Abstract
Glycinin and β-conglycinin are major soybean allergens involved in food hypersensitivity. However, the mechanism of immune responses induced by glycinin and β-conglycinin has not been fully understood. Balb/c mice were oral sensitised with different doses (0.1, 1.0 and 10 mg/day) of soybean glycinin and β-conglycinin for five weeks. Allergen-specific immunoglobulin (Ig), serum histamine and T-cell responses were tested to assess the allergenic activity of glycinin and β-conglycinin. Mice sensitised with 0.1 or 1.0 mg/day allergens induced high levels of specific IgE, IgG1 and serum histamine compared with mice treated with saline. Furthermore, specific T-cell proliferation and significant up-regulation of interleukin (IL)-4, IL-5 and interferon (IFN)-γ were observed in splenocytes from mice gavaged with 0.1 or 1.0 mg/day soybean proteins. Low doses of glycinin or β-conglycinin can induce allergic reactions in BALB/c mice, which might be associated with increased IgE and cytokine production.
Introduction
Soybeans contain approximately 36% to 38% protein with a wide range of applications both in the feed and food industries (Friedman & Brandon, Citation2001). The two major proteins in soybeans are glycinin and β-conglycinin, which stored in vacuoles of seed cells, and account for about 40% of the total seed proteins (Golubovic, van Hateren, Ottens, Witkamp, & van der Wielen, Citation2005). Glycinin and β-conglycinin are of interest, in addition to their anti-nutritional properties, because most of them have been observed to be allergenic (i.e. via IgE responses) in human or laboratory animals (Hou & Chang, Citation2004; Maruyama et al., Citation2001; Petruccelli, Chirdo, & Anon, Citation2005). Surveys have found the prevalence of soy allergy to be nearly 0.5% in the infants of western countries, although most of the allergic infants become tolerant of soy by the age of 3 years (Bock, Citation1987; Bruno et al., Citation1997; Sicherer, Sampson, & Burks, Citation2000). However, with the increasing consumption of soy-containing products, the incidence of soy-caused allergies is expected to increase (FAO, Citation1995).
Soy foods have a long history of safe usage in Asian populations and are generally recognised as safe (US Food and Drug Administration, Citation1999). Merritt and Jenks (Citation2004) suggested that dietary soy protein with isoflavones in soy infant formulas had no adverse effect on human health. However, food allergy caused by the ingestion of soybean products has been documented in many reports about clinical symptoms, which ranged from mild gastrointestinal distress to suspected anaphylaxis (Adachi et al., Citation2003; Helm et al., Citation2000). Nevertheless, unlike peanut allergy, it is uncommon to induce severe and fatal reactions in the soy allergy (Sicherer, Sampson, & Burks, Citation2000).
Much of our understanding of the responses induced by allergens has been obtained from the study of animal models. As a result, pigs (Hao, Zhan, Guo, Piao, & Li, Citation2009; Huang, Xu, Yu, Gao, & Liu, Citation2010; Sun, Li, Dong, Qiao, & Ma, Citation2008; Wang, Qin, Sun, Zhao, & Zhang, Citation2010; Zhao, Qin, Sun, Zhang, & Wang, Citation2010), rats (Guo, Piao, Ou, Li, & Hao, Citation2007; Han, Ma, & Yin, Citation2010) and mice (Gizzarelli et al., Citation2006; Oakes, Piller, & Bost, Citation2009) have been used for study of allergic reactions to soybean allergens. However, to our knowledge, there are few reports available on allergenic effects of purified soybean glycinin and β-conglycinin in mice. In addition, several studies have demonstrated that immune responses after oral sensitisation are influenced by the dosage of allergens (Adel-Patient, Bernard, Ah-Leung, Creminon, & Wal, Citation2005; Capobianco et al., Citation2008), but this has not been reported in soybean-induced anaphylaxis.
Our previous study has demonstrated anaphylactic reactions in BALB/c mice caused by soybean glycinin and β-conglycinin, including increased specific serum antibodies and histamine release (Liu, Feng, Xu, Wang, & Liu, Citation2008). In the present study, we aim to elucidate the effects of various doses of soybean allergens (glycinin or β-conglycinin) on the specific antibody and T-cell responses induced in BALB/c female mice using the established model. Features obtained in our study may provide valuable information for the development of a suitable in vivo model of soybean allergens and for exploring the underlying mechanisms related to soy-induced allergic reactions.
Material and methods
Mice and reagents
Second-generation female BALB/c mice (six-week-old, body weight, 20.0±2.1 g) purchased from the Zhejiang Chinese Medical University (Hangzhou, China) were kept in stainless steel wire cages (4 mice/cage) with soy-free feed and water ad libitum. To avoid oral tolerance, pregnant mice and mice after birth were both fed soy-free chow. Cages were put in a specific pathogen-free room maintained at 22±3°C, with a relative humidity of 55% to 65% and controlled lighting (10 h to 12 h/day) according to the guidelines of the Animal Care and Use Ethics Committee of the Zhejiang University (Hangzhou, China). Purified glycinin and β-conglycinin were offered by Professor Shuntang Guo of the Food Institute of China Agricultural University (Patent No. 200410029589.4, China). The protein samples contained over 95% glycinin and β-conglycinin. The purity was validated by sulfate polyacrylamide gel electrophoresis followed by Quantity OneTM Software (Bio-Rad, Hercules, USA) analysis.
Intragastric antigen sensitisation
Mice were sensitised with glycinin or β-conglycinin as previously described (Liu et al., Citation2008). Briefly, fifty-six mice were divided into seven groups (n=8/group). Mice were sensitised by an intragastric administration of 0.1 mg, 1 mg or 10 mg of soybean glycinin or β-conglycinin/1 ml/mouse daily for 5 weeks without an adjuvant using an 18-gauge stainless steel gavage needle (Popper & Sons, Inc., NY, USA), while control animals received an equal volume of saline. Mice were bled from the retro-orbital plexus on days 0, 7, 14, 21, 28 and 35 using a 2-ml Clot Activator tube (Kremsmunster, Austria) 1 hour after the administration of soybean allergens. Samples were centrifuged for 20 minutes at 2000×g and kept at 4°C to obtain sera. All samples were stored at 20°C until further analysis.
Measurement of soybean allergen-specific IgE, IgG1 and IgG2a levels
Serum allergen-specific IgE, IgG1 and IgG2a were detected by an indirect sandwich-type enzyme-linked immunosorbent assay (ELISA). In brief, 96-well microtiter plates (Costar, Corning Inc., NY, USA) were coated with 100 µl glycinin or β-conglycinin solution (50 µg/ml soybean allergens in 50 mM carbonate buffer (NaHCO3/Na2CO3, pH 9.6)) for 24 hours at 4°C. Plates were washed and blocked with 5% skim milk powder/phosphate buffered saline (PBS) at 37°C for 1 hour. Serum samples diluted in PBS containing 0.05% (s/v) Tween 20 (PBS/Tween) or standards (Sigma Chemical Co., Milan, Italy) were added and incubated for 1 hour at 4°C in 3 duplicates (diluted 1:20 for IgE measurement, 1:40 for IgG1 or IgG2a). Plates were again washed, and the bound antibodies were detected by adding a peroxidase-labelled rat monoclonal anti-mouse IgE (Bethyl, Inc., TX, USA) for 5 hours at room temperature or peroxidase-labelled rat monoclonal anti-mouse IgG1 and IgG2a (Bethyl, Inc., TX, USA) for 1 hour. Secondary antibodies were diluted 1:500. The peroxidase substrate (ortho-phenylenediamine chloride; Sigma-Aldrich, Milan, Italy) was then added, and absorbance at 490 nm was measured by a microtiter reader (Bio-Rad 550, Hercules, USA) after incubated at 37°C for 10 minutes. The data were expressed in optical density units. The ELISA assays were standardised by adding an irrelevant protein (e.g. ovalbumin) as a positive control to test specificity.
Measurement of serum histamine level
Concentrations of serum histamine on days 0, 7, 14, 21, 28 and 35 were determined using an enzyme immunoassay kit (Beckman Coulter) according to the manufacturer's instructions.
Spleen cell culture
After the last blood sampling at week 5, mice from each group were killed by vertebral dislocation, and individual spleens were aseptically removed and minced. After erythrocyte lysis, spleen cells were resuspended in complete medium (RPMI 1640 containing 10% fetal bovine serum, 2 mM L-glutamine, 25 mM Hepes, 100 IU/ml penicillin, 100 mg/ml streptomycin). Spleen lymphocytes from individual mice were used in further analysis of proliferation assays and cytokine measurements.
Spleen cell proliferation
Lymphocyte proliferation assays were performed in triplicate according to 3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) method (Mosmann, Citation1983). Aliquots (100 µl) of splenocyte suspensions at a concentration of 5×106 cells/well were daily stimulated with 50 µl of 0.1, 1, 10 mg/ml glycinin or β-conglycinin, respectively (spleen cells that separated from mice received different daily dosages of glycinin, or β-conglycinin were stimulated in vitro with 50 µl of the same concentration), whereas splenocytes from the mice treated with saline were added with 50 µl of saline as controls (un-stimulated). All cell cultures were incubated in 96-well flat bottom plates (Corning Costar Corp., Cambridge, USA). The plates were covered and incubated for 48 hours at 37°C in a 5% CO2 incubator with 95% humidity. Then, 20 µl of freshly prepared MTT in serum-free RPMI1640 (5 mg/day) was added to each well after 44 hours. Cultures were incubated for a further 4 hours and then solubilisation reagent [100 µl of acidified isopropanol: distilled water (2:1, v/v)] was added. The absorbance was measured at 570 nm, based on the optical wavelengths for differentiating MTT using a microtiter reader (Bio-Rad 550, Hercules, USA) (Kong, Hu, Rui, Wang, & Li, Citation2004; Zhou et al., Citation2005). The stimulation index was calculated as the absorbance ratio of stimulated cells to that of un-stimulated cells.
Quantification of specific cytokines in splenocytes
Spleen lymphocytes prepared as noted above were cultured in 24-well plates (5×106 cells/ml/well), and treated with 50 µl of glycinin or β-conglycinin (0.1, 1 or 10 mg/ml), or un-stimulated (saline treated), respectively, as described above. The cell-free supernatants were collected after 3 days of culture at 37°C with 5% CO2, and aliquots were stored at −80°C until analyzed. Levels of cytokines IFN-γ, IL-4 and IL-5 were determined using commercially available ELISA kits according to the manufacturer's instructions (Biosource, Camarillo, CA, USA). Assays were performed in triplicate.
Statistical analysis
All data in experiment were expressed as the mean±standard deviation (SD) and analyzed by using SPSS 16.0 for Windows (SPSS Inc., Chicago, USA). The differences between the groups were analyzed by one-way ANOVA followed by Turkey's post hoc test. Values of p<0.05 were considered as significant.
Results
Induction of soybean allergen–specific antibody response by oral sensitisation
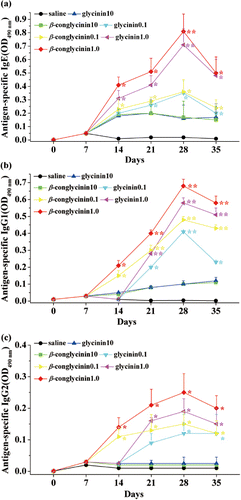
To evaluate soybean protein–induced anaphylactic reactions in sensitised mice, dose-responses of antigen-specific IgE, IgG1 and IgG2a were compared kinetically by ELISA with three different amounts of glycinin or β-conglycinin (0.1, 1.0 and 10 mg/mouse/day) on days 0, 7, 21 and 35 (). The administration of glycinin or β-conglycinin induced soybean-specific IgE antibody after 2 weeks of sensitisation. A clear different IgE level was detected among the three dosages of allergen (p<0.05), with the maximum level in mice administered with 1.0 mg/day of glycinin or β-conglycinin on day 28 (). β-conglycinin, 0.8±0.09; glycinin, 0.72±0.1; p<0.01 vs. control). Then, IgE levels induced by gavage with 0.1 or 1.0 mg/day of soybean proteins were somewhat decreased on day 35. Control mice, immunised with saline, did not produce any detectable level of allergen-specific IgE (). Similarly, levels of specific IgG1 started to increase after week 2, reaching its plateau level in mice treated with 1.0 mg/day of glycinin or β-conglycinin (). glycinin, 0.52±0.04; β-conglycinin, 0.66±0.02; p<0.01 vs. control). Specific IgG2a also increased from day 7 to day 28, but had a low production level (). No allergen-specific IgG responses were induced in control group of mice gavaged with saline. Additionally, no signals could be detected when testing ELISA specificity by irrelevant proteins (e.g., ovalbumin) (data not shown).
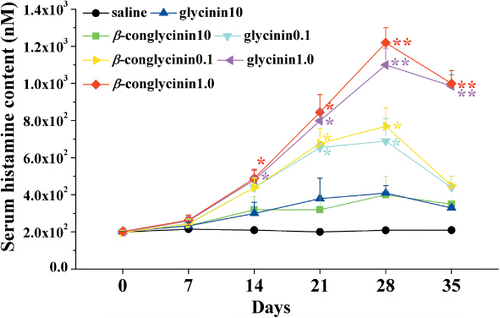
Induction of serum histamine release
We also measured the serum histamine levels 1 hour after soybean allergens sensitisation on days 0, 7, 14, 21, 28 and 35, because histamine is closely associated with the anaphylactic reactions. We found that serum histamine level was rapidly increased (p<0.05) 2 weeks after oral sensitisation with the maximal release at day 28 in mice gavage with 1.0 mg/day glycinin or β-conglycinin (glycinin, 1100±90 nM; β-conglycinin, 1220±80 nM; p<0.01 vs. control). As observed for Ig responses, histamine release was less intense after 28 days, but still showed a significant increase in the 1.0 mg/day-sensitised mice than in the control group gavaged with saline ().
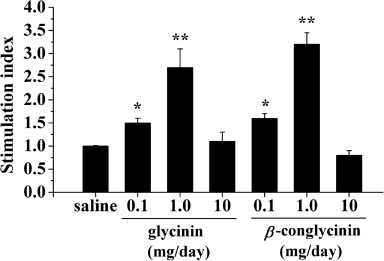
Induction of spleen cell proliferative responses by oral sensitisation
To determine whether oral sensitisation with soybean proteins affected systemic T-cell responses, we assessed spleen cell proliferative responses to glycinin or β-conglycinin stimulation in vitro. As shown in , splenocyte from groups of mice gavaged with 0.1 and 1.0 mg/day glycinin or β-conglycinin depicted significant proliferation activity in response to antigen stimulation (). No differences were observed among splenocytes from the 0.1, 10 mg/day and saline-treated groups (p>0.05).
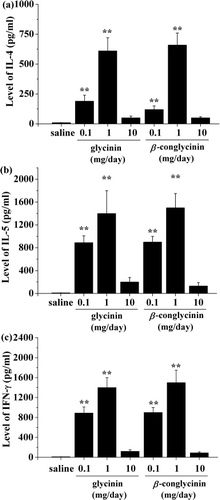
T-cell cytokine profile induced by soybean protein stimulation
To determine the primed pathway of systemic T help (Th)1/Th2 responses, the cytokines produced by splenocyte were measured in culture supernatants. In vitro stimulation with 50 µl of 0.1 and 1 mg/ml glycinin or β-conglycinin induced significant levels of IL-4, IL-5 and IFN-γ compared with control (). Among the three different doses of allergens, splenocytes from mice gavaged with 1 mg/day glycinin or β-conglycinin exhibited the strongest proliferation responses with levels of IL-4 (613.5 pg/ml and 651.5 pg/ml), IL-5 (1384.0 pg/ml and 1530.3 pg/ml) and IFN-γ (1372.0 pg/ml and 1432.1 pg/ml), respectively. Cytokine production after allergen stimulation was not changed in mice administered with 10 mg/day glycinin or β-conglycinin compared with the control mice (p>0.05). Additionally, almost undetectable levels of cytokines were found in culture supernatants of saline-treated cells (un-stimulated cells).
Discussion
The development of in vivo murine models against soybean proteins provides an optimal tool for revealing the possible mechanism of anaphylactic symptoms induced by allergen ingestion. Recently, we have established a suitable BALB/c mice model by oral administration of soybean allergens (Liu et al., Citation2008). In the present study, we demonstrated in the same model that systemic anaphylactic symptoms were induced in sensitised BALB/c mice, and reported the characterisation of the antigen-specific cellular responses in vitro. In addition, we also examined the effect of oral feeding dose on sensitisation in BALB/c mice.
IgE plays an important role in type I hypersensitivity reactions by inducing mast cell degranulation and release of histamine after antigen sensitisation or challenge. IgG1 is considered to be a Th2-associated antibody, whereas IgG2a is recognised as a Th1-associated antibody (Snapper & Paul, Citation1987). Our results showed that mice sensitised with 0.1 and 1 mg/day glycinin or β-conglycinin induced high levels of allergen-specific IgE and IgG1, which are in accordance with Gizzarelli et al. (Citation2006), who sensitised BALB/c mice with soybean extracts and cholera toxin as an adjuvant by oral administration. Christensen, Bruun, and Frokiaer (Citation2003) also reported that soybean protein induced strong levels of specific IgE and IgG1 vs. low levels of IgG2a. This pattern indicates the skewing of the immune responses towards a prevalent Th2-type. On the other hand, Liu et al. (Citation2008) demonstrated an increased level of IgE and IgG1 in mice sensitised with 1.0 mg/day soybean allergens. We further found that low dose given orally induced much stronger antigen-specific IgE, IgG1 and IgG2 responses than 10 mg/day administration of soybean proteins. In fact, the increase in the allergen doses may affect specific IgE levels and plasma histamine concentrations, suggesting an optimisation procedure for allergen administration (Capobianco et al., Citation2008). Our result confirms the statement that oral immunisation requires a low antigen dose to prevent the induction of oral tolerance (Akiyama et al., Citation2001; van Wijk & Knippels, Citation2007). Compared with the data of Liu et al. (Citation2008), we interestingly found that the kinetics of antibody production demonstrated a falling from the fourth week while the antigen intake was done for a week further (five weeks). This is consistent with the serum histamine release observed by Liu et al. (Citation2008), suggesting that mice might develop oral tolerance after the continuous administration of antigens (Stokes et al., Citation1983). However, there is no published evidence that feeding normal laboratory chow (containing soybean proteins) is detrimental to adult mice. This discrepancy may be due to the pre-limitation of soybean proteins in the diets of the pregnant mice and their newborn mice. Additionally, more general aspects, including the origin of mouse strain and the maintenance conditions, also exert an influence on optimal in vivo sensitisation. The variability of these factors may underline the difficulty to develop animal models of food allergy.
Histamine regulates body temperature by function on histaminergic neurons in the central nervous system (Makabe-Kobayashi et al., Citation2002). The increased histamine level could reflect mast cell degranulation and is considered to be one of the major indicators of rapid allergic reactions. Interestingly, BALB/c mice displayed a maximal increase of serum histamine level at day 28 when induced by 1.0 mg/day soybean allergens compared with those gavaged with 0.1 or 10 mg/day. This appears to correlate mainly with the increase of specific IgE and IgG1 in BALB/c mice, suggesting a dose-dependent difference in histamine release in our model. Our result also consists with the serum histamine level responding to ovalbumin in B10A mice, which also occurred in a dose-dependent manner (Akiyama et al., Citation2001).
Besides the effect on B cells, oral administration of glycinin or β-conglycinin influenced T-cell proliferative responses. We found that splenocytes from mice treated with 0.1 and 1.0 mg/day soybean proteins (glycinin or β-conglycinin) exhibited significant proliferative responses compared with those from other groups. Previous study has reported that splenocytes may develop resistance to spontaneous apoptosis by specific antigen or cytokines induced by allergens (Budd, Citation2002), which might be consistent with our results.
To investigate the role of Th1/Th2 cytokines in systemic immune responses in BALB/c mice orally induced by soybean glycinin and β-conglycinin, IL-4, IL-5 and IFN-γ in splenocytes were measured. It is well known that IFN-γ, IL-4 and IL-5 play pivotal roles in the development, differentiation and maintenance of the allergic response. Therefore, the identification of the cytokine profiles from spleen cells in vitro may help to determine the pathway whereby allergen-specific antibodies were induced. In the current study, a significant production of IL-4, IL-5 and IFN-γ were observed in vitro cell culture when stimulated by 50 µl of 0.1 mg/ml or 1 mg/ml soybean proteins. IFN-γ belongs to Th1 cytokines, which favors IgG2a production, while IL-4 and IL-5 are strong promoters of switching on IgE and IgG1 production, which belongs to Th2 cytokines (Howard et al., Citation1983). Thus, the result seems to suggest that a mixed Th1/Th2 response was stimulated. It is uncommon, to our knowledge, to find a high IFN-γ level combined with a low content of IgG2a after sensitisation. Although similar results were reported by Hofman (Citation1995), who demonstrated that serum IL-4 and IFN-γ levels were twice and four times as high in children with food allergy as those in the control group, the results may still not be fully explained by the T lymphocytes differentiation paradigm (Mosmann & Coffman, Citation1989). Stevens et al. (Citation1988) suggested that other factors may be involved for inducing the levels of IgG2a production in vivo. Therefore, further studies into other splenocytes development pathway will be necessary. Additionally, our study focused on systemic immune responses induced by soybean allergens. Cytokine expression in other tissues, such as mesenteric lymph nodes, which provides valuable information of local immune responses, may also help to explain the pattern in cytokine response induced by allergen-specific stimulation of spleen cells.
The model exhibited that lower doses of soybean allergens induced more severe immune responses than the higher sensitising dose (10 mg/day). Especially, 10 mg/day administration of soybean allergens was inefficient in priming the BALB/c mice for subsequent systemic immune responses. This is consistent with other studies showing that high allergen doses induce tolerance (Capobianco et al., Citation2008; Hao et al., Citation2009). These data provide evidence that the administration of the higher dose of glycinin or β-conglycinin through the oral route is capable of eliciting prevalent type 2-dependent antibody responses in BALB/c mice, which prevents persistent tissue injury (Cardoso et al., Citation2008). Similarly, Akiyama et al. (Citation2001) suggested that a low antigen dose and rapid antigen intake are required for oral immunisation. But the mechanism of this effect is still not fully understood due to the shortage of literatures. The animal diet and the maintenance conditions also exert an influence on optimal in vivo sensitisation (Capobianco et al., Citation2008). On the other hand, low and high doses are relative and may be changed in the different animals. Therefore, we can not guarantee that an increase of soybean meal in feed composition will reduce the allergic diarrhoea in animals. Further studies will be required to clarify the effects of different allergen doses on gut immune responses in our model.
Additionally, several mechanisms whereby oral tolerance occurs have been described, including T-cell active suppression and deletion (Faria & Weiner, Citation2005). There is solid evidence that a high dose of antigen induces clonal deletion and active suppression (Whitacre, Gienapp, Orosz, & Bitar, Citation1991). However, the underlying cellular mechanisms of oral tolerance and possible sites of oral tolerance induction remain to be answered. Our result suggests that a long-lasting administration of high-dose soybean proteins would be used to develop a tolerance model. In future studies, optimisation of oral administration techniques will be needed for building such a model.
We acknowledged that the results are somewhat in conflict with Gizzarelli et al. (Citation2006) and Oakes et al. (Citation2009), who detected no allergen-specific response in mice immunised with soybean protein alone. The discrepancy is probably due to the differences in animal sensitisation procedures, as mice used in these experiments received allergens on separated days. Other main contributing factors could also affect the outcome of the anaphylactic response, including different source of allergens, composition of food, and conditions of maintenance of the mice (Chatel, Song, Bhogal, Orson, Citation2003). Moreover, minipig models have already been established to investigate the anaphylactic reactions induced by soybean glycinin or β-conglycinin without any adjuvant (Sun et al., Citation2008).
Taken together, our study demonstrated that low doses (0.1 or 1.0 g/day) of glycinin or β-conglycinin for BALB/c mice could induce allergic reactions, including high levels of allergen-specific IgE and IgG1 associated with a concomitant increase of IL-4, IL-5 and IFN-γ production in spleen cells stimulated in vitro. Our results also suggest that BALB/c mice are suitable in vivo models for investigating the allergic responses caused by soybean protein.
Acknowledgements
We thank Jiang-Wu Tang and Xin-Ming Wu for their valuable technical supports to this study. The work was supported by the Key Science Project “973”Award (Grant Agreement 2004CB117506), the Key Science Project Award (Grant Agreement 30671524) from National Science and Technology Committee, and Zhejiang Provincial Natural Science Foundation (Grant No. Q12C200002), PR China.
References
- Adachi, M., Kanamori, J., Masuda, T., Yagasaki, K., Kitamura, K., Mikami, B., & Utsumi, S. (2003). Crystal structure of soybean 11S globulin: Glycinin A3B4 homohexamer. Proceedings of the National Academy of Sciences of the United States of America, 100, 7395–7400.
- Adel-Patient, K., Bernard, H., Ah-Leung, S., Creminon, C., & Wal, J. M. (2005). Peanut- and cow's milk-specific IgE, Th2 cells and local anaphylactic reaction are induced in Balb/c mice orally sensitized with cholera toxin. Allergy, 60, 658–664.
- Akiyama, H., Teshima, R., Sakushima, J., Okunuki, H., Goda, Y., Sawada, J., & Toyoda, M. (2001). Examination of oral sensitization with ovalbumin in Brown Norway rats and three strains of mice. Immunology Letters, 78, 1–5.
- Bock, S. A. (1987). Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics, 79, 683–688.
- Bruno, G., Giampietro, P. G., Del Guercio, M. J., Gallia, P., Giovannini, L., Lovati, C., & Businco, L. (1997). Soy allergy is not common in atopic children: a multicenter study. Pediatric Allergy and Immunolog, 8, 190–193.
- Budd, R. C. (2002). Death receptors couple to both cell proliferation and apoptosis. Journal of Clinical Investigation, 109, 437–441.
- Capobianco, F., Butteroni, C., Barletta, B., Corinti, S., Afferni, C., Tinghino, R., & Di Felice, G. (2008). Oral sensitization with shrimp tropomyosin induces in mice allergen-specific IgE, T cell response and systemic anaphylactic reactions. International Immunology, 20, 1077–1086.
- Cardoso, C. R., Teixeira, G., Provinciatto, P. R., Godoi, D. F., Ferreira, B. R., Milanezi, C. M., & Silva, J. S. (2008). Modulation of mucosal immunity in a murine model of food-induced intestinal inflammation. Clinical and Experimental Allergy, 38(2), 338–349.
- Chatel, J. M., Song, L., Bhogal, B., & Orson, F. M. (2003). Various factors (allergen nature, mouse strain, CpG/recombinant protein expressed) influence the immune response elicited by genetic immunization. Allergy, 58, 641–647.
- Christensen, H. R., Bruun, S. W., & Frokiaer, H. (2003). Antigenic specificity of serum antibodies in mice fed soy protein. International Archives of Allergy and Immunology, 132, 58–67.
- Faria, A. M., & Weiner, H. L. (2005). Oral tolerance. Immunological Reviews, 206, 232–259.
- FAO. (1995). Food and agriculture organization of the United Nations. Report of the FAO technical consultation on food allergies, Rome, Italy.
- Friedman, M., & Brandon, D. L. (2001). Nutritional and health benefits of soy proteins. Journal of Agricultural and Food Chemistry, 49, 1069–1086.
- Gizzarelli, F., Corinti, S., Barletta, B., Iacovacci, P., Brunetto, B., Butteroni, C., & Tinghino, R. (2006). Evaluation of allergenicity of genetically modified soybean protein extract in a murine model of oral allergen-specific sensitization. Clinical and Experimental Allergy, 36, 238–248.
- Golubovic, M., van Hateren, S. H., Ottens, M., Witkamp, G. J., & van der Wielen, L. A. M. (2005). Novel method for the production of pure glycinin from soybeans. Journal of Agricultural and Food Chemistry, 53, 5265–5269.
- Guo, P. F., Piao, X. S., Ou, D. Y., Li, D. F., & Hao, Y. (2007). Characterization of the antigenic specificity of soybean protein beta-conglycinin and its effects on growth and immune function in rats. Archives of Animal Nutrition, 61, 189–200.
- Han, P. F., Ma, X., & Yin, J. D. (2010). The effects of lipoic acid on soybean -conglycinin-induced anaphylactic reactions in a rat model. Archives of Animal Nutrition, 64, 254–264.
- Hao, Y., Zhan, Z. F., Guo, P. F., Piao, X. S., & Li, D. F. (2009). Soybean beta-conglycinin-induced gut hypersensitivity reaction in a piglet model. Archives of Animal Nutrition, 63, 188–202.
- Helm, R. M., Cockrell, G., Connaughton, C., Sampson, H. A., Bannon, G. A., Beilinson, V., & Burks, A. W. (2000). A soybean G2 glycinin allergen – 1. Identification and characterization. International Archives of Allergy and Immunology, 123, 205–212.
- Hofman, T. (1995). IL-4 and IFN-gamma level in blood serum of children with food allergy. Rocz Akad Med Bialymst, 40, 462–467.
- Hou, D. H., & Chang, S. K. C. (2004). Structural characteristics of purified glycinin from soybeans stored under various conditions. Journal of Agricultural and Food Chemistry, 52, 3792–3800.
- Howard, M., Mizel, S. B., Lachman, L., Ansel, J., Johnson, B., & Paul, W. E. (1983). Role of interleukin-1 in anti-immunoglobulin-induced B-cell proliferation. Journal of Experimental Medicine, 157, 1529–1543.
- Huang, Q., Xu, H. B., Yu, Z., Gao, P., & Liu, S. (2010). Inbred Chinese wuzhishan (WZS) minipig model for soybean glycinin and beta-conglycinin allergy. Journal of Agricultural and Food Chemistry, 58, 5194–5198.
- Kong, X. F., Hu, Y. L., Rui, R., Wang, D. Y., & Li, X. G. (2004). Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. International Immunopharmacology, 4, 975–982.
- Liu, X., Feng, J., Xu, Z. R., Wang, Y. Z., & Liu, J. X. (2008). Oral allergy syndrome and anaphylactic reactions in BALB/c mice caused by soybean glycinin and beta-conglycinin. Clinical and Experimental Allergy, 38, 350–356.
- Makabe-Kobayashi, Y., Hori, Y., Adachi, T., Ishigaki-Suzuki, S., Kikuchi, Y., Kagaya, Y., & Ohtsu, U. (2002). The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. Journal of Allergy and Clinical Immunology, 110, 298–303.
- Maruyama, N., Adachi, M., Takahashi, K., Yagasaki, K., Kohno, M., Takenaka, Y., & Utsumi, S. (2001). Crystal structures of recombinant and native soybean beta-conglycinin beta homotrimers. European Journal of Biochemistry, 268, 3595–3604.
- Merritt, R. J., & Jenks, B. H. (2004). Safety of soy-based infant formulas containing isoflavones: The clinical evidence. Journal of Nutrition, 134, 1220S–1224S.
- Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55–63.
- Mosmann, T. R., & Coffman, R. L. (1989). TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annual Review of Immunology, 7, 145–173.
- Oakes, J. L., Piller, K. J., & Bost, K. L. (2009). An antibody response to cholera toxin, but not soy proteins, following oral administration of adjuvanted soybean formulations. Food and Agricultural Immunology, 20, 305–317.
- Petruccelli, S., Chirdo, F. G., & Anon, M. C. (2005). Immunochemical reactivity of soybean β-conglycinin subunits. Food and Agricultural Immunology, 16, 17–28.
- Sicherer, S. H., Sampson, H. A., & Burks, A. W. (2000). Peanut and soy allergy: A clinical and therapeutic dilemma. Allergy, 55, 515–521.
- Snapper, C. M., & Paul, W. E. (1987). B-cell stimulatory factor-I (Bsf-1) interleukin-4 acts on resting murine B-cells to enhance IgG1 secretion upon subsequent addition of bacterial lipopolysaccharide. Federation Proceedings, 46, 920–920.
- Stevens, T. L., Bossie, A., Sanders, V. M., Fernandezbotran, R., Coffman, R. L., Mosmann, T. R., & Vitetta, E. S. (1988). Regulation of antibody isotype secretion by subsets of antigen-specific helper T-cells. Nature, 334, 255–258.
- Stokes, C. R., Swarbrick, E. T., & Soothill, J. F. (1983). Genetic-differences in immune exclusion and partial tolerance to ingested antigens. Clinical and Experimental Immunology, 52, 678–684.
- Sun, P., Li, D. F., Dong, B., Qiao, S. Y., & Ma, X. (2008). Effects of soybean glycinin on performance and immune function in early weaned pigs. Archives of Animal Nutrition, 62, 313–321.
- US Food and Drug Administration. (1999). Food labeling, health claims, soy protein and coronary heart disease. Federal Register, 57, 699–733.
- van Wijk, F., & Knippels, L. (2007). Initiating mechanisms of food allergy: Oral tolerance versus allergic sensitization. Biomedicine & Pharmacotherapy, 61, 8–20.
- Wang, T., Qin, G., Sun, Z., Zhao, Y., & Zhang, B. (2010). Comparative study on the residual rate of immunoreactive soybean glycinin (11S) in the digestive tract of pigs of different ages. Food and Agricultural Immunology, 21, 201–208.
- Whitacre, C. C., Gienapp, I. E., Orosz, C. G., & Bitar, D. M. (1991). Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. Journal of Immunology, 147, 2155–2163.
- Zhao, Y., Qin, G. X., Sun, Z. W., Zhang, B., & Wang, T. (2010). Effects of glycinin and beta-conglycinin on enterocyte apoptosis, proliferation and migration of piglets. Food and Agricultural Immunology, 21, 209–218.
- Zhou, J. Y., Wang, J. Y., Chen, J. G., Wu, J. X., Gong, H., Teng, Q. Y., & Shen, H. G. (2005). Cloning, in vitro expression and bioactivity of duck interleukin-2. Molecular Immunology, 42, 589–598.