Abstract
An immunochromatographic assay (ICA) was developed and applied for the residue determination of tylosin and tilmicosin in muscle, liver, fish and eggs. The assay is based on a competitive format, and its sensitivity is improved by using a novel criterion to screen the optimal amount of nanogold-labelled monoclonal antibody. Owing to the novel nanogold particle labelling method, 10 min was sufficient to perform the ICA, the visual detection limits were 10 and 20 ng/g for tylosin and tilmicosin, respectively. The standard curves based on the tylosin and tilmicosin matrix calibration ranged from 0.1 to 100 ng/mL, with IC50 values of 2.9 and 4.1 ng/mL for tylosin and tilmicosin, respectively. The recovery rates ranged from 71.5 to 103.2%, with coefficient of variation <14.1%, when tylosin and tilmicosin were spiked in various biological matrices with the concentrations of 10–100 ng/g. The results of ICA were consistent with those of ELISA kit and high-performance liquid chromatography in the analysis of tylosin in the incurred tissues, demonstrating the practical applicability of the developed assay in real samples. Over all, to our knowledge, this is the first report of qualitative and semi-quantitative residue determination for tylosin and tilmicosin by ICA.
Introduction
Tylosin and tilmicosin are the most commonly used premix macrolide antibiotics (), which constitute a very important class of antibiotics which is highly active against Gram-positive and Gram-negative cocci. Moreover, tylosin and tilmicosin have also been proved to be active against non-classical pathogens such as Helicobacter pylori (Bogialli, Ciampanella, Curini, Di Corcia, & Lagan, Citation2009). For those reasons, tylosin and tilmicosin are widely used in veterinary medicine for the treatment of a wide range of infections in domestic animals, especially for respiratory diseases treatment. If recommended drug withdrawal times are not respected, there would be a significant risk of detecting macrolide residues in edible tissues, milk or eggs, and human exposure to these foods contaminated by those drugs may lead to allergic reactions (Tong, Rao, Zhu, Jiang, & Ding, Citation2009). Therefore, tylosin and tilmicosin have been banned as feed additives in the European Union since 1999 (European Commission, Citation1998). Within China, maximum residue limits (MRLs) have also been established. Therefore, it is necessary to develop a simple and efficient method to screen tylosin and tilmicosin antibiotics in feedstuff.
Various techniques have been developed for the detection of tylosin and tilmicosin residues in food animal tissues, such as high-performance liquid chromatography (HPLC) (Clark, Dowling, & Boison, Citation2009; Keng & Boison, Citation1992; Montesissa, De Liguoro, Santi, Capolongo, & Biancotto, Citation1999; Nozal et al., Citation2006; Thompson, Noot, Calvert, & Pernal, Citation2003; Zheng et al., Citation2011), thin-layer chromatography (Vincent, Gizzi, von Holst, De Jong, & Michard, Citation2007), liquid chromatography–tandem mass spectrometry (Bogialli et al., Citation2009; Thompson, Pernal, Noot, Melathopoulos, & van den Heever, Citation2007) and fluorescence immunoassay (Bang-Ce, Songyang, Peng, & Xiao-Hong, Citation2008). However, most of them require expensive equipment, highly skilled personnel and extensive sample pre-treatment. Those methods are unsuitable for routine screening and high-throughput field detection. Microbiological assays (Litterio, Calvinho, Flores, Tarabla, & Boggio, Citation2007; Pol-Hofstad, Driessen-Van Lankveld, Tomassen, De Jong, & Van Egmond, Citation2008) are commonly used for the measurement of tylosin in animal feed, but they are lengthy and not sufficiently specific for analytical purposes. In addition, some researchers have established enzyme-linked immunosorbent assay (ELISA) for tylosin and tilmicosin (Beier, Creemer, Ziprin, & Nisbet, Citation2005; Jackman, Spencer, Silverlight, Marsh, & Bellerby, Citation1997; Peng et al., Citation2012; Silverlight, Brown, & Jackman, Citation1999; Wicker et al., Citation1994). Nevertheless, available information about ELISA techniques are not sufficient to prove that ELISA could be useful for simultaneous determination of tylosin and tilmicosin in different animal species and in a wide range of tissues.
In past few years, the immunochromatographic assay (ICA) technique for the diagnosis of low molecular mass analytes has been developed rapidly, and becomes user-friendly in terms of simplification, visual evaluation and on-site screening (Chen, Wang, Tang, Zhu, & Xiao, Citation2008; Guo, Liu, Gui, & Zhu, Citation2009; Verheijen, Stouten, Cazemier, & Haasnoot, Citation1998; Wang et al., Citation2007; Zhang et al., Citation2006). Several reports on ICA method for antibiotic residues detection (Guo et al., Citation2010; Xie et al., Citation2009; Zhang et al., Citation2008; Zhao et al., Citation2008) have proved that ICA is a promising tool for the qualitative/semi-quantitative test to identify ‘positive’ samples, and it decreases the number of samples needed by instrumental methods. However, most of these studies are with limited capacity by detecting only a single analyte in complex samples.
In the current study, a monoclonal antibody (mAb) was employed to prepare the immunogold probe for detecting tylosin and tilmicosin in various biological matrices (muscle, liver, fish and eggs) without complicated sample preparation and cleanup. On the basis of proposed method, a series of satisfactory results were obtained. By the application of ICA method, fast and selective sample screening within a few minutes can be achieved, and skilled personnels are not necessary either. To our knowledge, this is the first report on ICA method based on mAb for simultaneous residue determination of tylosin and tilmicosin.
Materials and methods
Materials and reagents
Tylosin, tilmicosin, desmycosin, 5-O-mycaminosyltylonolide, spiramycin, erythromycin, kitasamycin, azithromycin, josamycin, oleandomycin, roxithromycin, avermectins, ivermectin, incomplete Freund's adjuvant, complete Freund's adjuvant, ovalbumin (OVA), bovine serum albumin (BSA), goat anti-mouse IgG, polyethylene glycol 2000 (PEG 2000), polyvinylpyrrolidone (PVP, K-30), gold chloride and trisodium citrate dihydrate were purchased from Sigma (St. Louis, MO, USA). Nitrocellulose membrane (HF135, 25 mm width), glass fibre conjugate pad (CFSP223000), sample pad and adsorbent pad were from Millipore Corp. (Bedford, MA, USA). A mouse mAb isotyping kit was purchased from Southern Biotech Co. (Beijing, China). The ELISA kit for tylosin analysis was obtained from RANDOX Laboratories Ltd. (Ardmore, Diamond Road, Crumlin, Co., Antrim, UK). All chemicals and reagents used were of analytical grade or better, and the percentage concentrations were defined by weight unless otherwise specified.
Equipment
ZX1000 Dispensing Platform and CM4000 Guil-lotine Cutter (BioDot, Irvine, CA, USA) were used to prepare test strips. A TSR3000 Test Strip Reader (BioDot, Irvine, CA, USA) was used to analyse the intensity of test strips. Transmission electron microscopy (TEM) images were recorded with a Hitachi H600 transmission electron microscope (Hitachi Instrument Co., Tokyo, Japan). The UV–visible spectrums were obtained using an 8453UV/Visible Spectrophotometer (Agilent Tech, Santa Clara, CA, USA). The ICA was validated with an Agilent 1100 HPLC system (Agilent Tech, Santa Clara, CA, USA) reader device.
Preparation of desmycosin–BSA conjugates
O-carboxymethoxylamine (CMO, 21.9 mg) and NaHSO4 (8.4 mg) were diluted in 4 mL triple-distilled water. Then the solution was added drop by drop to the desmycosin solution (154 mg desmycosin diluted in 4 mL methanol), followed by magnetic stirring for 2.5 h at room temperature. After the reaction, the solution was vacuum dried. Then, 4 mL dichloromethane, 2 mL ethanol and 5 g anhydrous sodium sulphate were added to the concentrated mixture which was incubated overnight by shaking. After filtration, the solution was evaporated to dry at 60°C, and then the product of desmycosin–CMO was obtained.
As much as 44.2 mg desmycosin–CMO, 12.4 mg N,N-dicyclohexylcarbodiimide and 6.9 mg N-hydroxysuccinimide were dissolved in 3 mL N,N-dimethylformamide in response to magnetic stirring 12 h for reaction at room temperature. Three hundred and forty milligram BSA (or 220 mg of OVA) was dissolved in 17 mL 0.1 M sodium phosphate buffer (pH 8.0). Desmycosin–CMO solution was added drop by drop to BSA solution with the magnetic stirring under room temperature for 12 h. The mixture was then centrifuged at 2000×g for 5 min and the supernatant was collected at the end and dialysed against 10 mM phosphate buffered (pH 7.4) for 3 days. Then desmycosin–CMO–BSA (or desmycosin–CMO–OVA) was obtained, lyophilised and stored at −20°C until use.
Preparation of mAbs
Five female Balb/c mice (8 week old) were immunised with desmycosin–CMO–BSA conjugates. Serum samples from immunised mice were collected individually 1 week after the final immunisation, and the titre antiserum was determined by indirect non-competitive ELISA. The mouse with serum showing optimum relative inhibition was selected for the subsequent fusion. Cell fusion procedures were performed as previously described with some modifications (Chen et al., Citation2008; Zeng et al., Citation2007). Briefly, hybridoma cell lines were produced through the fusion of myeloma cells (Sp2/0), and spleen cells obtained from the immunised mice with PEG 2000 as the fusing agent. The ratio of spleen cells to myeloma cells in suspension was 5–10:1. Fused cells were resuspended in the selective medium containing hypoxanthine, aminopterin and thymidine (HAT; supplemented with 20% foetal calf serum), and distributed into 96-well tissue culture plates containing a feeder layer of peritoneal cells. When hybridoma colonies appeared, they were expanded in the HAT medium. Ten days after fusion, hybridoma culture supernatants were screened by an indirect competitive ELISA (icELISA) using desmycosin as the competitor. Desmycosin–CMO–OVA conjugate was used as the coating antigen. The hybridoma cells that secreted antibodies with high inhibition titre were selected and subcloned four times by limiting dilution. Then, the hybridoma cells were injected into Balb/c mice, and the ascites were produced and collected. The mAbs were purified from ascites using a protein G affinity column (Amersham Biosciences, Uppsala, Sweden) according to the manufacturer's protocol. Isotypes of the purified mAb were determined with a mouse mAb isotyping kit (Southern Biotech Co., Beijing, China). The titre and 50% binding inhibition concentration (IC50) were determined by icELISA coated with antigen as described below.
ELISA procedure
Indirect ELISA was developed to determine the purified mAb titre, and icELISA was developed for the construction of calibration curves. Briefly, microwell plates were coated with 100 µL of desmycosin–CMO–OVA conjugate (0.5 µg/mL) diluted in carbonate buffer (pH 9.6), and the plates were incubated overnight at 4°C, washed three times and blocked for 1 h at 37°C with a 200 µL blocking buffer. After the plates were washed three times, 100 µL of purified mAb diluted in phosphate buffer was added to each well (indirect ELISA). For the calibration curves, 50 µL of the diluted mAb and 50 µL of standard samples or extracts were added in each well (icELISA). After incubation for 45 min at 37°C, the plates were washed three times with the washing buffer. This was followed by the addition in each well of 100 µL of peroxidase-labelled goat anti-mouse antibodies diluted in washing buffer (1:10,000), and the plates were incubated for 45 min at 37°C. Finally, the plates were washed four times with washing buffer, and 100 µL of 3,3′,5,5′-tetramethyl-benzidine solution was added to each well. Colour development was stopped after 20 min by adding 50 µL of 2 M H2SO4, and the absorbances were read at 450 nm using an ELX800 Universal Microplate Reader.
Preparation of colloidal gold probe
Colloidal gold solutions were prepared according to the procedure described in literature (Guo et al., Citation2010). Briefly, 100 mL of a 0.01% (w/w) aqueous solution of chlorauric acid (HAuCl4·3H2O) in a round-bottom flask (500 mL volume) was boiled under a vigorous stirring, and 4 mL of 1% (w/v) sodium citrate solution was then added to the solution. The colour of the solution turned into deep blue within 20 s and changed to wine red. After boiling for 15 min, the heating source was turned off, and the colloidal gold solution was stirred gently at room temperature for 30 min. The colloidal gold obtained was scanned by UV–Visible Spectrophotometer at 200–700 nm, showing only one absorption maximum at 522 nm. The average diameter of these uniform-sized particles was 24.3±2.2 nm (n=100) measured by TEM. Then, the colloidal gold solution was stored at 4°C until use.
Exactly 0.4 mg of the purified mAb (4.1.6/5A2) diluted in 5 mL of distilled water was added gently into 100 mL of gold colloid solution (pH 8.0–9.0), with gentle stirring for 15 min. Then 10% BSA aqueous solution was added to block excess reactivity of the gold colloid, followed by stirring for 30 min. After centrifugation at 12,000×g at 4°C for 30 min, the supernatants were removed and the remaining pellets were suspended in 2 mM borate buffer (pH 9.0) containing 0.1% PEG 2000 and 0.02% NaN3. The process was repeated twice, and the pellets were finally resuspended in 10 mL of borate buffer. The mAb conjugates were put into labelled tubes and then stored at 4°C until use.
Preparation of immunochromatographic strips
The sample and the conjugate pads were treated with PBS and the above conjugate storage buffer, respectively, and then vacuum dried at 37°C for 4 h. A total of 5 µL/cm of colloidal gold-labelled mAb (4.1.6/5A2) diluted five times with PBS containing 2.5% sucrose, 0.3% PVP, 2% BSA, 1% mycose and 0.02% sodium azide was jetted on the treated conjugate pad by using the BioDot platform, and then lyophilised to dryness. The pad was stored in a desiccator at room temperature. A total of 1.6 µL/cm of desmycosin–CMO–BSA (1 mg/mL) conjugate and goat anti-mouse IgG (1.5 mg mL−1) were sprayed onto the bottom and the top of nitrocellulose membrane as the test (T line) and control lines (C line), respectively, by BioDot platform, and then vacuum dried at 37°C for 2 h. Afterwards, the sample pad, detector conjugated pad components, nitrocellulose membrane and absorbent pad were laminated into a sheet of plastic backing orderly, and cut into test strip using a strip cutter. The assembly was then stored at room temperature in plastic bags containing silica gel desiccant.
Sample preparation
Samples, such as porcine muscles, porcine liver, chicken muscle, chicken liver, egg and fish, were obtained from a local supermarket, and the contents of tylosin and tilmicosin were determined by HPLC. Tylosin and tilmicosin free samples determined by HPLC were used as negative control. Sample tested (2±0.005 g) was homogenised, mixed with 4 mL of ethyl acetate, vortexed for 2 min and then centrifuged at 2000×g for 10 min. The supernatants were collected and the debris was treated one more time as above. The final supernatants were evaporated at 60°C in water bath with a gentle nitrogen flow. The extractions were resuspended in 1 mL of 10 mM PBS (pH 7.4) and mixed thoroughly before analysis.
Principle and procedure of the ICA
The assay was based on competitive reaction theory. When a control sample (negative sample) was placed on the sample pad, the detector reagents in the conjugate pads were dissolved and then trained up by the liquid through capillary action force. In the absence of analyte, colloidal gold mAb 4.1.6/5A2 would be trapped by the immobilised desmycosin–CMO–BSA (T line) on the nitrocellulose membrane. On the other hand, if the sample contains sufficient target analytes, no band was observed in the corresponding location. In any assay, the control line should always be developed to ensure the system is working properly.
Standard curves and specificity tests
To prepare the standard curves of the test strips to tylosin and tilmicosin, standard samples of the analytes were prepared in 0.01 mM phosphate buffer (pH 7.4) at concentrations 0, 1, 5, 10, 20, 40, 60, 80 and 100 ng/mL. The colour intensities on the test lines were determined by using ICA reader device (TSR3000 membrane strip reader, BioDot, Irvine, CA, USA). Data were expressed as relative optical density (ROD). The fifty percent of inhibition (IC50 was calculated by using the following formula: IC50=B/B 0×100%, B and B 0 represent the ROD values obtained for the positive and the negative samples, respectively. The specificity of test strips was tested in a cross-reaction study with related compounds. The standard solutions of tylosin, tilmicosin, desmycosin, 5-O-mycaminosyltylonolide, spiramycin, erythromycin, kitasamycin, azithromycin, josamycin, oleandomycin, roxithromycin, avermectins and ivermectin were prepared at concentrations of 1, 10, 100, 1000 and 10,000 ng/mL, respectively, and were tested by the optimal lateral flow strips. The values of cross-reactivity (CR) were determined by CR (%)=(IC50 of desmycosin/IC50 of competitor)×100%.
Recovery of spiked samples
Negative porcine muscles, porcine liver, chicken muscle, chicken liver, egg and fish confirmed by HPLC were spiked with tylosin or tilmicosin with concentrations at 10, 25, 50 and 100 ng/g. The analyses were performed at eight replicates for each concentration (n=8).
Comparison of ICA with HPLC for the analyses of sample from local markets
Parallel comparison tests among ICA and HPLC were accomplished simultaneously by determining 30 samples (five porcine muscle samples, five porcine liver samples, five chicken muscle samples, five chicken liver samples, five fish samples and five egg samples), which were randomly collected from local markets in Hechuan and Wanzhou city (Chongqing, China) during March 2011. These samples were analysed and compared by ICA and HPLC method. The sample pre-treatment and process of HPLC analysis were carried out as reported in a previous study (Clark et al., Citation2009; Prats, Francesch, Arboix, & Perez, Citation2002).
Animal experiments
To further evaluate the validity of the quantitative response of the strip, groups of Duroc castrated pigs weighing 13–15 kg were housed individually in our animal facility for 1 week. Eighteen pigs were randomly divided into control and test groups. The control group (n=3) was mock treated with another drug, intramuscularly. The test group (n=15) was treated intramuscularly with tylosin at a dose of 10 mg/kg bodyweight daily for five consecutive days. The animals were euthanised at days 0, 1 and 3 after the withdrawal of the tylosin treatments. The muscle and liver samples were collected separately and stored at −20°C until analysis by ICA, ELISA kit and HPLC.
Results and discussion
Identification of artificial antigens
The protocol of the hapten carrier preparation is shown in (desmycosin–CMO–BSA). There were several significant spectral changes between the conjugates and its carrier protein. The maximum absorbance wavelength of BSA and desmycosin were 280 and 290 nm, respectively. However, the maximum absorbance wavelength of desmycosin–CMO–BSA conjugate was 286 nm according to the spectrum. This blue shift indicated the successful combination of the carrier protein and the hapten. The same spectral change can also be observed in desmycosin–CMO–OVA after conjugation. These results demonstrated that the artificial antigens have been successfully produced. The molar ratio of hapten to the carrier protein for desmycosin–CMO–BSA and desmycosin–CMO–OVA was 5.6:1 and 3.3:1, respectively. Of note, the conjugation of haptens to its carrier is the key step to produce a mAb in this research. Some haptens of macrolide antibiotics have been reported (Beier et al., Citation2005; Jackman et al., Citation1997; Peng et al., Citation2012; Silverlight et al., Citation1999; Wicker et al., Citation1994). Here, we designed and synthesised a new hapten with the combination of immunising/coating and the spacer-arm connected to desmycosin, which is distinct from previous reported ones.
Characterisation of the mAb
The conjugate desmycosin–CMO–BSA was used as immunogen to immunise the mice, while desmycosin–CMO–OVA were coated onto ELISA plates to determine the titre and inhibition level of antisera, and both high titres and low IC50 values of antisera were obtained. After cell fusions, one clone of hybridroma cell line 4.1.6/5A2, which produced antibody highly competitive between free desmycosin and desmycosin–CMO–OVA, was selected by icELISA. The purified mAb concentration was determined by Bio-Rad protein assay kit. It was found that this mAb 4.1.6/5A2 was IgG2a isotype with a kappa light chain by mouse mAb isotyping kit, and this hybridoma was employed for further evaluation of mAb specificity and subsequent immunoassay development. The titre of the purified mAb was 1.28×105 determined by indirect ELISA. The standard curves plotted by icELISA analysis showed that the working concentration ranged from 0.01 to 100 ng/mL. The IC50 values of desmycosin, tylosin and tilmicosin by icELISA were calulated, and they were 2.3, 3.8 and 6.1 ng/ml, respectively.
Test sensitivity
The test strip was based on competitive immunoassay principle (). Negative tests provide the most intense coloured test line because of the inverse relationship between analytes (desmycosin, tylosin and tilmicosin) concentration and colour development. The smallest amount of analytes that resulted in no colour development at the test line (positive test) was considered as the cut-off level (). The absence of control line means the test was invalid (). As shown in , using the ICA of lateral-flow immunoassay for a single antibiotic, extraction solutions of concentrations of 0, 1, 5, 10, 20, 40, 60, 80 and 100 ng/mL were tested to get the visual limit of detection of 5, 10 and 20 ng/mL for desmycosin, tylosin and tilmicosin, respectively.
![Figure 2. The illustrations of immunochromatographic assay (ICA) results (A) and the visual limit of detection of single antibiotics [(B) desmycosin, (C) tylosin, (D) tilmicosin] with ICA test: upper line, C line; lower line, T line. The standard solutions of desmycosin, tylosin and tilmicosin at each final concentration of 0, 1, 5, 10, 20, 40, 60, 80 and 100 ng/mL (numbers across the top of strips from left to right) were tested.](/cms/asset/a3a430e4-24c0-4e20-b7cc-4fbb7dfd96d5/cfai_a_716025_f0002_oc.jpg)
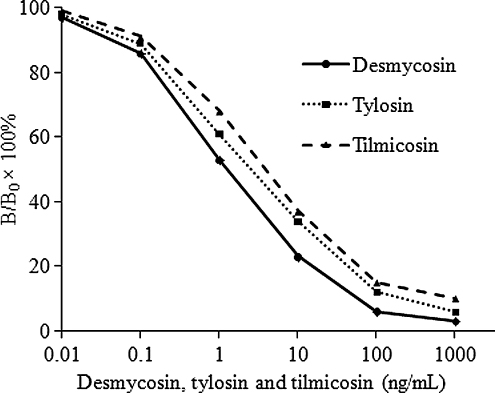
To prepare the ICA standard curves of desmycosin, tylosin and tilmicosin, standard samples of the analytes were prepared in 0.01 mM phosphate buffer (pH 7.4). The inhibition curves for the quantitative analyses are shown in . Good linearity was obtained from 0.1 to 100 ng/mL with acceptable correlation coefficients. The IC50 values were calculated to be 1.6, 2.9 and 4.1 ng/mL for desmycosin, tylosin and tilmicosin, respectively.
Cross-reactivities of ICA
To evaluate the specificity of the strip format assay, several analogous compounds were tested. As shown in , the mAb (4.1.6/5A2) showed CR towards tylosin (55.2%), tilmicosin (36.4%), desmycosin (100%), 5-O-mycaminosyltylonolide (4.5%). However, the mAb did not exhibit measurable CR (<0.02) with other antibiotics such as spiramycin, erythromycin, kitasamycin, azithromycin, josamycin, oleandomycin, roxithromycin, avermectin. Thus, the ICA could be utilised for residue detection as a preliminary screening method.
Table 1. Cross-reactivity (CR) of macrolide antibiotics by the ICA based on mAb.
Recovery and reliability of the test strip assay
The results of recovery and repeatability for tylosin or tilmicosin in tissues are shown in . Close agreements were obtained between the measured values and the fortified concentrations at the three spiked levels. The recovery for tylosin ranged from 71.5 to 103.2% and that of tilmicosin ranged between 72.6 and 101.5%. The repeatability of the assay was investigated by calculating the coefficient of variations which were less than 14.9%.
Table 2. Recoveries of tylosin and tilmicosin spiked in tissues by the ICA (n=64Footnotea).
Comparison between ICA and HPLC for the analysis of samples from local markets
To evaluate the practicability of this strip test in real samples, the strip was applied to 30 samples gathered from markets, and then the results were confirmed by HPLC. A sample without contamination from tylosin or tilmicosin (determined by HPLC to be tylosin and tilmicosin free) was used as the negative control. Results () showed that most of the examined samples were contaminated by tylosin or tilmicosin. The visual evaluation results of the immunostrip were consistent with those of HPLC (), and also in conformity with the visual detection of limits. Moreover, consistent results were obtained with five replications, showing good reproducibility and strip-to-strip performance.
Table 3. Results of tylosin or tilmicosin analysis by HPLC and ICA test in animal muscle, liver, fish and egg samples.
Analysis of animal experiment sample by ICA, ELISA and HPLC
An overview of the results obtained from ICA, ELISA and HPLC analyses is presented in . According to the data, 1–3 days of withdrawal were required for the drug to reach a concentration below the MRLs (100 ng/g) in porcine muscle and liver. For the quantitative analyses, a side-by-side comparison of ICA with ELISA kit and HPLC method showed high correlation (R2=0.99). However, the ICA results to tylosin are closer to that of HPLC compared with that of ELISA kit on positive samples collected at the intraday and first day of withdrawal. In the overall analyses, the positive and negative consistent rates between the test strip and HPLC method were 100%.
Table 4. Result analyses of HPLC, ELISA kit and ICA for the detection of tylosin in porcine muscle and liver samples from animal experiments (n=5).
Conclusions
In our study, a semi-quantitative immunochemical rapid test for residue analysis has been developed, based on mAb (4.1.6/5A2) with strong CR to tylosin and tilmicosin. This study has reported an ICA method for the determination of tylosin and tilmicosin in muscle, liver, fish and eggs for the first time. The ICA test had a visual limit of detection of 10 and 20 ng/mL for tylosin and tilmicosin, respectively. This method showed good consistency with confirmatory ELISA kit and HPLC. The developed ICA can be a suitable tool for rapid, qualitative and semi-quantitative detection of tylosin and tilmicosin in various biological matrices.
Acknowledgements
This project was supported by the Scientific and Technological Research Project of Chongqing China (CSTC2011ggB10009) and National Natural Science Foundation of Chongqing education committee (KJ110605).
References
- Bang-Ce, Y., Songyang, L., Peng, Z., & Xiao-Hong, L. (2008). Simultaneous detection of sulfamethazine, streptomycin, and tylosin in milk by microplate-array based SMM-FIA. Food Chemistry, 106(2), 797–803.
- Beier, R.C., Creemer, L.C., Ziprin, R.L., & Nisbet, D.J. (2005). Production and characterization of monoclonal antibodies against the antibiotic tilmicosin. Journal of Agricultural and Food Chemistry, 53(25), 9679–9688.
- Bogialli, S., Ciampanella, C., Curini, R., Di Corcia, A., & Lagan, A. (2009). Development and validation of a rapid assay based on liquid chromatography-tandem mass spectrometry for determining macrolide antibiotic residues in eggs. Journal of Chromatography A, 1216(40), 6810–6815.
- Chen, Y., Wang, Z., Tang, S., Zhu, Y., & Xiao, X. (2008). Rapid enzyme-linked immunosorbent assay and colloidal gold immunoassay for kanamycin and tobramycin in swine tissues. Journal of Agricultural and Food Chemistry, 56(9), 2944–2952.
- Clark, C.R., Dowling, P.M., & Boison, J.O. (2009). Development and validation of a method for determination of tilmicosin residues in equine plasma and tissues using HPLC. Journal of Liquid Chromatography & Related Technologies, 32(19), 2839–2856.
- European Commission. (1998). Council Regulation (EC) No 2821/98 of 17 December 1998 amending, as regards the withdrawal of the authorisation of certain antibiotics. In: Directive 70/524/EEC concerning additives in feeding stuffs. Council Regulation (EC) No 2821/98: OJL 351-4-OJL 351/8.
- Guo, Y.R., Liu, S.Y., Gui, W.J., & Zhu, G.N. (2009). Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Analytical Biochemistry, 389(1), 32–39.
- Guo, Y., Ngom, B., Le, T., Jin, X., Wang, L., Shi, D., et al. (2010). Utilizing three monoclonal antibodies in the development of an immunochromatographic assay for simultaneous detection of sulfamethazine, sulfadiazine, and sulfaquinoxaline residues in egg and chicken muscle. Analytical Chemistry, 82(18), 7550–7555.
- Jackman, R., Spencer, Y., Silverlight, J., Marsh, S., & Bellerby, P. (1997). Development of antibodies to tilmicosin and their use in the immunolocalization of the antibiotic in porcine lung tissue. Journal of Veterinary Pharmacology and Therapeutics, 20(Suppl.), 131–132.
- Keng, L.J.Y., & Boison, J.O. (1992). High performance liquid chromatographic determination of tylosin in bovine muscle, kidney and liver. Journal of Liquid Chromatography & Related Technologies, 15(12), 2025–2034.
- Litterio, N., Calvinho, L.F., Flores, M., Tarabla, H.D., & Boggio, J.C. (2007). Microbiological screening test validation for detection of tylosin excretion in milk of cows with low and high somatic cell counts. Journal of Veterinary Medicine Series A, 54(1), 30–35.
- Montesissa, C., De Liguoro, M., Santi, A., Capolongo, F., & Biancotto, G. (1999). Tylosin depletion in edible tissues of turkeys. Food Additives & Contaminants, 16(10), 405–410.
- Nozal, M.J., Bernal, J.L., Martín, M.T., Jiménez, J.J., Bernal, J., & Higes, M. (2006). Trace analysis of antibacterial tylosin A, B, C and D in honey by liquid chromatography-electrospray ionization-mass spectrometry. Journal of Separation Science, 29(3), 405–413.
- Peng, D., Ye, S., Wang, Y., Chen, D., Tao, Y., Huang, L., et al. (2012). Development and validation of an indirect competitive enzyme-linked immunosorbent assay for the screening of tylosin and tilmicosin in muscle, liver, milk, honey and eggs. Journal of Agricultural and Food Chemistry, 60(1), 44–51.
- Pol-Hofstad, I., Driessen-Van Lankveld, W., Tomassen, M., De Jong, J., & Van Egmond, H. (2008). Collaborative study of a microbiological screening method (three-plate) for the banned antimicrobial growth promotors tylosin, virginiamycin, spiramycin, zinc bacitracin and avoparcin in animal feed. Food Additives and Contaminants, 25(12), 1465–1474.
- Prats, C., Francesch, R., Arboix, M., & Perez, B. (2002). Determination of tylosin residues in different animal tissues by high performance liquid chromatography. Journal of Chromatography B, 766(1), 57–65.
- Silverlight, J., Brown, A., & Jackman, R. (1999). Antisera to tilmicosin for use in ELISA and for immunohistochemistry. Food and Agricultural Immunology, 11(4), 321–328.
- Thompson, T.S., Noot, D.K., Calvert, J., & Pernal, S.F. (2003). Determination of lincomycin and tylosin residues in honey using solid-phase extraction and liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Journal of Chromatography A, 1020(2), 241–250.
- Thompson, T.S., Pernal, S.F., Noot, D.K., Melathopoulos, A.P., & van den Heever, J.P. (2007). Degradation of incurred tylosin to desmycosin – Implications for residue analysis of honey. Analytica Chimica Acta, 586(1), 304–311.
- Tong, J., Rao, Q., Zhu, K., Jiang, Z., & Ding, S. (2009). Simultaneous determination of five tetracycline and macrolide antibiotics in feeds using HPCE. Journal of Separation Science, 32(23–24), 4254–4260.
- Verheijen, R., Stouten, P., Cazemier, G., & Haasnoot, W. (1998). Development of a one step strip test for the detection of sulfadimidine residues. Analyst, 123(12), 2437–2441.
- Vincent, U., Gizzi, G., von Holst, C., De Jong, J., & Michard, J. (2007). Validation of an analytical method for the determination of spiramycin, virginiamycin and tylosin in feeding-stuffs by thin-layer chromatography and bio-autography. Food Additives and Contaminants, 24(4), 351–359.
- Wang, X., Li, K., Shi, D., Xiong, N., Jin, X., Yi, J., et al. (2007). Development of an immunochromatographic lateral-flow test strip for rapid detection of sulfonamides in eggs and chicken muscles. Journal of Agricultural and Food Chemistry, 55(6), 2072–2078.
- Wicker, A.L., Mowrey, D.H., Sweeney, D.J., Coleman, M.R., Morris, D.K., & Brockus, C.L. (1994). Particle concentration fluorescence immunoassay for determination of tylosin in premix, feeds, and liquid feed supplement: Comparison with turbidimetric assay. Journal of AOAC International, 77(5), 1083–1095.
- Xie, H.L., Ma, W., Liu, L.Q., Chen, W., Peng, C., Xu, C.L., et al. (2009). Development and validation of an immunochromatographic assay for rapid multi-residues detection of cephems in milk. Analytica Chimica Acta, 634(1), 129–133.
- Zeng, K., Yang, T., Zhong, P., Zhou, S., Qu, L., He, J., et al. (2007). Development of an indirect competitive immunoassay for parathion in vegetables. Food Chemistry, 102(4), 1076–1082.
- Zhang, G., Wang, X., Yang, J., Yang, Y., Xing, G., Li, Q., et al. (2006). Development of an immunochromatographic lateral flow test strip for detection of β-adrenergic agonist clenbuterol residues. Journal of Immunological Methods, 312(1), 27–33.
- Zhang, G., Wang, X., Zhi, A., Bao, Y., Yang, Y., Qu, M., et al. (2008). Development of a lateral flow immunoassay strip for screening of sulfamonomethoxine residues. Food Additives and Contaminants, 25(4), 413–423.
- Zhao, Y., Zhang, G., Liu, Q., Teng, M., Yang, J., & Wang, J. (2008). Development of a lateral flow colloidal gold immunoassay strip for the rapid detection of enrofloxacin residues. Journal of Agricultural and Food Chemistry, 56(24), 12138–12142.
- Zheng, Y., Liu, Y., Guo, H., He, L., Fang, B., & Zeng, Z. (2011). Molecularly imprinted solid-phase extraction for determination of tilmicosin in feed using high performance liquid chromatography. Analytica Chimica Acta, 690(2), 269–274.