, the detection of five quinoxaline-1,4-dioxides in pig feed and chicken feed was reproducible. The quinoxaline-1,4-dioxide negative feed samples (determined by HPLC) were spiked with different concentrations of quinoxaline-1,4-dioxides and simultaneously analysed by ciELISA as described above. Intra-assay variability for the ciELISA to detect quinoxaline-1,4-dioxides and inter-assay reproducibility from the repeated experiment were determined. As shown in , the recovery values ranged from 78.8% to 90.2% for QCT, 71.6% to 88.7% for CYA, 73.6% to 91.3% for CBX, 76.4% to 92.9% for MEQ and 75.6% to 88.6% for OLA, respectively. The coefficients of intra-assay variations (CVs) were 4.1–9.6%, and the inter-assay CVs were 6.5–10.8%. Therefore, the reproducibility of the ciELISA for the detection of quinoxaline-1,4-dioxides appeared to be better than that reported with other methods (Situ & Elliott, Citation2005; Wu et al., 2006, 2009).
Table 2. Accuracy and precision of the method for quinoxaline-1,4-dioxides in different animal feed types spiked at three levels (n =5).
Validation of the assay with HPLC
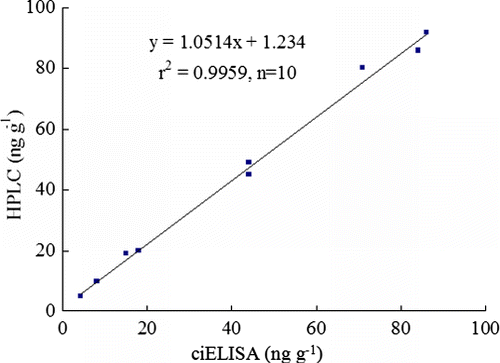
In order to verify the applicability of this ELISA method, ten fortified feed samples including QCT (spiked at concentrations of 5 and 50 ng g−1 sample, respectively), CYA (spiked at concentrations of 50 and 100 ng g−1 sample), CBX (spiked at concentrations of 10 and 20 ng g−1 sample), MEQ (spiked at concentrations of 5 and 20 ng g−1 sample) and OLA (spiked at concentrations of 80 and 100 ng g−1 sample) were measured by HPLC and ciELISA simultaneously, and the measured results were compared. As shown in , good correlation was obtained between ciELISA (x) and HPLC (y) with the linear regression equation of y=1.0514x+1.234 (r2=0.9959, n=10). These results suggested that five quinoxalines in feed samples could be simply, rapidly and accurately detected by ciELISA.
Conclusions
In this study, the hapten derivative with a spacer-arm (PABA) was synthesised and coupled to the carrier proteins. A PAb against the quinoxaline-1,4-dioxides was prepared and a highly sensitive and specific ciELISA for rapid multi-residues detection of the five quinoxaline-1,4-dioxides in animal feed was developed. Recovery rates varied from 71.6% to 92.9%, and the coefficient of variation was from 4.1% to 10.8%, which demonstrated high-accuracy and precision of the assay. The method was also validated by HPLC, which had good correlation. Therefore, the optimised ciELISA may become a new convenient and satisfactory analytical tool for monitoring quinoxaline-1,4-dioxide residues in animal feed.
Acknowledgements
This project was supported by the Scientific and Technological Research Project of Chongqing China (CSTC2011ggB10009), Natural Science Foundation of Chongqing education committee (KJ110605).
References
- Bories, G.F. (1979). High-performance liquid chromatographic determination of olaquindox in feeds. Journal of Chromatography A, 172, 505–508.
- Chen, Q., Tang, S., Jin, X., Zou, J., Chen, K., Zhang, T., et al. (2009). Investigation of the genotoxicity of quinocetone, carbadox and olaquindox in vitro using Vero cells. Food and Chemical Toxicology, 47, 328–334.
- Commission regulation No. 2788/98. (1998). Official Journal of the European Union, Commun. L347, 3–4, 31–32.
- Fuh, M.R., Chan, S.A., Wang, H.L., & Lin, C.Y. (2000). Determination of antibacterial reagents by liquid chromatography-electrospray-mass spectrometry. Talanta, 52, 141–151.
- Graaf, G.J., & Spierenburg, T.J. (1985). Liquid chromatographic determination of carbadox and desoxycarbadox in medicated feeds and in porcine gastrointestinal tract. Journal of the Association of Official Analytical Chemists, 68, 658–660.
- Hutchinson, M.J., Young, P.B., & Kennedy, D.G. (2005). Confirmatory method for the analysis of carbadox and olaquindox in porcine feedingstuffs using LC-electrospray MS-MS. Food Additives and Contaminants, 22, 113–119.
- Le, T., Yu, H., Guo, Y.C., Ngom, B., Shen, Y.A., & Bi, D.R. (2009). Development of an indirect competitive ELISA for the detection of doxycycline residue in animal edible tissues. Food and Agricultural Immunology, 20, 111–124.
- Lin, S.Y., & Jeng, S.L. (2001). High-performance liquid chromatographic determination of carbadox, olaquindox, furazolidone, nitrofurazone, and nitrovin in feed. Journal of Food Protection, 64, 1231–1234.
- Mcgary, E.D. (1986). Quantitative determination of sulphamethazine and carbadox in animal feeds by paired ion high-performance liquid chromatography. Analyst, 111, 1341–1342.
- Ramos, F.J., & Silveira, I.N. (1991). Column liquid chromatographic determination of carbadox and olaquindox in feeds. Journal of Chromatography A, 558, 125–130.
- Roybal, J.E., Munns, R.K., & Shimoda, W. (1985). Liquid chromatographic determination of carbadox residues in animal feed. Journal of the Association of Official Analytical Chemists, 68, 653–657.
- Situ, C., & Elliott, C.T. (2005). Simultaneous and rapid detection of five banned antibiotic growth promoters by immunoassay. Analytica Chimica Acta, 529, 89–96.
- Thorpe, V.A. (1988). A collaborative study: High-pressure liquid chromatographic determination of carbadox and pyrantel tartrate in animal feeds. Journal of Chromatographic Science, 26, 545–550.
- Wu, C.-M., Li, Y., Shen, J.-Z., Cheng, L.-L., Li, Y.-S., Yang, C.-Y., et al. (2009). LC-MS-MS quantification of four quinoxaline-1,4-dioxides in swine feed. Chromatographia, 70, 1601–1605.
- Wu, Y., Wang, Y., Huang, L., Tao, Y., Yuan, Z., & Chen, D. (2006). Simultaneous determination of five quinoxiline 1,4-dioxides in animal feeds using ultrasonic solvent extraction and high-performance liquid chromatography. Analytica Chimica Acta, 569, 97–102.