Abstract
In this study, an enhanced test strip, based on a monoclonal antibody for the cadmium-ethylenediaminetetraacetic acid (EDTA) complex, but not metal-free EDTA, has been developed. This colorimetric sensor was sensitive and specific for the detection of cadmium in aqueous samples containing excess EDTA. Through a process of silver enhancement, the visual detection limit for Cd(II) was 5 µg/L under optimised conditions and the limit of detection for semi-quantitative detection could be as low as 0.35 µg/L by using a scanning reader. The calibration curve showed that the colour intensity decreased as the Cd(II) concentration increased in the range of 0.5–5 µg/L. The other metal ions did not interfere with the determination of Cd(II). The recoveries of drinking water samples were from 98 to 108%. Consequently, the assay could be employed as a potential on-site screening tool for the detection of Cd(II) in water samples.
1. Introduction
Heavy metals pollution is a global issue because these metals are highly toxic and dangerous to the environment and human beings. Cadmium is one of the major heavy metal pollutants. Industrial waste, fertiliser and mine drainage are the main sources of cadmium pollution (McLaughlin & Singh, Citation1999). In humans, ingested cadmium accumulates and damages the kidneys, liver, bone and blood (Nordberg, Citation1974). Clean drinking water is indispensable to human life, but water is also highly vulnerable to pollution. The US Environmental Protection Agency (EPA) has defined the Maximum Contaminant Level Goal and Maximum Contaminant Level for cadmium in drinking water as 5 ng/mL because this level should not cause any of the potential health problems described above. Regular monitoring of cadmium pollution in drinking water is necessary for the proper management of cadmium in the environment in order to protect human health.
At present, the most frequently used methods for cadmium analysis are atomic absorption spectroscopy, inductively coupled plasma-mass spectroscopy (ICP-MS) and inductively coupled plasma atomic emission spectrometry (ICP-AES). Although these methods are sensitive, accurate and highly specific, they are expensive, and they are not suitable for use as on-site screening tools because of the complexity of the methods, the size and immobility of the instruments and the requirement for highly skilled operators.
Rapid and simple methods for detection of heavy metal ions have been extensively researched and has attracted increasing attention globally (Aragay, Pons, & Merkoçi, Citation2011; Cho et al., Citation2012; Liu et al., Citation2011; Mathew, Sajanlal, & Pradeep, Citation2012). Since the first monoclonal antibodies against the EDTA chelate of indium were reported (Reardan et al., Citation1985), the connection between immunology and inorganic chemistry has shown extensive practical use in trace element analysis. Many immunoassays based on antibodies have since been developed for toxic metal ions, including cadmium (Blake et al., Citation1996), lead (Khosraviani et al., Citation2000), mercury (Wang et al., Citation2012; Wylie et al., Citation1992), chromium (Sasaki, Oguma, Namiki, & Ohmura, Citation2009a), copper (Zhao et al., Citation2010), and uranium ions (Blake et al., Citation2004). Most of these methods are competitive ELISA. Metal ions are too small to be detected directly by antibodies, however, if the ions are chelated to a specific chelating agent the combination may be detected. To achieve this, antibodies must be raised to the metal-chelate combination, thus all antibodies specific for cadmium in fact recognised a chelated form of cadmium (Sasaki et al., Citation2009b; Zhu et al., Citation2007). These antibodies have been applied to the detection of cadmium in water and human serum successfully (Darwish & Blake, Citation2001, Citation2002).
A fast, simple and cheap strip detection platform has recently been developed. This platform combined with suitable antibodies (Abe et al., Citation2011; Abe, Sakurai, Okuyama, Sasaki, & Tawarada, Citation2009; Tang et al., Citation2010) or nucleic acids (Fang et al., Citation2010; Guo et al., Citation2012; Mazumdar, Liu, Lu, Zhou, & Lu, Citation2010; Torabi & Lu, Citation2011) has proven to be a powerful alternative tool for the detection of heavy metals. However, these systems were unreliable for low-level heavy metal contamination. For example, an immunochromatographic assay for the quantification of cadmium in plant samples, based on an anti-Cd-EDTA antibody, had a detection range from 0.01 to 0.1 mg/L at a 20% mean coefficient of variance, which is inadequate for the detection of cadmium in drinking water. The silver-enhancement process is versatile and has been extensively used in many detection methods (Cao, Jin, & Mirkin, Citation2002; Chiao, Shyu, Hu, Chiang, & Tang, Citation2004; Hou, Chen, Cheng, & Huang, Citation2007; Li et al., Citation2012; Rodríguez-Lorenzo, De La Rica, Álvarez-Puebla, Liz-Marzán, & Stevens, Citation2012; Yang et al., Citation2011). To overcome the limitations of the low sensitivity of the basic lateral flow assay, this technique has been combined with silver enhancement enabling the detection of trace amounts of Cd(II) in drinking water samples. The visual limit of detection (LOD) is 5 µg/L, and the LOD for semi-quantitative detection is 0.35 µg/L by using a scanning reader.
2. Materials and methods
2.1. Materials
All metal ions were atomic absorption standards. Cd(II), Hg(II), Cu(II), Pb(II), Cr(III), Mn(II), Co(II), Fe(III), Zn(II), Al(III), Mg(II) and Ca(II) (1000 µg/mL in 1% HNO3 or 5% HCl) obtained from the National Institute of Metrology PR China (Beijing, China). 1-(4-Isothiocyanobenzyl) ethylenediamine-N, N, N′, N′-tetraacetic acid (ITCBE) was obtained from Dojindo Laboratories (Shanghai, China). Bovine serum albumin (BSA), keyhole limpet haemocyanin (KLH), goat anti-mouse IgG, silver enhancer reagent and chloroauric acid (HAuCl4 .4H2O) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-Cd(II)-EDTA monoclonal antibody was produced in our laboratory. All plasticware was soaked overnight in 3 M HCl, and glassware was mixed-acid washed and rinsed liberally with purified water (Millipore, 18.2 MΩ·cm resistivity) before use.
The Dispensing Platform (BioJet Quanti3000 dispenser) and the CM4000 Guillotine Cutting Module (BioDot Inc., Irvine, CA, USA) were used to manufacture the test strips. The BioDot TSR3000 Membrane Strip Reader (Gene Company Limited, Shanghai Branch, Shanghai, China) was used to test the colour intensity of the test line. ICP-MS (Thermo Fisher Scientific, Waltham, MA, USA) was used to confirm the detection results.
2.2. Synthesis of protein-chelate conjugates
Protein-chelate conjugates were prepared according to the following method. Both 2.0 mM ITCBE and 3 mM cadmium were added to 10.0 mL 0.2 M Hepes buffer (pH 9.0) containing BSA (5 mg) or KLH (10 mg) while stirring. The mixtures were reacted overnight at room temperature. Unreacted low-molecular weight molecules were then removed by buffer exchange through centrifugal ultrafiltration (Ultrafiltration tube 4mL 3KD) at 8500 rpm for 15 min repeated three times. The resulting protein-chelate conjugates were stored in aliquots of 500 µL (1 mg/mL) in Hepes-buffered saline (HBS, 10 mM sodium Hepes, 3 mM KCl, 137 mM NaCl, pH 7.4) at −20°C.
2.3. Preparation of colloidal gold nanoparticles (GNPs)
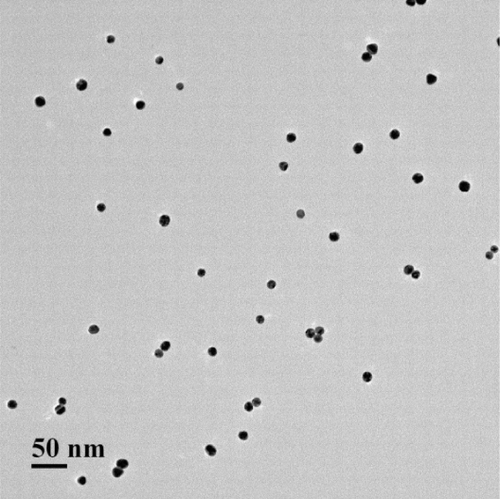
All glassware were thoroughly soaked with aqua regia (HCl/HNO3, volume ratio 3:1), washed with Millipore-Q water and air-dried before use. The GNPs were synthesised according to the previous classical method (Frens, Citation1973). Typically, 250 mL of 0.01% HAuCl4 solution was boiled for 5 min under constant stirring, and then 5 mL of 1% trisodium citrate solution was quickly added. After the colour of the solution changed to wine-red within several minutes, the reaction solution was boiled for another 5 min. The solution was then allowed to cool to room temperature and stored at 4°C. The transmission electron microscopy images () show that the diameter of the prepared colloidal gold was approximately 20 nm.
2.4. Preparation of colloidal gold probe
The colloid gold solution (1%, w/v) was adjusted to pH 8.2 with 0.1 M K2CO3. A total of 0.6 mL of ammonium sulphate precipitation purified anti-Cd(II)-EDTA monoclonal antibody (0.2 mg/mL, in 2 mM borate buffer, pH 8.2) was added to 10 mL pH-adjusted colloid gold solution. After gently mixing for 30 min, the gold particles were blocked with 10% BSA solution for 2 h and then recovered by centrifuging at 12,000 rpm for 20 min. After centrifugation, the gold pellets were suspended in 1 mL 10 mM Tris/HCI buffer (pH 8.2) containing 0.5% (w/v) BSA, 0.5% (w/v) PEG, and 0.01% (w/v) NaN3. The colloidal gold probe was centrifuged, resuspended, and then stored at 4°C until use.
2.5. Immunochromatography for the detection of Cd(II)
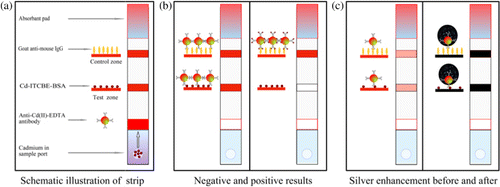
The fabrication of the immunochromatographic test strip is shown in . The Cd(II)-EDTA-BSA (0.5 mg/mL) antigen and goat anti-mouse IgG (0.4 mg/mL) (in phosphate-buffered saline, PBS, pH 7.4) were dispersed on the Nitrocellulose (NC) membrane as the test line and control line, respectively, by the dispensing platform and air-dried overnight. NC membranes bind proteins electrostatically through interaction of the strong dipole of the nitrate ester with the strong dipole of the peptide bonds of the protein. The purpose of drying the membrane is to fix protein to the NC. The goat anti-mouse IgG was the control protein to combine with the colloidal gold probe to validate the method. The Cd(II)-EDTA-BSA was the detection protein and competed with Cd(II)-EDTA in the sample for the colloidal gold probe. The concentrated colloidal gold probe (10 µL/strip) was added to the glass fibre membrane (conjugate pad) and dried. The sample pad was soaked in sucrose solution (5% w/v, in 20 mM Tris/HCl, pH 7.4) and dried. The sucrose protects the colloidal gold probe from aggregation and increases the mobility of colloidal gold probe on NC membranes when an aqueous sample is introduced. The NC membrane, conjugate pad, absorbent pad and sample pad were sequentially laminated to the adhesive card. The integrated strips were cut into individual strips 3.85 mm wide.
2.6. Cadmium detection and silver enhancement
First, the samples with specific concentrations of Cd(II) were then mixed with 10-fold concentrated HEPES buffer solution (HBS; 100 mM HEPES, 1.37 M NaCl, 30 mM KCl, and 10 µM EDTA, pH 7.4) and incubated for 30 min at 37°C to produce Cd(II)-EDTA(Abe et al., Citation2011). The detection was performed by applying 80 µL of appropriate standard test Cd(II) ions solution to the sample port of the strip. The visual results were obtained on the basis of the presence/absence of the test line after 10 min. In order to detect trace levels of cadmium, silver-enhancement reagent was used to amplify the signal on the strip as follows. After cadmium analysis, the tested strips were washed once with PBS containing 0.1% (w/v) Tween 20 and twice with distilled water. The washed strips were then soaked into a silver enhancer under cover at room temperature for 3–10 min. After a quick rinse in distilled water, the strip was fixed with sodium thiosulphate solution for 3 min at room temperature. The colour intensity of the test line on the strip was recorded by the BioDot TSR3000 Membrane Strip Reader.
2.7. Cross-reactivity of cadmium test strip
Other metal-EDTAs may have different affinity with the antibody and possibly interfere with the accuracy of the detection results. Therefore, samples containing other heavy metals were also tested on the strip to determine cross-reactivity.
2.8. Practicability of Cadmium test strip for drinking water sample
To evaluate the practicability of the cadmium test strip, drinking water was used as a diluent. The drinking water sample was taken from a bottled water purchased at the local market. All of the drinking water samples were filtered using a 0.45 µm nylon filter, and the pH was adjusted to 7.4. The samples were then mixed with 10-fold concentrated HEPES buffer solution and incubated for 30 min at 37°C. Different concentrations of cadmium were added to the prepared water and detected three times.
3. Results
3.1. Characterisation of anti-Cd(II)-EDTA monoclonal antibody
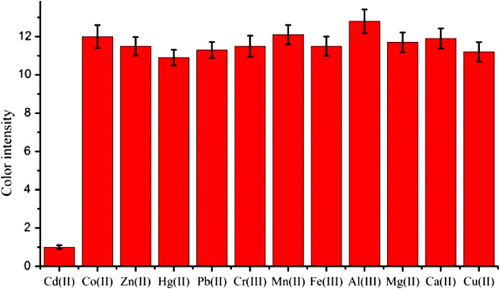
The monoclonal antibody 5H2 was produced in our lab; the experimental procedure is shown in the supporting information. An indirect competitive assay was performed, and the concentration of Cd(II) ions that produced 50% inhibition in the signal (IC50) was found to be 2 µg/L (see , Supporting information). The affinity constant was 1.1×1010 L/mol. The results of cross-reactivity demonstrated that the mAb had negligible cross-reactivity with other heavy metals, including Hg(II), Cu(II), Pb(II), Cr(III), Mn(II), Fe(III), Al(III), Mg(II), Co(II), Zn(II) and Ca(II).
3.2. Determination of test results
A schematic description of the immunochromatographic test device is shown in . In the NC membrane, the goat anti-mouse IgG and Cd(II)-ITCBE-BSA are separately dispersed onto the control zone (C) and test zone (T). The sample solution was applied to the sample port and rehydrated the dried anti-Cd(II)-EDTA monoclonal antibody-labelled gold nanoparticles. If there is no target in the sample, the gold probe will interact with the Cd(II)-ITCBE-BSA, and the test zone will appear as a red line because of the aggregated gold nanoparticles. As the concentration of Cd(II) in the sample increases, part of the gold probe would be combined and would not be able to interact with the Cd(II)-ITCBE-BSA test line. On the test zone, the colour intensity decreases as the concentration of the analyte increases in the sample. When the concentration of Cd(II) exceeds a certain value, no red line will appear on the test zone. Regardless of the presence of the Cd(II) in the sample solution, the control zone combines with the gold probe to validate the results. A negative result is judged by the appearance of two red lines, the control and test zones and a positive result is judged by the appearance of only a single line in the control zone (). If no line appears on the test strip or only one line appears in the test region, the test is invalid.
The principle of detection of small molecules on the strip is competition between immobilised antigen on the NC membrane and mobilised antigen in solution. The intensity of the colour on the test zone was inversely proportional to the amount of Cd(II) in the original sample, and this principle was used for semi-quantitative detection.
3.3. Optimisation of the strip determination
Many parameters need to be optimised in the strip detection system, such as the concentration of Cd(II)-ITCBE-BSA on the test zone, the amount of K2CO3 for pH adjustment, the concentration of antibody used to label GNPs and the concentration of BSA for blocking unoccupied sites on the gold probe.
First, a series of concentrations of Cd(II)-ITCBE-BSA (1, 0.75, 0.5, 0.25, 0.1, 0.05 mg/mL) dispersed on the NC membrane at the same deposition speed were compared, and 0.25 mg/mL Cd(II)-ITCBE-BSA was found to be optimal for capture concentration. When a lower concentration of capture reagent is used, the amount of the colloidal gold probe aggregated on the test zone was too little to be seen by the naked eye. Most of the gold nanoparticles were trapped under the surface of the NC membrane (Qin et al., Citation2012). A high concentration of the capture reagent on the test zone means that the red line disappeared only when a very high concentration Cd(II) is present and, hence, the LOD is very high.
The density of the antibody on the surface of gold nanoparticles will also affect the sensitivity of the detection. The optimal concentration of antibody to gold nanoparticles for the preparation of gold probes was found to be 4 µg/mL.
The sample pad was impregnated with a running buffer solution. The buffer solution contained: 0.5% BSA, 2% sucrose, in 100 mM HEPES (pH 9.0), 1.37 M NaCl and 30 mM KCl.
3.4. Sensitivity and stability of the detection
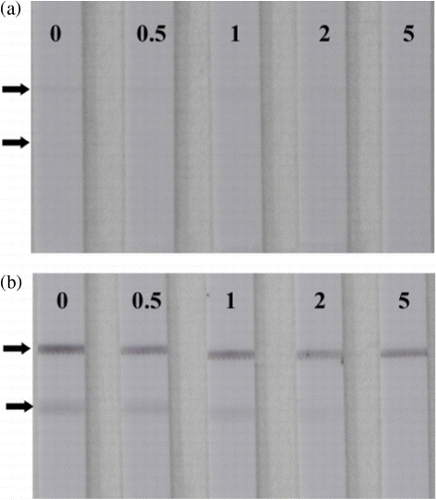
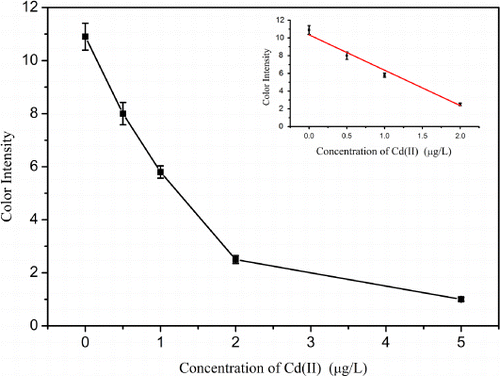
Using the optimised conditions, cadmium standard solutions at different concentrations (0–20 µg/L) were analysed using the strip sensor. As shown in , the intensity of the red colour decreased as the concentration of cadmium increased. When the concentration of cadmium in the sample solution reached 20 µg/L, the test line disappeared. Thus, the visual detection limit of the conventional method was 20 µg/L. In order to improve the limit, the tested strip was treated with silver enhancer. Compared with the sandwich LFA (Chiao et al., Citation2004), the competitive LFA was applied to detect small molecules characterised by a “turn-off” effect on the colour intensity. The intensities of the test zones will decrease as the concentration of the analyte increase. However, the silver enhancement will deepen the intensities of the test zones, and the visual LOD will increase. In order to increase sensitivity of the competitive LFA with the silver enhancement, the concentration of Cd(II)-ITCBE-BSA and the amount of the gold-labelled antibody need to be optimised further. As shown in , only a very faint line could be observed when the concentration of Cd(II)-ITCBE-BSA was adjusted to 0.1 mg/mL, and the concentration of the gold-labelled antibody probe used in the single strip was diluted 10-fold (the volume was still 10 µL). However, this result could not be distinguished easily with confidence because the negative test line was very faint (). As shown in , the detection result was obvious and clear when such “invalid” strip results were treated with silver enhancer. Using silver enhancement, 0.5 µg/L cadmium was detectable, and the line was not observed when the concentration of Cd(II) reached 5 µg/L. The sensitivity of this method was four-fold greater than the conventional method. The standard curve showed that the colour intensity decreased linearly as the concentration increased (). The LOD for semi-quantitative detection was as low as 0.35 µg/L by using a scanning reader. The control experiment involved the use of Pb(II)-ITCBE-KLH as the capture reagent, and no line was revealed in the test zone before and after silver enhancement. This indicated that the non-specific binding of the reagents was very low.

The stability of the antibody-based strips was evaluated. All strips were sealed in hermetic bags with desiccating agent stored for 10, 20, 30 and 40 days at room temperature, respectively. Sample solutions without Cd(II) ions were used. A visual comparison of the intensity of the colour on the test zone was performed, and no significant differences were observed on the test lines between the 10-, 20- and 30-day strips. The colour intensity of the test line and control line of the 40-day strip was slightly weaker than that of the new strips, which showed that the strips were still useable after 40 days of storage.
The intra- and inter-assay repeatability of this method was evaluated by the coefficient of variations (CVs) at different Cd(II) ions concentrations. The intra-assay CV was obtained by analysing each sample three times on the same day. The average of the individual CVs was recorded as the intra-assay CV. The inter-assay CV was determined by analysing the same sample three times in three different days. The results are summarised in Table S1b and indicate that the intra- and inter-assay CVs are 2.2–4.2 and 2.3–4.6%, respectively.
3.5. Specificity confirmation
The false positive and negative detection results may be a problem in the practical detection. In particular, the metal ions are widespread and species abundant in the environment. Thus, it was necessary to confirm other metal ions that have no cross-reaction with Cd(II). From the results of ELISA, the antibody has a negligible cross-reactivity with Hg(II) (<2%) which may be caused by the similar three-dimensional structures between Cd(II)-EDTA and Hg(II)-EDTA (Jones, Yu, Delehanty, & Blake, Citation2002). To test the assay specificity, the colour intensity of the test lines were investigated when the following metal ions were added (500 µg/L): Co(II), Zn(II), Hg(II), Pb(II), Cr(III), Mn(II), Fe(III), Al(III), Mg(II), Ca(II) and Cu(II). As shown in , this strip sensor has a good selectivity for Cd(II). This was due to the low affinity of the antibody for other metal-chelator complexes.
3.6. Detection of Cd(II) in drinking water samples
The accuracy and applicability of this strip sensor were also tested. Drinking water samples spiked with different concentrations of Cd(II) were used as simulated samples. The average recoveries of the determinations were used to evaluate the accuracy of the assay. As shown in Table S2, the recoveries were from 98 to 108%, which meet the requirements for detection.
4. Conclusion
In the present study, a colloidal gold probe-based silver enhancement immunochromatographic assay has been successfully developed for the detection of cadmium in drinking water samples. This method is fast, simple and sensitive. The visual detection limit was 5 µg/L, and the LOD for semi-quantitative detection was as low as 0.35 µg/L by using a scanning reader. This satisfies the requirement regulated by the US EPA for drinking water. In summary, this biosensor could be a potential alternative tool for the detection of aqueous Cd(II) in drinking water.
Supporting Information
A silver enhanced and sensitive strip sensor for Cadmium detection
Mouse Immunization, Hybridoma Production, and Purification of Monoclonal Antibodies.
Five 6-week old BALB/c mice were injected with 100 µg of the Cd(II)-EDTA-KLH conjugate emulsified with an equal volume of Freund's complete adjuvant. The subsequent injection was repeated three times at 3-week intervals with Freund's incomplete adjuvant and the injection dose was reduced in half. After the third injection, antibody response of each mouse was determined using indirect competitive ELISA following the antibody titre test. Cd(II) was tested for the ability to inhibit binding to the immobilized Cd(II)-EDTA-BSA conjugate in a HBS amended with 1 mM EDTA. The mice showing the highest antibody responses were given a final boost of 20 µg of the KLH conjugate in phosphate-buffered saline (pH 7.4) 4 days prior to fusion.
A mouse with antibody to Cd(II)-EDTA was selected to cell fusion according standard operating procedure (Pontecorvo, 1975). Supernatants from replicating hybridomas were collected and screened for antibodies to Cd(II)-EDTA using competitive inhibition ELISA. Positive hybridoma clones were subcloned twice by limiting dilution. BALB/c mice primed with Freund's incomplete adjuvant were injected with 1.8×106 hybridoma cells and ascites fluid was collected 12-14 days later. The ascites fluid was purified by bitter-ammonium sulphate law.
The protocol of the indirect competitive ELISA
The general procedure was performed as follows. Microwell plates with 96 wells were coated (100 µl/well) with Cd(II)-ITCBE-BSA at 1.5 µg/mL in HBS buffer (HBS, 10 mM sodium Hepes, 3 mM KCl, 137 mM NaCl, pH 9.0) for 2 h at 37 °C. After being washed three times with phosphate buffered saline (PBS, 137 mM NaCl, 3 mM KCl, 10 mM phosphate, pH 7.4) containing 0.05% Tween 20(PBST), the plates were blocked with 2% BSA in HBS for 2 h at 37 °C. After being washed again, the plates were air-dried 15 min at 37°C. 50µl purified antibody (1:8000) was incubated in the presence of 50µl different concentrations of lead ions in HBS buffer amended with 1 mM EDTA. After 0.5 h at 37°C, the plates were washed with PBST and the amount of antibody captured by the Cd(II)-ITCBE-BSA was bound by the goat anti-mouse IgG-horseradish peroxidase(HRP) conjugate. After washing to separate the unbound goat anti-mouse IgG-HRP conjugate, TMB substrate was added and oxidised by HRP into the final product. The results were read by the microplate reader at 450 nm.
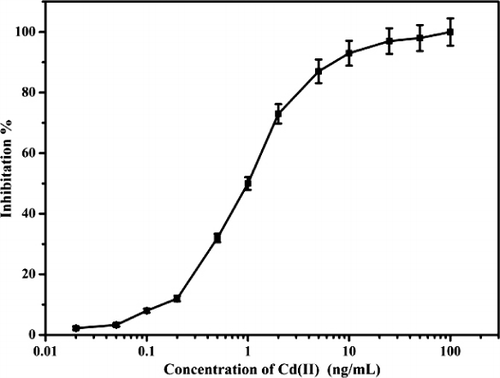
Table S1. The intra- and inter-assay of the visual sensor for Cd(II).
Table S2. Recovery test of Cd(II) in drinking water samples.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (21071066, 91027038, 21101079, 21175034), the Key Programs from MOST (2012BAC01B07, 2012BAD29B05, 2012AA06A303, 2012BAD29B04, 2011BAK10B07, 2011BAK10B05, 2011BAK10B01, 2010AA06Z302, 2010DFB3047, 2011ZX08012-001, 2012BAK17B10, 2012BAK08B01, 2012YQ090194), and grants from Jiangsu Province, MOF and MOE (NCET-12-0879, BE2011626, 201210036, 201310135, 311002).
References
- Abe, K., Nakamura, K., Arao, T., Sakurai, Y., Nakano, A., Suginuma, C., & Sasaki, K. (2011). Immunochromatography for the rapid determination of cadmium concentrations in wheat grain and eggplant. Journal of the Science of Food and Agriculture, 91(8), 1392–1397. doi:10.1002/jsfa.4321
- Abe, K., Sakurai, Y., Okuyama, A., Sasaki, K., & Tawarada, K. (2009). Simplified method for determining cadmium concentrations in rice foliage and soil by using a biosensor kit with immunochromatography. Journal of the Science of Food and Agriculture, 89(6), 1097–1100. doi:10.1002/jsfa.3541
- Aragay, G., Pons, J., & Merkoçi, A. (2011). Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chemical Reviews, 111(5), 3433–3458. doi:10.1021/cr100383r
- Blake, D. A., Chakrabarti, P., Khosraviani, M., Hatcher, F. M., Westhoff, C. M., Goebel, P., & Blake, R. C. (1996). Metal binding properties of a monoclonal antibody directed toward metal-chelate complexes. Journal of Biological Chemistry, 271(44), 27677–27685. doi:10.1074/jbc.271.44.27677
- Blake, R. C., Pavlov, A. R., Khosraviani, M., Ensley, H. E., Kiefer, G. E., Yu, H., & Blake, D. A. (2004). Novel monoclonal antibodies with specificity for chelated uranium(VI): Isolation and binding properties. Bioconjugate Chemistry, 15(5), 1125–1136. doi:10.1021/bc049889p
- Cao, Y. C., Jin, R., & Mirkin, C. A. (2002). Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science, 297(5586), 1536–1540. doi:10.1126/science.297.5586.1536
- Chiao, D. J., Shyu, R. H., Hu, C. S., Chiang, H. Y., & Tang, S. S. (2004). Colloidal gold-based immunochromatogrphic assay for detection of botulinum neurotoxin type B. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 809, 37–41. doi:10.1016/j.jchromb.2004.05.033
- Cho, E. S., Kim, J., Tejerina, B., Hermans, T. M., Jiang, H., Nakanishi, H., & Grzybowski, B. A. (2012). Ultrasensitive detection of toxic cations through changes in the tunnelling current across films of striped nanoparticles. Nature Materials, 11(11), 978–985. doi:10.1038/nmat3406
- Darwish, I. A., & Blake, D. A. (2001). One-step competitive immunoassay for cadmium ions: Development and validation for environmental water samples. Analytical Chemistry, 73(8), 1889–1895. doi:10.1021/ac0012905
- Darwish, I. A., & Blake, D. A. (2002). Development and validation of a one-step immunoassay for determination of cadmium in human serum. Analytical Chemistry, 74(1), 52–58. doi:10.1021/ac010510r
- Fang, Z., Huang, J., Lie, P., Xiao, Z., Ouyang, C., Wu, Q., Zeng, L. (2010). Lateral flow nucleic acid biosensor for Cu2 + detection in aqueous solution with high sensitivity and selectivity. Chemical Communication (Cambridge), 46(47), 9043–9045. doi:10.1039/c0cc02782k
- Frens, G. (1973). Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature Physical Science, 241, 20–22. Retrieved from http://www.nature.com/nature-physci/journal/v241/n105/abs/physci241020a0.html.
- Guo, Z. Y., Duan, J., Yang, F., Li, M., Hao, T. T., Wang, S., & Wei, D. Y. (2012). A test strip platform based on DNA-functionalized gold nanoparticles for on-site detection of mercury (II) ions. Talanta, 93, 49–54. doi:10.1016/j.talanta.2012.01.012
- Hou, S. Y., Chen, H. K., Cheng, H. C., & Huang, C. Y. (2007). Development of zeptomole and attomolar detection sensitivity of biotin-peptide using a dot-blot goldnanoparticle immunoassay. Analytical Chemistry, 79(3), 980–985. doi:10.1021/ac061507g
- Jones, R. M., Yu, H. N., Delehanty, J. B., & Blake, D. A. (2002). Monoclonal antibodies that recognize minimal differences in the three-dimensional structures of metal-chelate complexes. Bioconjugate Chemistry, 13(3), 408–415. doi:10.1021/bc0155418
- Khosraviani, M., Blake II, R. C., Pavlov, A. R., Lorbach, S. C., Yu, H., Delehanty, J. B., & Blake, D. A. (2000). Binding properties of a monoclonal antibody directed toward lead-chelate complexes. Bioconjugate Chemistry, 11(2), 267–277. doi:10.1021/bc9901548
- Li, H., Chen, C. Y., Wei, X., Qiang, W., Li, Z., Cheng, Q., & Xu, D. (2012). Highly sensitive detection of proteins based on metal-enhanced fluorescence with novel silver nanostructures. Analytical Chemistry, 84(20), 8656–8662. doi:10.1021/ac301787x
- Liu, S., Clever, G. H., Takezawa, Y., Kaneko, M., Tanaka, K., Guo, X. F., & Xu, D. (2011). Direct conductance measurement of individual metallo-DNA duplexes within single-Molecule break junctions. Angewandte Chemie-International Edition, 50(38), 8886–8890. doi:10.1002/anie.201102980
- Mathew, A., Sajanlal, P. R., & Pradeep, T. (2012). Selective visual detection of TNT at the sub-Zeptomole level. Angewandte Chemie International Edition, 51(38), 9596–9600. doi:10.1002/anie.201203810
- Mazumdar, D., Liu, J., Lu, G., Zhou, J., & Lu, Y. (2010). Easy-to-use dipstick tests for detection of lead in paints using non-cross-linked gold nanoparticle-DNAzyme conjugates. Chemical Communication (Cambridge), 46(9), 1416–1418. doi:10.1039/b917772h
- McLaughlin, M., & Singh, B. (1999). Cadmium in soils and plants. The Netherlands: Kluwer Academic Publishers Dordrecht. doi:10.1007/978-94-011-4473-5
- Nordberg, G. F. (1974). Health hazards of environmental cadium pollution. Ambio, 3, 55–56. Retrieved from http://www.jstor.org/stable/4312046.
- Qin, Z., Chan, W. C., Boulware, D. R., Akkin, T., Butler, E. K., & Bischof, J. C. (2012). Significantly improved analytical sensitivity of lateral flow immunoassays by using thermal contrast. Angewandte Chemie, 124(18), 4434–4437. doi:10.1002/ange.201200997
- Reardan, D. T., Meares, C. F., Goodwin, D. A., McTigue, M., David, G. S., Stone, M. R., & Frincke, J. M. (1985). Antibodies against metal chelates. Nature, 316(6025), 265–268. doi:10.1038/316265a0
- Rodríguez-Lorenzo, L., de la Rica, R., Álvarez-Puebla, R. A., Liz-Marzán, L. M., & Stevens, M. M. (2012). Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nature Materials, 11(7), 604–607. doi:10.1038/nmat3337
- Sasaki, K., Oguma, S., Namiki, Y., & Ohmura, N. (2009a). Monoclonal antibody to trivalent chromium chelate complex and its application to measurement of the total chromium concentration. Analytical Chemistry, 81(10), 4005–4009. doi:10.1021/ac900419c
- Sasaki, K., Yongvongsoontorn, N., Tawarada, K., Ohnishi, Y., Arakane, T., Kayama, F., & Ohmura, N. (2009b). Cadmium purification and quantification using immunochromatography. Journal of Agricultural and Food Chemistry, 57(11), 4514–4519. doi:10.1021/jf900155t
- Tang, Y., Zhai, Y. F., Xiang, J. J., Wang, H., Liu, B., & Guo, C. W. (2010). Colloidal gold probe-based immunochromatographic assay for the rapid detection of lead ions in water samples. Environmental Pollution, 158(6), 2074–2077. doi:10.1016/j.envpol.2010.03.001
- Torabi, S. -F., & Lu, Y. (2011). Small-molecule diagnostics based on functional DNA nanotechnology: A dipstick test for mercury. Faraday Discussions, 149, 125.doi: 10.1039/c005404f
- Wang, Y. Z., Yang, H., Pschenitza, M., Niessner, R., Li, Y., Knopp, D., & Deng, A. P. (2012). Highly sensitive and specific determination of mercury(II) ion in water, food and cosmetic samples with an ELISA based on a novel monoclonal antibody. Analytical and Bioanalytical Chemistry, 403(9), 2519–2528. doi:10.1007/s00216-012-6052-1
- Wylie, D. E., Lu, D., Carlson, L. D., Carlson, R., Babacan, K. F., Schustercan, S. M., & Wagner, F. W. (1992). Monoclonal antibodies specific for mercuric ions. Proceedings of the National Academy of Sciences, 89(9), 4104–4108. doi:10.1073/pnas.89.9.4104
- Yang, W., Li, X. B., Liu, G. W., Zhang, B. B., Zhang, Y., Kong, T., & Wang, Z. (2011). A colloidal gold probe-based silver enhancement immunochromatographic assay for the rapid detection of abrin-a. Biosensors & Bioelectronics, 26(8), 3710–3713. doi:10.1016/j.bios.2011.02.016
- Zhao, H. W., Xue, C. G., Nan, T. G., Tan, G. Y., Li, Z. H., Li, Q. X., & Wang, B. M. (2010). Detection of copper ions using microcantilever immunosensors and enzyme-linked immunosorbent assay. Analytica Chimica Acta, 676(1–2), 81–86. doi:10.1016/j.aca.2010.07.041
- Zhu, X. X., Xu, L., Lou, Y., Yu, H. N., Li, X., Blake, D. A., & Liu, F. Q. (2007). Preparation of specific monoclonal antibodies (MAbs) against heavy metals: MAbs that recognize chelated cadmium ions. Journal of Agricultural and Food Chemistry, 55(19), 7648–7653. doi:10.1021/jf071025l