Abstract
Détente Instantanée Contrôlée (DIC) technology was used to reduce immunoglobulin E (IgE) reactivity of legume proteins. Soybean, roasted peanut, chickpea and lentil seeds were treated at three or six bars for 60 or 180s. The effect of this treatment on the IgE-binding pattern of the legume proteins – separated by sodium-dodecyl-sulphate polyacrylamide gel electrophoresis – was monitored by five individual paediatric legume allergic and – two individual negative control human sera. A highly cross-reactive legume positive serum was selected for the two-dimensional electrophoreses immunoblots to compare the IgE reactive protein patterns, before and after the DIC treatment. The number of the identified IgE reactive spots was highly reduced for soybean (0/7) and chickpea (2/7), and moderately reduced for lentil (4/7) when the seeds were treated at a higher pressure (6 bar) and for a longer time (180 s), but this treatment was not effective for roasted peanut (6/8) where the intensity of the IgE reactive resistant spots were even stronger.
1. Introduction
Legumes are essential constituents of human diets, contributing to both dietary protein and processed vegetable oil for human consumption. Additionally, legumes are important source of animal feeds and industrial oils (Desrochers & Brauer, Citation2001). In most species, seed protein content varies from 20% to 30% of total dry weight. Oil content is more variable, ranging from 1% (e.g. lentil) to approximately 50% (e.g. peanut). Legumes have desirable characteristic such as abundance of carbohydrates, ability to lower the serum cholesterol, high fiber, low fat, high concentration of polyunsaturated fatty acids and a long shelf life (Scarafoni, Magni, & Duranti, Citation2007). Despite these advantages and their nutritional value, legumes have an unfavourable property that they are also an important source of food allergens (Riascos, Weissinger, Weissinger, & Burks, Citation2010).
In the Mediterranean area, many Asian countries, India and Canada, lentils (Lens culinaris) and chickpeas (Cicer arietinum) are among the most frequent foods associated with immunoglobulin E (IgE)–mediated hypersensitivity reactions (Clemente et al., Citation1999; López-Torrejón et al., Citation2003). In United States, the United Kingdom and Japan, peanut (Arachis hypogea) and soybean (Glycine max) are the two major legumes involved in food allergy (Burks et al., Citation1992a, Citation1992b). Chickpeas and lentils are mostly consumed whole, split or milled, and have traditionally been used in the preparation of salads, soups, snacks and condiments (Boye et al., Citation2010). Soy products are widely used in the food industry, in particular as texturiser, emulsifier and protein filler. Peanuts are generally eaten as snacks after roasting and are frequently constituents in snack bars, chocolates and breakfast cereals (EU informall database, CitationMills). This wide range of utilisation warns the allergic people to pay more attention to their healthy diet and follow the labelling on the products to avoid the allergic reactions. According to the European legislation (2006/142/EC European Community), soybeans, peanuts and products thereof are listed in the directive on labelling of food allergens (Commission Directive, Citation2006). They are also listed as major food allergens by the Food and Drug Administration labelling regulations (FDA, Citation2004). Lentil and chickpea allergies are less prevalent than the others mentioned, so these legumes and products thereof are not required to be labelled on the products. For the treatment of food allergy, at present, the avoidance of the offending food is the most feasible solution.
The majority of legume allergens belong to the seed storage protein group, which mainly includes the cupin superfamily composed of protein families such as 7S vicilin-type and 11S legumin-type globulins, and the 2S albumin seed storage protein family as a member of prolamin superfamily. Moreover, proteases and protease inhibitors, oleosins and other proteins, such as seed-specific biotinylated proteins, hull proteins, plant-specific proteins and lectins, can also be allergens in legumes (Breiteneder & Radauer, Citation2004; Riascos et al., Citation2010; Radauer & Breiteneder, Citation2007).
To date, 33 soybean (Glycine max) proteins have been identified as allergens (from 7 to 71 kDa) (Wilson, Blaschek, Gonzalez de Mejia, Citation2005). The P 34, also referred as Gly m Bd 30K (thiol protease), has been identified as an immunodominant allergen that shares sequence homology (70%) with Ara h 1. Moreover, the acidic and basic glycinin polypeptides (11S) are allergenic and resistant to processing (Christensen, Bruun, & Frokiaer, Citation2003; Maruyama et al., Citation2003). The 7S globulin fraction (β-conglycinin) includes the and
subunits, which are also allergenic proteins (Ogawa, Samoto, & Takahashi, Citation2000).
Considerable effort has been spent in identifying and characterising allergens from peanut because of its severity and lifelong persistence (Sicherer & Sampson, Citation2007). The two major peanut allergens, Ara h 1 (64 kDa) and Ara h 2 (isoforms 16.5 kDa and 18 kDa), are recognised by 70–90% of sensitised subjects (Burks, Sampson, & Bannon, Citation1998) and correspond to the vicilin (7S) and 2S families of storage proteins, respectively (Burks et al., Citation1991; Burks et al., Citation1992a, Citation1992b). Ara h 3 (40 kDa, oligomeric masses 360 kDa) has been considered to play a lesser allergenic role and it belongs to the 11S legumin–like proteins (Rabjohn et al., Citation1999).
According to the EU informall database (CitationMills), the major chickpea allergens are 11S storage proteins (23 and 35–40 kDa) and Cic a 2S albumin (10 and 12 kDa). The last 2S albumin, generally acts as seed storage protein, but can also have trypsin and chymotrypsin inhibitor activity (Vioque et al., Citation1999).
A major lentil allergen Len c 1 has been isolated and identified as a 48 kDa vicilin. Its processing fragments, corresponding to subunits 12–16 kDa and 26 kDa are also relevant lentil IgE-binding proteins (López-Torrejón et al., Citation2003; Sánchez-Monge et al., Citation2000).
Plant protein allergenicity may be variably affected by thermal processing. Their effects on food protein allergenicity have been reviewed in several recent articles (Mills, Sancho, Rigby, Jenkins, & Mackie, Citation2009; Paschke, Citation2009; Sathe & Sharma, Citation2009). The molecular basis of changes in allergenic activity is the inactivation or disruption of epitope structures, the formation of new epitopes or an enhanced access to cryptic epitopes by denaturation of the native allergen, which may increase or decrease the IgE immune reactivity of a protein (Besler, Steinhart, & Paschke, Citation2001).
Previous studies have reported that boiling in autoclave (2.6 bar up to 30 min) markedly reduced lupin, lentil and chickpea allergenicity (Alvarez-Alvarez et al., Citation2005; Cuadrado et al., Citation2009). Moreover, the use of instant controlled pressure drop (Détente Instantanée Contrôlée [DIC]) treatment (6 bar for 3 min), combining heat and steam pressure as autoclaving with instant cooling once pressure drops, greatly decreased IgE reactivity of the heat resistant major lupin allergens (Lup-1 and Lup-2) and in vitro immune reactivity of other legumes (Guillamón et al., Citation2008; Guillamón et al., Citation2010). The advantages of DIC, such as short processing time, relatively simple heat treatment and temperature are highly controlled (Haddad & Allaf, Citation2007; Haddad, Louka, Gadouleau, Juhel, & Allaf, Citation2001). The possibility of treating and maintaining whole seeds, as the end product for industrial applications, would permit the use of the seed in various culinary preparations. The end product has a porous texture and thus has better functional properties.
The aim of the present work was to study the effect of DIC treatment on IgE-binding ability of soybean, roasted peanut, chickpea and lentil proteins by assessing the defatted legume meals separated by polyacrylamide gel electrophoresis in the presence of sodium-dodecyl-sulphate (SDS-PAGE) and two-dimensional electrophoreses (2-DE) followed by immunoblot using clinically proved legume-sensitive human IgE-sera.
2. Materials and methods
2.1. Materials
2.1.1. Plant materials
Roasted peanuts (A. hypogaea var. Virginia) were purchased from Aperitivos Medina (Madrid, Spain), lentil seeds (L. culinaris var. Magda) were provided by the Instituto Técnico Agronómico Provincial (ITAP, Albacete, Spain) and chickpea seeds (C. arietinum var. Athenas) and soybean seeds (G. max var. Ostrumi) were obtained from the Instituto de Formación e Investigación Agraria y Pesquera (IFAPA, Córdoba, Spain). All these seeds were subjected to DIC technology by the Université de la Rochelle, Laboratoire Maîtrise des Technologies Agro-Industrielles, La Rochelle, France.
2.1.2. Human sera
Individual anonym sera of five paediatric patients sensitised to legumes (pea, bean, soybean, lentil and peanut) having gastrointestinal and skin symptoms and positive IgE-RAST (>0.35 kU/ml) prick or patch tests after a repeated legume challenge were selected for the identification of IgE-binding activity. Sera of two patients without sensitisation of legume allergens were used as controls.
2.2. Methods
2.2.1. DIC treatment
DIC treatment was carried out following a factorial experimental design previously described (Haddad & Allaf, Citation2007; Haddad et al., Citation2001). Briefly, the moistened whole seeds are placed in a processing chamber and exposed to steam pressure (up to 8 bar) at high temperature (up to 170°C), over a relatively short time (few seconds to some minutes). This high temperature-short time stage is followed by an instant pressure drop towards a vacuum at about 50 mbar. This abrupt pressure drop, at a rate ΔP/Δt higher than 5 bar s−1, simultaneously provokes an autovaporisation of a part of the water in the product and an instantaneous cooling of the products, which stops thermal degradation. Whole seeds of soybean, roasted peanut, chickpea and lentil were treated at different pressure, time and initial water content conditions. For roasted peanuts, a central composite design of 12 points was used with four repetitions of central point while for the other legumes, a 22 point central composite design with eight repetitions of central point was used. From all these DIC-treated samples, 3 and 6 bar for 60 and 180 s with constant initial water content of 50 g water per 100 g dry matter were selected for electrophoresis and immunoblot analysis.
2.2.2. Protein separation and immunoblot
Defatted and grained meals of untreated (control) and DIC-treated legumes were used for these analyses. SDS-PAGE (1-DE) was performed according to Laemmli protocol (Citation1970) following the Instruction Manual of Mini-PROTEAN 3Cell (BioRad) using 6%/15% stacking/resolving gel. One milligram of defatted flours was extracted by 100 µl sample buffer (BioRad) and same amount (8–10 µl) of samples was loaded onto the gel (loaded total crude protein: 0.01–0.03 mg). Separation was done at 200 V for approximately 65 minutes at room temperature. After electrophoresis, the separated proteins were fixed on gel with 20% trichloroacetic acid (TCA) for 20 minutes, and then the gel was soaked in acetic acid/ethanol washing liquid three times for 10 minutes. Staining was performed with Coomassie Brilliant Blue R-250 (Reanal, Hungary). The background staining was removed by 10% acetic acid.
In case of 2-DE PAGE, the isoelectric focusing, as the first dimension, was run in the immobilised pH gradient gel (IPG strip, 7 cm) using an isoelectric focusing chamber (PROTEAN IEF Cell, BioRad). A 0.8 mg dry matter of sample was used for each strip, which were dissolved in 250 µl rehydration buffer (8 M urea, 1% CHAPS, 0.02 M DTT) (Hanson, Schulenberg, Patton, & Capaldi, Citation2001). After reaching the equilibrium, each strip was incubated in 1.5 ml equilibration solution [I. buffer: 6 M urea, 0.375 M Tris-HCl (pH 8.8), 2% w/v DTT, 2% SDS, 20% glycerol and II. buffer: 6 M urea, 0,375 M Tris-HCl (pH 8.8), 2.5% w/v iodoacetamide, 2% SDS, 20% glycerol] for 10 min in I. buffer and 10 min in II. buffer at room temperature with gentle shaking before being transferred to the second dimension. In the second dimension, the separation of proteins was carried out by SDS-PAGE (Hanson et al., Citation2001) in thin layer gels (75 mm×83 mm×1.5 mm) by using a Mini-PROTEAN II cell (BioRad). The total acrylamide content of the running gel was 12%. After completion of the run, the gels were fixed in 20% TCA and stained with Coomassie Brilliant Blue R-250. Imaging of the gels was carried out with BioRad Gel Doc 2000 system (Szabó, Hajós, & Matuz, Citation2002).
For 2-DE immunoblot, the 2-DE PAGE–separated proteins were subsequently transferred to polyvinylidene fluoride membrane (Millipore, 0.45 µm) by semi-dry blotting (BIO-RAD Trans Blot SD Semi-Dry Transfer Cell) for 60 minutes at 0.25 V, 0.08 mA/cm2. The transblotting was performed by the description of Millipore membrane brochure. The immune reactive proteins were identified with individual legume positive (serum No.1-5 in case of 1-DE, serum No.1 in case of 2-DE analyses) and control human sera. The binding patterns were visualised using a substrate solution containing 4-chloronaphtol (Sigma), H2O2 and ethanol in 16 mM phosphate buffered saline solution. Image analyses of gels and blots were carried out with BIO-RAD Gel Doc 2000 system.
3. Results and discussion
Important alterations in protein structure may occur during thermal processing, which can dramatically affect the results of digestibility, solubility and other parameters. After heat processing, proteins can form oligomers, become denatured, degraded, aggregated, cross-linked, fragmented and re-assembled, and these changes most often cause a reduction in solubility. Consequently, thermal processing can alter the overall IgE-binding capacity of proteins (Maleki, Citation2004).
In our study, DIC technology was used to assess the thermal effect on IgE reactivity of non-treated control versus the DIC-treated legume samples. The effect of pressure (3 bar and 6 bar) and treatment time (60 s and 180 s) were screened on the gels where the proteins were separated according to their molecular weights by SDS-PAGE and by immunoblots. The control (non-DIC–treated) legumes were composed of numerous bands, out of which only the IgE-reactive bands were monitored.
It was found that the DIC treatment at lower pressure and shorter treatment time (3 bar, 60 s) did not result in significant changes in the number of recognised IgE-reactive bands. The first visible reduction in the corresponding bands was observed at 3 bar, 180 s and the most effective treatment was at 6 bar, 180 s except the roasted peanut where such a reduction was not observed. The immunoblot patterns of the studied legume samples with five individual legume positive sera resulted in high similarity. The two negative sera, which were used as a control did not recognise IgE-reactive bands (data not shown).
The 2-DE separation allows identifying the IgE reactive proteins according to their isoelectric points as well (Schmidt et al., Citation2009). Therefore, a highly cross-reactive legume positive serum was selected out from the five individual sera (previously selected by 1-DE analyses) for the 2-DE immunoblots to compare the IgE-reactive spot patterns, before and after the DIC treatment. The 2-DE results are presented via two effectively treated samples (3 bar, 180 s; 6 bar, 180 s) versus non-treated controls (Figures – and Tables 1–4). The control (non-DIC–treated) legumes were composed of numerous spots, out of which only the IgE-reactive spots were monitored. The spots corresponding to IgE reactive proteins in the immunoblots are designated with the same identification numbers – which were given by the gel documentation system – in the 2-DE PAGE gels. The intensity of the IgE reactive spots was measured on the 2-DE PAGE gels, and in non-treated samples the intensity was accepted as 100%. The intensity of the spots in the DIC–treated samples was related to the corresponding spots of non-treated sample and expressed in percentage as well.
The 2-DE results of soybean are presented in and , where seven IgE-reactive spots were recognised in the control soybean (, and ). Two spots were eliminated at 3 bar, 180 s and the remaining spots became more intensive (156–288%) or less intensive (24% and 65%) than the corresponding spots in the non-treated sample (). The higher pressure and longer time treatment (6 bar, 180 s) was more effective because the selected spots disappeared to a great extent (5/7) and only two slight spots remained visible on the gel (c and ) These spots were observed with reduced intensity (28% and 71%), which were not even recognised by the applied individual serum on the immunoblot (d). Studies of Penas, Prestamo, Polo, and Gomez (Citation2005) prove our experiment as they found that high pressure (and enzymatic hydrolysis) reduced the allergenicity of soybean proteins.
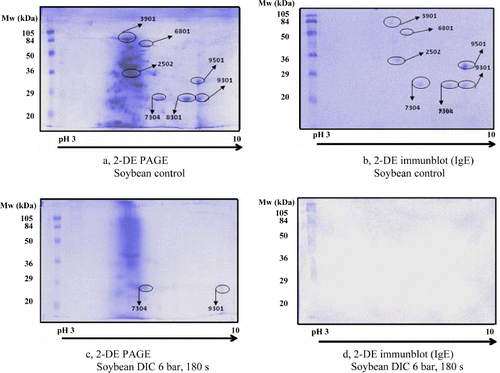
Table 1. Intensity of IgE reactive spots in soybean determined by 2-DE PAGE in the non-treated control sample versus DIC-treated samples.
Data on roasted peanut are presented in and . There were eight IgE-reactive spots on the roasted peanut control pattern (a and b; ). The number of spots (8/8) was not reduced due to DIC treatment conditions and the intensity of numerous spots was even increased (c and ). Legume allergic sera still recognised many resistant spots (6/8) (d).
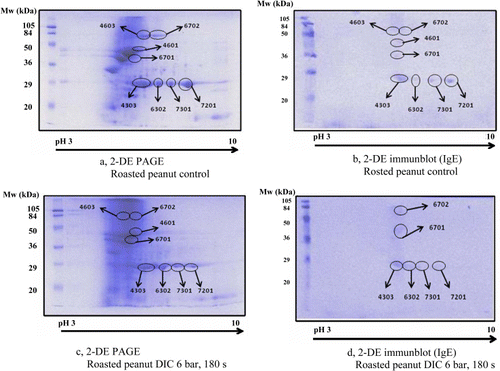
Table 2. Intensity of IgE reactive spots in roasted peanut determined by 2-DE PAGE in the nontreated control sample versus DIC-treated samples.
Chickpea 2-DE analyses are summarised in and . On the control sample seven IgE-reactive spots were identified (a and b; ). The milder intensive DIC treatment (3 bar, 180 s) did not affect the number of the IgE-reactive spots (7/7) but reduced the intensity (29%–91%) of the corresponding spots (). The efficiency of such a treatment has increased at higher pressure (6 bar, 180 s) and only two IgE-reactive spots remained visible on the gel (2/7) with lowered intensity of the corresponding spots (14% and 52%) (c and ) that were recognised by the positive serum as well (d).
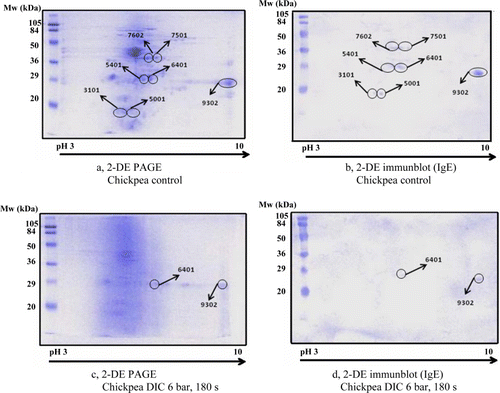
Table 3. Intensity of IgE-reactive spots in chickpea determined by 2-DE PAGE in the non-treated control sample versus DIC-treated samples.
Lentil 2-DE separations and immunoblot data are summarised in and . There were seven spots identified by legume positive serum in the control sample. (a and b; ). The DIC treatment at lower pressure (3 bar, 180 s) did not result in any changes in the number of IgE-reactive spots (7/7) but decreased their intensity (38%–94%) except in two cases where the intensity of the spots was even increased (167% and 401%) (). At higher pressure treatment (6 bar, 180 s), some spots disappeared (2/7) and the intensity of the remaining spots decreased (37%–64%) except one where the intensity still remained high (477%) (c; ). Four spots out of these five were recognised by the positive serum (d).
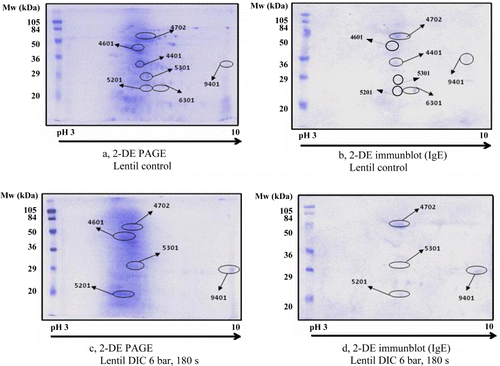
Table 4. Intensity of IgE-reactive spots in lentil determined by 2-DE PAGE in the non-treated control sample versus DIC-treated samples.
The control serum did not recognise IgE-reactive spots in the investigated legume-protein extracts (data not shown).
Various studies are published on the efficiency of DIC treatment on the loss of undesirable components such as antinutritive factors, including proteins. For example, Haddad et al. (Citation2001) applied DIC treatment on lupins, and analysed the antinutritive phytate content in the seed and after the treatment. Phytate content was highly decreased (16%–19%) after 60 s of DIC treatment. Trypsin inhibitor, one of the most important antinutrients and minor allergens in legumes, was also analysed after DIC treatment in soybean, which caused a 94% decrease in its activity after 180 s of DIC treatment (Haddad & Allaf, Citation2007). However, the literature is still not so widespread, only small number of studies can be found on the immune reactivity of the DIC-treated proteins. Cuadrado et al. (Citation2011) analysed legumes by 1 DE immunoblots and concluded that DIC treatment affected a reduction in the overall in vitro IgE binding of peanut, lentil and chickpea and resulted in a drastic reduction in soybean. These results are in agreement with our data.
Summarising all data, it can be concluded that the DIC treatment could be a promising technology to improve the nutritional quality and safety of soybean, chickpea and lentil proteins leading to a decreased IgE-binding ability of the main protein allergens present naturally in legume seeds. Of course the optimisation of the treatment parameters to reduce or eliminate legume allergenic proteins should be necessary. At the same time and further assessment would be required on the safety of the DIC-treated legumes regarding the gut resistance of the aggregated proteins that were not able even to enter into the gel. At the same time, our results are suggesting that the applied DIC treatment will not support the reduction of peanut protein–related allergenic risk.
Acknowledgements
We wish to thank Mrs K. Háder Sólyom for assisting in the practical laboratory work. This study was supported by the Bilateral Action funded by Education Ministry (HH2006-0039)/OMFB-00004/2008, ESP-31/2006) and by the research project (AGL2004-07971) funded by the National Programme I + D+i. The DIC technology is supported by innovation and SME EU Programme, coordinated by ABCAR-DIC Process (www.abcar-dic.com).
References
- Alvarez-Alvarez, J., Guillamón, E., Crespo, J. F., Cuadrado, C., Burbano, C., Rodríguez, J., … Muzquiz, M. (2005). Effects of extrusion, boiling, autoclaving and microwave heating on lupine allergenicity. Journal of Agricultural and Food Chemistry, 53, 1294–1298. doi:10.1021/jf0490145
- Besler, M., Steinhart, H., & Paschke, A. (2001). Stability of food allergens and allergenicity of processed foods. Journal of Chromatography B: Biomedical Sciences and Applications, 756, 207–228. doi:10.1016/S0378-4347(01)00110-4
- Boye, J. I., Aksay, S., Roufik, S., Ribéreau, S., Mondor, M., Farnworth, E., Rajamohamed, S. H. (2010). Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Research International, 43, 537–546. doi:10.1016/j.foodres.2009.07.021
- Breiteneder, H., & Radauer, C. (2004). A classification of plant food allergens. Journal of Allergy and Clinical Immunology, 113(5), 821–830. doi:10.1016/j.jaci.2004.01.779
- Burks, A. W., Sampson, H. A., & Bannon, G. A. (1998). Peanut allergens. Allergy, 53, 725–730. doi:10.1111/j.1398-9995.1998.tb03967.x
- Burks A. W., Williams L. W., Helm R. M., Connaughton, C., Cockrell, G., & O'Brien, T. (1991). Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges. Journal of Allergy and Clinical Immunology, 88, 172–179. doi:10.1016/0091-6749(91)90325-I
- Burks A. W., Williams, L. W., Connaughton, C., Cockrell, G., O'Brien, T. J., Helm, R. M. (1992a). Identification and characterization of a second major peanut allergen, Ara h II, with use of the sera of patients with atopic dermatitis and positive peanut challenge. Journal of Allergy and Clinical Immunology, 90, 962–969. doi:10.1016/0091-6749(92)90469-I
- Burks, A. W., Williams, L. W., Thresher, W., Connaughton, C., Cockrell, G., & Helm, R. M. (1992b). Allergenicity of peanut and soybean extracts altered by chemical or thermal denaturation in patients with atopic dermatitis and positive food challenges. Journal of Allergy and Clinical Immunology, 90, 889–897. doi:10.1016/0091-6749(92)90461-A
- Christensen, H. R., Bruun, S. W., & Frokiaer, H. (2003). Antigenic specificity of serum antibodies in mice fed soy protein. International Archives of Allergy and Immunology, 132, 58–67. doi:10.1159/000073265
- Clemente, A., Vioque, J., Sánchez-Vioque, R., Pedroche, J., Bautista, J., & Millán, F. (1999). Protein quality of chickpea (Cicer arietinum L.) protein hydrolysates. Food Chemistry, 67, 269–274. doi:10.1016/S0308-8146(99)00130-2
- Commission Directive 2006/142/EC of the European Parlament and of the Council. (2006, December 23). Official Journal of the European Union, L 368/110.
- Cuadrado, C., Cabanillas, B., Pedrosa, M. M., Muzquiz, M., Haddad, J., Allaf, K., … Burbano, C. (2011). Effect of instant controlled pressure drop on IgE antibody reactivity to peanut, lentil, chickpea and soybean proteins. International Archives of Allergy Immunology, 156(4), 391–404. doi:10.1159/000324443 [Epub 2011 Aug 9]
- Cuadrado, C., Cabanillas, B., Pedrosa, M. M., Varela, A., Guillamón, E., Muzquiz, M., … Burbano, C. (2009). Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. Molecular Nutrition & Food Research, 53, 1462–1468. doi:10.1002/mnfr.200800485
- Desrochers, N., & Brauer, P. M. (2001). Legume promotion in counselling: An e-mail survey of dieticians. Canadian Journal of Dietetic Practice and Research, 62, 193–198.
- FDA (US) Food and Drug Administration, Food Allergen Labeling and Consumer Protection Act of 2004 (Public Law 108-282, Title II). 2004
- Guillamón E., Burbano, C., Cuadrado, C., Muzquiz, M., Pedrosa, M. M., Sánchez, M., … Allaf, K. (2008). Effect of an instantaneous controlled pressure drop on in vitro allergenicity to Lupins (Lupinus albus var Multolupa). International Archives of Allergy and Immunology, 145, 9–14. doi:10.1159/000107461
- Guillamón, E., Rodríguez, J., Burbano, C., Muzquiz, M., Pedrosa, M. M., Cabanillas, B., … Cuadrado, C. (2010). Characterization of lupin major allergens (Lupinus albus L). Molecular Nutrition Food Research, 54, 1168–1676. doi:10.1002/mnfr.200800485
- Haddad, J., & Allaf, K. (2007). A study if the impact of instantaneous controlled pressure drop on the trypsin inhibitors of soybean. Journal of Food Engineering, 79, 353–357. doi:10.1016/j.jfoodeng.2006.01.066
- Haddad, J., Louka, N., Gadouleau, M., Juhel, F., & Allaf, K. (2001). Application du nouveau procédé de séchage/texturation par Détente Instantanée Contrôlée DIC aux poissons: Impact sur les caractéristiques physico-chimiques du produit fini. Science des Aliments, 21, 481–498. doi:10.3166/sda.21.481-498
- Hanson, B. J., Schulenberg, B., Patton, W. F., & Capaldi, R. A. (2001). Novel subfractionation approach for mitochondrial proteins: A three-dimensional mitochondrial proteome map. Electrophoresis, 22, 950–959. doi:10.1002/1522-2683()22:5%3C950::AID-ELPS950%3E3.0.CO;2-D
- Laemmli, V. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. doi:10.1038/227680a0
- López-Torrejón, G., Salcedo, G., Martin-Esteban, M., Diaz-Perales, A., Pascual, C. Y., & Sánchez-monge, R. (2003). Len c 1, a major allergen and vicilin from lentil seeds: Protein isolation and cDNA cloning. Journal of Allergy and Clinical Immunology, 112(6), 1208–1215. doi:10.1016/j.jaci.2003.08.035
- Maleki, S. J. (2004). Food processing: Effects on allergenicity. Current Opinion in Allergy and Clinical Immunology, 4, 241–245. doi:10.1097/00130832-200406000-00018
- Maruyama, N., Fukada, T., Saka, S., Inui, N., Kotoh, J., Miyagawa, M., … Utsumi, S. (2003). Molecular and structural analysis of electrophoretic variants of soybean seed storage proteins. Phytochemistry, 64, 701–708. doi:10.1016/S0031-9422(03)00385-6
- Mills, C.. 2012EU informall database.University of Manchester. Retrieved from http://foodallergens.ifr.ac.uk/informall.html
- Mills, E. N. C., Sancho, A. I., Rigby, N. M., Jenkins, J. A., & Mackie, A. R. (2009). Impact of food processing on the structural and allergenic properties of food allergens. Molecular Nutrition & Food Research, 53, 963–969. doi:10.1002/mnfr.200800236
- Ogawa, A., Samoto, M., & Takahashi, K. (2000). Soybeans allergens and hypoallergenic soybean products. Journal of Nutritional Science and Vitaminology, 46, 271–279. doi:10.3177/jnsv.46.271
- Paschke, A. (2009). Aspects of food processing and its effect on allergen structure. Molecular Nutrition & Food Research, 53, 959–962. doi:10.1002/mnfr.200800187
- Penas, E., Prestamo, G., Polo, F., & Gomez, R. (2005). Enzymatic proteolysis under high pressure of soybean whey: Analysis of peptides and a Gly m 1 in the hydrolysates. Food Chemistry, 99, 569–573. doi:10.1016/j.foodchem.2005.08.028
- Rabjohn, P., Helm, E. M., Stanley, J. S., West, C. M., Sampson, H. A., Burks, A. W., & Bannon, G. A. (1999). Molecular cloning and epitope analysis of the peanut allergen Ara h 3. Journal of Clinical Investigation, 103, 535–542. doi:10.1172/JCI5349
- Radauer, C., & Breiteneder, H. (2007). Evolutionary biology of plant food allergens. Journal of Allergy and Clinical Immunology, 120(3), 518–525. doi:10.1016/j.jaci.2007.07.024
- Riascos, J. J., Weissinger, A. K., Weissinger, S. M., & Burks, A. W. (2010). Hypoallergenic legume crops and food allergy: Factors affecting feasibility and risk. Journal of Agricultural and Food Chemistry, 58, 20–27. doi:10.1021/jf902526y
- Sánchez-Monge, R., Pascual, C. Y., Díaz-Perales, A., Fernández-Crespo, J., Martin-Esteban, M., & Salcedo, G. (2000). Isolation and characterization of relevant allergens from boiled lentils. Journal of Allergy and Clinical Immunology, 106, 955–961. doi:10.1067/mai.2000.109912
- Sathe, S. K., & Sharma, G. M. (2009). Effects of food processing on food allergens. Molecular Nutrition & Food Research, 53, 970–978. doi:10.1002/mnfr.200800194
- Scarafoni, A., Magni, C., & Duranti, M. (2007). Molecular nutraceutics as a mean to investigate the positive effects of legume seed proteins on human health. Trends in Food Science & Technology, 18, 454–463. doi:10.1016/j.tifs.2007.04.002
- Schmidt, H., Gelhaus, C., Latendorf, T., Nebendahl, M., Petersen, A., Krause, S., … Janssen, O. (2009). 2-D DIGE analysis of the proteome of extracts from peanut variants reveals striking differences in major allergen contents. Proteomics, 9, 3507–3521. doi:10.1002/pmic.200800938
- Sicherer, S. H., & Sampson, H. A. (2007). Peanut allergy: Emerging concepts and approaches for an apparent epidemic. Journal of Allergy and Clinical Immunology, 120, 491–503. doi:10.1016/j.jaci.2007.07.015
- Szabó, E., Hajós, Gy., & Matuz, J. (2002). Identification of major allergens of cereal proteins by electrophoretic methods. Polish Journal of Food and Nutrition Sciences, 11(52) SI2, 131–134.
- Vioque, J., Sanchez-Vioque, R., Clemente, A., Pedroche, J., Bautista, J., & Millan, F. (1999). Purification and partial characterization of chickpea 2S albumin. Journal of Agricultural and Food Chemistry, 47, 1405–1409. doi:10.1021/jf980819k
- Wilson, S., Blaschek, K., & Gonzalez de Mejia, E. (2005). Allergenic proteins in soybean: Processing and reduction of P34 allergenicity. Nutrition Reviews, 63, 47–58. doi:10.1301/nr.2005.feb.47-58
