Abstract
Aflatoxin M1 (AFM1) levels in milk and milk products (kasar cheese, tulum cheese, dil cheese, cream cheese, white cheese) consumed in Turkey were determined in this study by using enzyme-linked immunosorbent assay (ELISA). Extraction procedure was optimised to obtain high extraction efficiency. Recovery experiment was also performed to test the suitability of the extraction system and it was observed that % recovery values for AFM1 were higher than 92%. The method used in this study was simple and suitable for the detection of AFM1 in these matrices. Results found from the samples were compared with the requirements of Turkish Food Codex (TFC). As much as 42.16% of cheese samples analysed were contaminated while 61/77 milk samples were found to be contaminated with AFM1 in the range of 0.005–0.410 µg/L. It was found that five white cheese, one kasar cheese and four milk samples exceeded the criteria of 0.5 μg/kg for cheese and 0.05 μg/L for milk set by TFC.
Introduction
Aflatoxin species as mycotoxin are difuranocoumarin derivatives which are produced by Aspergillus flavus and Aspergillus parasiticus through a polyketide pathway (Afsah-Hejri, Jinap, Arzandeh, & Mirhosseini, Citation2011; Bakirdere et al., Citation2012; Turner, Subrahmanyam, & Piletsky, Citation2009). Aflatoxin species are known to be mutagen, carcinogen and teratogen compounds and ingestion through the dietary channel is the main source of these mycotoxins for both human and animals. It might be very dangerous to take these mycotoxins even in very trace levels for a long-time period (Bakirdere et al., Citation2012; Papp, H-Otta, Záray, & Mincsovics, Citation2002). In the event that aflatoxin B1 (AFB1) contaminated feed are taken by cattle, AFB1 is transformed into Aflatoxin M1 (AFM1) as its hydroxylated product and then secreted in the milk. Hepatotoxic and carcinogenic effects of AFM1 are known in literature (Kanungo, Pal, & Bhand, Citation2011). During the milk pasteurisation, storage and the preparation of various dairy products, AFM1 is known to be stable (Badea et al., Citation2004; Codex Committee, Citation2001). AFM1 is known as a strong genotoxic and hepatotoxic agent and AFM1 has been evaluated as possible human carcinogen (group 2B) and evident carcinogen (group 1) in the case of mixing with aflatoxins B1 and G1 [International Agency for Research on Cancer (IARC), Citation1997]. Maximum residual limits for certain contaminants were set by European Regulation 1881/2006. Legal limits for aflatoxins change significantly from country to country as well as mycotoxin type and matrix (European Commission, Citation2006; Rahmani, Jinap, & Soleimany, Citation2010). Turkish legislation imposes maximum permissible levels of AFM1 as 0.05 µg/L in milk and 0.5 µg/kg for cheese samples (Turkish Food Codex, Citation2008).
Due to the carcinogenicity of this compound, determination of AFM1 in any matrices related with the human at trace levels is very crucial. There are some methods in literature to analyse the samples for their AFM1 contents. Thin layer chromatography (TLC; Shundo & Sabino, Citation2006), high performance liquid chromatography (HPLC; Shundo & Sabino, Citation2006), ultra performance liquid chromatography (UPLC; Frenich, Martínez Vidal, Romero-González, & Aguilera-Luiz, Citation2009), liquid chromatography–tandem mass spectrometry (LC-MS/MS; Aguilera-Luiz, Plaza-Bolaños, Romero-González, Martínez Vidal, & Garrido Frenich, Citation2011), enzyme-linked immunosorbent assay (ELISA; Rastogi, Dwivedi, Khanna, & Das, Citation2004; Yaroglu, Oruc, & Tayar, Citation2005) are some of the methods used in literature for the determination of AFM1 in variety of matrices. ELISA is commercially available for the determination of mycotoxins including AFM1. In ELISA measurement system, specific antibodies are binding to a solid support. Due to its benefits, this measurement system is very popular in literature for both quantification and screening of aflatoxin species (Goryacheva, de Saeger, Eremin, & Van Peteghem, Citation2007). Very low detection limits can be obtained by using this system for AFM1. Although ELISA method has some drawbacks such as matrix interferences compared with the chromatographic methods, many advantages of ELISA system like low detection limits, low sample volume requirement, high sample throughput, high speed, high sensitivity and easy application raise its popularity over other methods. It is also know that chromatographic methods require not only expensive instrumentation but expertise in this field as well. Hence, ELISA demanding minimum sample clean-up is the practical alternative method for routine analysis of food products (Bakirdere et al., Citation2012).
The main objective of this work was to determine the AFM1 levels in milk and milk products (kasar cheese, tulum cheese, dil cheese, cream cheese, white cheese) consumed in Turkey to reveal the reliableness of these products in this region.
Experimental procedure
Materials and methods
Chemicals and instrumentation
All of the chemicals used were in analytical reagent grade. Sodium phosphate dibasic anhydrous (Fluka), sodium hydrogen phosphate-2-hydrate (Riedel), absolute methanol (J.T.Baker), sodium chloride (Merck), dichlorometan (Merck), n-heptane (Merck) were used as chemicals in the extraction and sample preparation studies. All of the working solutions were daily prepared and stored at proper conditions. Ridascreen brands of AFM1 ELISA test kit was used in measurement step. In the ELISA measurement, Bio-Tek ELx800 brand ELISA instrument was used at 450 nm. Nüve NS 112 water purification system was used for the production of pure water. In the extraction studies, BagMixer brand of stomacher instrument was used to extract the AFM1 contents from the matrix. Rocker hat plate was used to dry up the extracts. In the centrifugation, Nüve 1215 brand instrument was used. Whatman filter paper was used in the filtration studies.
Sample collection and storage
Kasar cheese (40), tulum cheese (16), dil cheese (22), cream cheese (21), white cheese (67) and milk samples (77) were obtained from the west part of Turkey (Kocaeli, Sakarya, Düzce) for the analysis. Samples were taken in original packages (if any) and transferred to the laboratory. Samples were stored in a refrigerator (2–4°C) until time for measurements. Sampling points are shown in . It is clear in that all the sample points are in the Black Sea Region. In this region of Turkey, temperatures range from −8°C to 40°C with an average humidity of 72%. Quite often, it is cloudy which seems to suit to form the aflatoxin species.
Methods
Extraction of AFM1 from cheese samples
Optimum extraction conditions were applied to cheese samples to extract the AFM1 in high extraction yield. In the extraction of AFM1 from the cheese matrix, about 2.0 g of representative cheese sample were weighed and put into stomacher bag. Forty millilitres of dichloromethane was added to sample and extraction was carried out throughout 15 min at room temperature by using stomacher instrument. After the extraction procedure, the entire extract was filtered by using Whatman filter paper. Ten millilitres of extract was taken and dried up under nitrogen gas by using hat plate and then 0.5 mL of methanol, 0.50 mL of PBS buffer and 1.0 mL n-heptane were added to dried sample. Sample was centrifuged at 2700 rpm for 15 min and then n-heptane was removed from the matrix. One hundred microlitres aliquot of the lower methanolic phase was taken via Pasteur pipette and 400 µL of buffer 1 solution was added to dilute the extract. One hundred microlitres extract was used in the ELISA kit study.
For milk, samples were cooled at 108°C for 60 min and then centrifuged at 3500 rpm throughout 10 min for degreasing. Upper cream layer was separated from the milk sample by using Pasteur pipette to obtain skimmed milk. One hundred microlitres of milk sample were used in ELISA measurements.
ELISA measurement system
The principle of the ELISA system is based on the antigen–antibody reaction. Plate well is coated with the specific antibody added to plate, target toxin, if any, compete for the antibody, thereby preventing antibodies against AFM1. Both free AFM1 and AFM1 enzyme conjugate compete for the AFM1 antibody. In the washing step, unbounded enzyme conjugate is removed. After the addition of substrate/chromogen to the wells, chromogen is converted to a blue colour product by the effect of enzyme conjugate. Stop solution converts the colour from blue to yellow and photometric measurement is performed at 450 nm (CitationRidascreen manual).
In the AFM1 measurements, 5.0, 10, 20, 40 and 80 ng/L of standards were used to obtain a calibration curve. Before the analysis, AFM1 enzyme conjugate solution and washing buffer were prepared according to the description in the test manual. In the test protocol, microtiter wells were inserted to plate and 100 µL of each standard and extraction solutions were separately added to the wells. The plate was manually shaken and incubated at room temperature for 30 min. After the incubation period, wells were washed three times with 250 µL of washing solution. One hundred microlitres diluted enzyme conjugate was added and then plate was again manually shaken and incubated at room temperature for 15 min. Wells were washed with 250 µL of washing solution three times. After the washing step, 100 µL of substrate/chromogen was added to each well. Plate was gently shaken and then samples were incubated for 15 min in the dark medium. One hundred microlitres of stop solution was added to the each well to stop the reaction and then the plate was shaken manually. Measurements were performed at 450 nm as soon as possible after adding a stop buffer.
Result and discussion
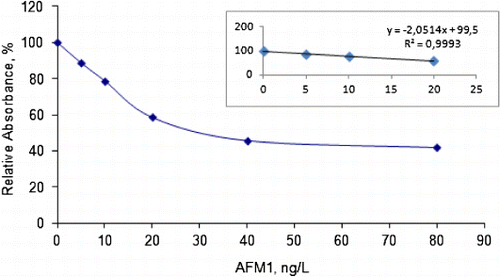
Consumption of milk and milk products is very high in Turkey like in other parts of the world. Hence, determination of AFM1 in these samples is very important. In this study, milk and cheese samples were analysed for their AFM1 contents using the procedure given above. For each sample, three replicates were analysed. In the quantitative measurements, 5.0, 10, 20, 40, 80 ng/L of AFM1 standards were used to obtain calibration curve. In case, AFM1 concentration in the sample is not in the calibration range, samples were further diluted by sample dilution buffer. A typical calibration curve obtained from ELISA can be seen in .
Limit of detection for the method is 5.0 ng/L and 50 ng/kg for milk and cheese samples, respectively. Recovery experiments were also performed before each set of analysis for aflatoxins B1 carried out at the level of 60 ng/L. It was observed that recovery values for AFM1 were higher than 92% with relative standard deviation ranged between 3.4 and 9.2. Recovery experiments showed that dichloromethane as extraction solution is efficient for the extraction of AFM1 from the sample matrix.
Aflatoxin M1 results
AFM1 results for kasar cheese, tulum cheese, dil cheese, cream cheese, white cheese and milk (243 samples) can be seen in . According to Turkish Food Codex (TFC), AFM1 should not be present in milk and cheese samples at the level of 0.05 and 0.50 µg/kg, respectively (Turkish Food Codex, Citation2008). Determination of AFM1 as mutagen, carcinogen and teratogen compounds in milk and cheese samples is very important due to its toxicity. In literature, there are many studies in this field. Some of the studies related with the AFM1 are given in . As an example, Sarımehmetoglu, Kuplulu, and Celik (Citation2004) analysed 400 cheese samples consumed in Turkey (100 samples each of white, kashar, tulum and processed cheese) for their AFM1 contents. AFM1 levels were determined in different concentrations in the 327 (81.75%) cheese samples [white (82%), tulum (81%), kashar (85%) and processed cheese (79%)]. One hundred and ten cheese samples (27.5%) were found to have levels that exceed the legal limits set by the TFC (27% of white cheese, 24% of tulum cheese, 34% of kashar cheese and 25% of processed cheese) (Sarımehmetoglu et al., Citation2004). In our study, 2.5% of kasar cheese exceeded the TFC limit of 0.50 µg/kg and detectable levels of AFM1 were found in 50% of the samples interested as in the range of 0.05–0.70 µg/kg. Sixteen tulum cheese were analysed for their AFM1 contents. Although AFM1 is present in tulum cheese with the frequency of 18.75% (0.05–0.10 µg/kg), none of the tulum cheese exceeded the criteria of 0.50 µg/kg (Turkish Food Codex, Citation2008). It is clear in that detected AFM1 in dil cheese samples were found in three samples corresponding to 13.63% of the samples analysed and found in the range of 0.10–0.20 µg/kg. All of the dil cheese samples interested were found to meet TFC (0.50 µg/kg). In cream cheese samples, detectable AFM1 level was found to be in the range of 0.05–0.16 µg/kg in 21 samples. It was found that 38.09% of samples analysed are positive for AFM1 and AFM1 concentrations, if found, were far below the legal limits of 0.50 µg/kg. A total of 67 samples of white cheese were also analysed. Detectable AFM1 levels in the samples were as high as 53.73% and concentrations were between 0.05 and 2.10 µg/kg. Five contaminated samples (7.46%) were over the TFC limit for cheese as 0.50 µg/kg. It is clear that a number of the samples which were analysed in our study exceeded the Turkish legal limit and the results are much lower than the results found by Sarımehmetoglu et al. (Citation2004). In other study, Amer determined AFM1 in 150 cheese samples (50 of soft cheese, hard cheese and processed cheese) from Egyptian supermarkets in Alexandria city. It was found that AFM1 was found in 40% of soft cheese, 38% of hard cheese and 22% of processed cheese. In general, 33.3% of cheese samples were AFM1 positive (Amer & Ibrahim, Citation2010) while 42.16% of cheese samples were contaminated with AFM1 in our study.
Table 1. AFM1 results of milk and cheese samples.
Table 2. Some examples of AFM1 studies in the literature.
The survey of AFM1 in 77 whole milk samples showed that the levels of AFM1 in 94.8% of milk samples analysed were below maximum tolerance level accepted by TFC. Levels of AFM1 of 77 milk samples were in the range of 0.005–0.410 µg/L. The highest concentration in milk samples was found to be 0.410 µg/L. Although AFM1 was detected in 61 samples (79.22%), only 4 samples among them were contaminated at a level above the maximum permissible limit of 0.05 µg/L. In Argentina, 77 milk samples were analysed and 18 of 77 milk samples were found to be contaminated at a level <50 ng/kg (Lopes, Ramos, Ramadan, & Bulacio, Citation2003). In the another study, out of the 42 milk samples, 10 samples (24%) were contaminated by AFM1 in Brazil. In addition, 7% were found to be above the 0.5 µg/L (Sassahara, Netto, & Yanaka, Citation2005). It is clear that our milk samples analysed were contaminated with AFM1 in higher ratios when compared with these two studies given above.
These results show high occurrence of AFM1 in milk and milk products, which might be attributed to a good monitorisation in preventing the contamination of moulds and AFM1 in cheese production.
Conclusions
Determination of AFM1 in milk and different cheese samples were performed in this study. Results show that the extraction procedure and detection system used can be successfully applied as a rapid method for screening of AFM1 in milk and milk products. Detectable AFM1 concentration in kasar samples was found to be in the range of 0.05–0.70 µg/kg. AFM1 was not detected in 13 of 16 tulum (81.25%) and 19 of 22 dil cheese (86.36) samples. Five white cheese samples exceeded the criteria set by TFC while all of the cream cheese samples analysed were found to meet TFC. The detectable AFM1 in milk samples was found in 61 samples corresponding to 79.22% of the total milk samples analysed. Although 53.9% of the milk and cheese samples investigated were contaminated with AFM1, most of the samples interested meet the requirements set by the Turkish government. Four milk and six cheese samples exceeded the criteria of 0.50 µg/kg for cheese and 0.05 µg/L for milk set by Turkish Food Codex (Citation2008).
References
- Afsah-Hejri, L., Jinap, S., Arzandeh, S., & Mirhosseini, H. (2011). Optimization of HPLC conditions for quantitative analysis of aflatoxins in contaminated peanut. Food Control, 22, 381–388.
- Aguilera-Luiz, M. M., Plaza-Bolaños, P., Romero-González, R., Martínez Vidal, J. L., & Garrido Frenich, A. (2011). Comparison of the efficiency of different extraction methods for the simultaneous determination of mycotoxins and pesticides in milk samples by ultra high-performance liquid chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 399, 2863–2875.
- Amer, A. A., & Ibrahim, M. A. E. (2010). Determination of aflatoxin M1 in raw milk and traditional cheeses retailed in Egyptian markets. Journal of Toxicology and Environmental Health Sciences, 2, 50.
- Badea, M., Micheli, L., Messia, M. C., Candigliota, T., Marconi, E., Mottram, T., & Palleschi, G. (2004). Aflatoxin M1 determination in raw milk using a flow-injection immunoassay system. Analytica Chimica Acta, 520, 141–148.
- Bakirdere, S., Bora, S., Bakirdere, E. G., Aydin, F., Arslan, Y., Komesli, O. T., Yildirim, E. (2012). Aflatoxin species: Their health effects and determination methods in different foodstuffs. Central European Journal of Chemistry, 10, 675–685.
- Codex Committee. 2001. Codex Committee on Food Additives and Contaminants, 2001 (CL CX/FAC 01/20). Comments submitted on the Draft Maximum Level for Aflatoxin M1 in Milk. the Netherlands: FAO/WHO.
- Colak, H., Hampikyan, H., Ulusoy, B., & Ergun, O. (2006). Comparison of a competitive ELISA with an HPLC method for the determination of aflatoxin M1 in Turkish white, kasar and tulum cheeses. European Food Research and Technology, 223, 719–723.
- European Commission. (2006). Commission regulation (EC) no 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Official Journal of European Union, L70/12, 19–23.
- Fallah, A. A. (2010). Aflatoxin M-1 contamination in dairy products marketed in Iran during winter and summer. Food Control, 21, 1478–1481.
- Frenich, A. G., Martínez Vidal, J. L., Romero-González, R., & Aguilera-Luiz, M. M. (2009). Simple and high-throughput method for the multimycotoxin analysis in cereals and related foods by ultra-high performance liquid chromatography/tandem mass spectrometry. Food Chemistry, 117, 705–712.
- Goryacheva, I. Y., de Saeger, S., Eremin, S. A., & Van Peteghem, C. (2007). Immunochemical methods for rapid mycotoxin detection: Evolution from single to multiple analyte screening: A review. Food Additives and Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 24, 1169–1183.
- International Agency for Research on Cancer (IARC). (1997). Monographs on the evaluation of carcinogenic risks to humans (Vol. 56). Lyon: Author.
- Kanungo, L., Pal, S., & Bhand, S. (2011). Miniaturised hybrid immunoassay for high sensitivity analysis of aflatoxin M1 in milk. Biosensors and Bioelectronics, 26, 2601–2606.
- Kim, H. J., Lee, J. E., Kwak, B. M., Ahn, J. H., & Jeong, S. H. (2010). Occurrence of aflatoxin m-1 in raw milk from south korea winter seasons using an immunoaffinity column and high performance liquid chromatography. Journal of Food Safety, 30, 804–813.
- Lopes, C. E., Ramos, L. L., Ramadan, S. S., & Bulacio, L. C. (2003). Presence of aflatoxin M1 in milk for human consumption in Argentina. Food Control, 14, 31–34.
- Nuryono, N., Agus, A., Wedhastri, S., Maryudani, Y. B., Setyabudi, F. M. C. S., Bohm, J., & Razzazi-Fazeli, E. (2009). A limited survey of aflatoxin M1 in milk from Indonesia by ELISA. Food Control, 20, 721–724.
- Papp, E., H-Otta, K., Záray, G., & Mincsovics, E. (2002). Liquid chromatographic determination of aflatoxins. Microchemical Journal, 73, 39–46.
- Prado, G., de Oliveira, M. S., Lima, A. S., & Moreira, A. P. A. (2008). Occurrence of aflatoxin M-1 in parmesan cheese consumed in Minas Gerais, Brazil. Cience E Agrotecnologia, 32, 1906–1911.
- Rahimi, E., Karim, G., & Shakerian, A. (2009). Occurrence of aflatoxin M-1 in traditional cheese consumed in Esfahan, Iran. World Mycotoxin Journal, 2, 91–94.
- Rahmani, A., Jinap, S., & Soleimany, F. (2010). Validation of the procedure for the simultaneous determination of aflatoxins ochratoxin A and zearalenone in cereals using HPLC-FLD. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 27, 1683–1693.
- Rastogi, S., Dwivedi, P. D., Khanna, S. K., & Das, M. (2004). Detection of aflatoxin M1 contamination in milk and infant milk products from Indian markets by ELISA. Food Control, 15, 287–290.
- Ridascreen. Aflatoxin M1 30/15 ELISA test kit manual, Art. No.: R1111.
- Sarımehmetoglu, B., Kuplulu, O., & Celik, T. H. (2004). Detection of aflatoxin M-1 in cheese samples by ELISA. Food Control, 15, 45–49.
- Sassahara, M., Netto, D. P., & Yanaka, E. K. (2005). Aflatoxin occurrence in foodstuff supplied to dairy cattle and aflatoxin M1 in raw milk in the North of Paraná state. Food and Chemical Toxicology, 43, 981–984.
- Shundo, L., & Sabino, M. (2006). Aflatoxin M-1 in milk by immunoaffinity column cleanup with TLC/HPLC determination. Brazilian Journal of Microbiology, 37, 164–167.
- Tajkarimi, M., Aliabadi, F. S., Nejad, M. S., Pursoltani, H., Motallebi, A. A., & Mahdavi, H. (2007). Seasonal study of aflatoxin M1 contamination in milk in five regions in Iran. International Journal of Food Microbiology, 116, 346–349.
- Turkish Food Codex. 2008. Number 26879, Food Codex, No. 2008/26.
- Turner, N. W., Subrahmanyam, S., & Piletsky, S. A. (2009). Analytical methods for determination of mycotoxins: A review. Analytica Chimica Acta, 632, 168–180.
- Yaroglu, T., Oruc, H. H., & Tayar, M. (2005). Aflatoxin M-1 levels in cheese samples from some provinces of Turkey. Food Control, 16, 883–885.