Abstract
An indirect enzyme-linked immunosorbent assay (ELISA) was developed for the quantitative analysis of octylphenol. A linear carboxylated analogue of 4-nonylphenol was synthesised and characterised as hapten. The hapten was coupled to the carrier protein to produce a specific antibody. An indirect competitive ELISA method was established based on the polyclonal antibody, which exhibited an IC50 value of 51 ng/mL for octylphenol. A monoclonal antibody was produced with an IC50 value of 76 ng/mL for octylphenol. The immunoassay used with the monoclonal antibodies was applied to analyse water samples from Tai Lake in China.
Keywords:
1. Introduction
Alkylphenol polyethoxylates (APEOs) represent an important class of non-ionic surfactants which are widely used in detergents, textiles, paper and in many other industries as emulsifiers and as solubilising, wetting or dispersing agents (Chen et al., Citation2006). Large amounts of APEO have been continuously discharged into the environment and are quickly biodegraded to alkylphenols (APs), such as 4-nonylphenol (NP) and octylphenol (OP), which are more toxic and difficult to biodegrade (Hotta et al., Citation2010). These metabolites are very dangerous mainly due to their strong teratogenic effects and estrogenic effects in fish and mammals (Abdulla Bin-Dohaish el, Citation2012; Chaube, Gautam, & Joy, Citation2013; Ferrara, Fabietti, Delise, & Funari, Citation2005; Ghekiere, Verslycke, & Janssen, Citation2006; Meucci & Arukwe, Citation2005; Sabik, Gagne, Blaise, Marcogliese, & Jeannot, Citation2003; Soto, Justicia, Wray, & Sonnenschein, Citation1991; Uguz, Iscan, Erguven, Isgor, & Togan, Citation2003; Vaccaro et al., Citation2005). Moreover, Pachura-Bouchet, Blaise, and Vasseur (Citation2006) also found that these metabolites were toxic to invertebrates. The toxicity of AP increases with decreasing ethoxylate chain length, and OP has higher estrogenic activity than NP (Servos, Citation1999). Although little data have been obtained on human exposure, the above findings have raised increasing concern regarding the impact of APEO and their biodegradation products on the environment and the human health.
Currently, a large number of methods are used for the detection of APEO and their biodegradation products. Of these methods, chromatographic methods such as gas, liquid or high-pressure liquid chromatography (HPLC) are widely used (Ferrer et al., Citation2011; Lu, Chen, & Liu, Citation2009; Meulenberg, Citation2009; Stenholm, Hölmstrom, Hjärthag, & Lind, Citation2012; Takasu, Iles, & Hasebe, Citation2002; Wu & Marriott, Citation2011). Chromatography–tandem mass spectrometry was shown to be most effective (Andreu, Ferrer, Rubio, Font, & Pico, Citation2007; Cespedes et al., Citation2006; Koh et al., Citation2009; Vega-Morales, Sosa-Ferrera, & Santana-Rodriguez, Citation2010). Norton and Shamsi (Citation2007) found that capillary electrochromatography–electrospray ionisation–mass spectrometry offered several unique advantages over the traditional methods based on HPLC–MS. However, these methods were time-consuming and demanded sophisticated equipments. Therefore, simple and quick analytical methods are required to detect APEO and their biodegradation products in environmental and food samples.
Immunochemical methods, which have been used for decades, are rapid, simple and reliable tools for screening analysis and have gradually been incorporated in environmental and food analyses (Franek & Hruska, Citation2005; Franek et al., Citation2001). Enzyme-linked immunosorbent assay (ELISA) is an alternative to conventional instrumental analysis due to its sensitivity, cost-efficiency and short effective determination time (Zeravik, Skryjová, Nevoranková, & Fránek, Citation2004). Much work has been done to detect APEO, NP and OP using ELISA (Goda et al., Citation2004; Mart'ianov et al., Citation2005; Mart'ianov, Zherdev, Eremin, & Dzantiev, Citation2004; Matsui et al., Citation2007; Meulenberg, Citation2009; Samsonova, Rubtsova, & Franek, Citation2003). Samsonova, Uskova, Andresyuk, Franek, and Elliott (Citation2004) developed a biacore biosensor immunoassay using two types of antibodies for the determination of 4-NP in 31 shellfish samples. But the assay incorporating polyclonal antibodies with high cross-reactivity (CR) towards technical 4-NP achieved remarkably different results from the assay incorporating a monoclonal antibody very specific to 4-n-nonylphenol. Therefore, verification of the results by chromatographic methods was still needed, and moreover, the sensitivity of the assay was less than that of conventional ELISA. Estévez, Kreuzer, Sánchez-Baeza, and Pilarmarco (Citation2006) prepared an immunising hapten with a four-carbon atom spacer arm placed at the ortho position which preserved both the hydroxyl group and the branched nonyl chain of the NP. The assay reached a limit of detection (LOD) of 2.3±0.9 µg/L and an half maximal inhibitory concentration (IC50) value of 29±5 µg/L (for both, n=16). However, the recovery studies with real wastewater samples from different wastewater treatment plants showed that the sample preparation procedure used had a significant influence on the method accuracy. Meulenberg (Citation2009) reviewed the occurrence and immunochemical detection of phenolics in the environment and food. She concluded that there was a need for new bioassays and immunoassays for real-time and sensitive detection and optional immunoaffinity supports and sensors for the purification, concentration and real-time detection of target compounds. Therefore, more research is needed to develop a more sensitive and effective ELISA technique.
To date, many reported immunoassays are focused on the production of antibodies against NP, because NP is the main degradation product of APEO. But there is still an urgent need to produce an antibody which is highly specific to OP. The purpose of the present study is to develop a rapid and sensitive immunoassay method for OP determination. A linear carboxylated analogue of NP was synthesised as the hapten, and then used for further immune response.
2. Experimental methods
2.1. Chemicals and materials
Standard OP and NP technical mixtures were bought from Sigma Chemical Co. (St. Louis, MO, USA). Carrier proteins such as bovine serum albumin (BSA) and ovalbumin (OVA) were purchased from BoAo Co., Shanghai, China. Coupling agents including N-hydroxysulphosuccinimide sodium salt (Sulph-NHS) and 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide (EDC), and polyethylene glycol solution (PEG, Hyb-ri-Max, 50% (w/v)) as fusogen were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Freund's complete and incomplete adjuvant, goat anti-mouse IgG conjugated to horseradish peroxidase (HRP) and 3,3,5,5-tetramethylbenzidine (TMB) were also bought from Sigma Chemical Co. (St. Louis, MO, USA). RPMI-1640 cell culture medium, foetal calf serum, hypoxanthine–aminopterin–thymidine medium (HAT) and hypoxanthine–thymidine medium were purchased from Gibco BRL (Paisley, Scotland). The Sp2/0-Ag14 murine myeloma cell line was provided by the Cell Bank of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). BALB/c mice were obtained from the Comparative Medicine Center of Yangzhou University. All other chemicals and reagents were bought from Sinopharm Chemical Reagent Co, Ltd. (Shanghai, China).
2.2. Synthesis of hapten derivative
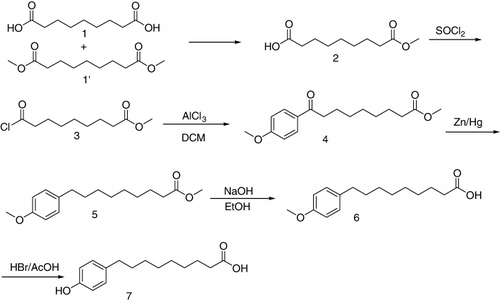
The hapten derivative was prepared as shown in (Mikhura, Formanovsky, Nikitin, Yakovleva, & Eremin, Citation2000). Nonanedioic acid (compound 1, 353 mg, 1.875 mmol) and dimethyl azelate (compound 1′, 405 mg, 1.875 mmol) were stirred overnight with tetrahydrofuran (THF) (5 ml) in a round-bottomed flask (25 ml) at ambient temperature, and the reaction mixture was then concentrated to give compound 2 (750 mg, yield 100%). After the addition of methyl hydrogen azelate (750 mg, 3.71 mmol) and sulphoxide chloride (1.5 ml) to the flask, the reaction liquid was refluxed with two drops of N,N-dimethylformamide (DMF) for 2 h. Following the evaporation of sulphoxide chloride, dichloromethane (DCM) was added to remove residual sulphoxide chloride to give compound 3 (820 mg, yield 100%). Anisole (441 mg, 4.08 mmol) and DCM (3 ml) were stirred with anhydrous aluminium chloride (1740 mg, 13.08 mmol) in a 50-ml round-bottomed flask in an iced water bath. A solution of compound 3 (818 mg, 3.708 mmol) in DCM (2 ml) was then added to this mixture and stirred for 1 h. The reaction mixture was then poured into iced water and the reaction was quenched with 3 M hydrochloric acid. The water phase was extracted twice with ethyl acetate. The organic phase was washed with salt water and then dehydrated with anhydrous sodium sulphate. The organic phase was concentrated and isolated by column chromatography to give the target compound 4 (1.0 g, yield 92.3%). Zinc (1.95 g), 5% mercuric acetate solution (3.2 ml), water (1.5 ml) and concentrated hydrochloric acid (3.4 ml) were added to a solution of compound 4 in toluene (2 ml) and then stirred and refluxed for 6 h. The reaction mixture was extracted three times with ethyl acetate (10 ml each time). The organic phase was dehydrated with anhydrous sodium sulphate and isolated by column chromatography to give the target compound 5 (800 mg, yield 94%). To a stirred mixture of compound 5 (220 mg, 0.79 mmol) and ethanol (2.5 ml), 10% sodium hydroxide solution (2.5 ml) was added. The reaction mixture was refluxed for 6 h and then concentrated. Water (2.5 ml) was added to the residue, and then acidification was adjusted with hydrochloric acid. The reaction mixture was extracted with ethyl acetate. The organic phase was dehydrated with anhydrous sodium sulphate and concentrated to give compound 6 (180 mg, yield 86.2%). Compound 6 (165 mg, 0.615 mmol), 48% HBr (1.5 ml) and glacial acetic acid (1.5 ml) were added to a round-bottomed flask (25 ml), refluxed for 6 h, then the reaction mixture was concentrated and isolated by column chromatography to give compound 7 (120 mg, yield 78%). Finally, the hapten derivative (compound 7, named NPA) was identified by Nuclear Magnetic Resonance (NMR) and Ultra Performance Liquid Chromatography coupled with Time-of-Flight Mass Spectrometry (UPLC-TOF-MS/MS).
2.3. Preparation of hapten–protein conjugates
The hapten derivative (12 mg) was dissolved in DMF (0.5 ml) in a brown bottle. Sulph-NHS (15 mg) and EDC (15 mg) were added to the hapten derivative solution, and reacted at ambient temperature for 2.5 h as solution A. BSA (38 mg) was dissolved in phosphate buffer solution (PBS, 0.01 M, pH 7.4) as solution B. Then, solution A was added dropwise to solution B and reacted at ambient temperature for 3 h. The reaction mixture was centrifuged, the sediment removed and the supernate obtained to give the artificial antigen mixture. The artificial antigen mixture was then transferred to a pre-treated dialysis bag, dialysed with 2×2 L 0.01 M PBS and 2×2 L distilled water for 3 days. Finally, hapten–BSA was freeze-dried to powder and used as the immunogen. The coating antigen (hapten–OVA) was conjugated in the same way.
2.4. Production and purification of polyclonal antibodies
Six female BALB/c mice (6–8 weeks) were immunised with the immunogen which was emulsified using Freund's adjuvant. The immunogen dissolved in PBS was fully emulsified with isovolumetric Freund's complete adjuvant for the first immunisation which was administered by hypodermic injection into the back of the neck in each mouse. The mice were injected with 100 µg immunogen each time. Three weeks later, the immunogen was emulsified with Freund's incomplete adjuvant for the second immunisation. After that, the enhanced immunisation was carried out every 2 weeks with the immunogen which was emulsified with Freund's incomplete adjuvant to meet the requirements of serum titre. The immune response was determined by intraperitoneal injection for 3 days. A mouse was sacrificed for cell fusion and ocular blood was collected at the same time and centrifuged to obtain serum for the polyclonal antibody.
2.5. Screening of polyclonal antibodies by an indirect competitive ELISA (icELISA)
The hapten–OVA solutions (0.5 µg/ml, 100 µL/well) were used to coat 96-well polystyrene microplates with carbonate coating buffer and incubated at 4°C overnight. The plates were then washed three times with Phosphate Buffered Saline containing Tween-20 (PBST) which contained 0.05% Tween 20 and blocked with blocking buffer (0.2% gelatin in carbonate buffer, 200 µL/well) for 2 h at 37°C. After washing, the OP standard solutions (50 µL/well) and diluents of antibodies (50 µL/well) were added in sequence to the plates and incubated for 30 min at 37°C, then washed four times with PBST. After that, goat anti-mouse IgG–HRP (100 µL/well) was added and the mixture was incubated at ambient temperature for 30 min. The mixture was washed five times with PBST, and the plates were incubated with TMB substrate solution (100 µL/well) for 15 min. The reaction was then terminated by adding 50 µL H2SO4 (2 M) and the absorbance was read at 450 nm using a microplate reader.
2.6. Production and purification of monoclonal antibodies
The immunised mouse with the highest specificity antibody was dissected. The spleen was separated aseptically and the splenocytes were fused with SP2/0 myeloma cells in a 1:10 ratio by adding 50% PEG 1500 drop by drop for 1 min. After that, cell fusion was stopped by adding cell culture solution, and the produced hybridoma cells were suspended in a selective medium which included RPMI 1640 medium, 20% Fetal Cattle Serum (FCS) and 2% HAT solution. The cells were then placed on 96-well plates and cultured for 9 days. The supernatants in the wells were measured for hybridoma selection using icELISA. After three cycles of cell sub-cloning and cell selections, the chosen cell line was enlarged in large-scale culture and injected intraperitoneally into the mice which were pre-treated with liquid paraffin. Ascites were extracted and then purified with caprylic acid and saturated ammonium sulphate (Temponi et al., Citation1989).
2.7. icELISA for monoclonal antibody
The hapten–OVA conjugate (0.3 µg/mL, 100 µL/well) was used as a coating antigen to coat the microplates with carbonate buffer and incubated overnight at 4°C. The plates were washed three times with PBST and blocked with a blocking buffer at ambient temperature for 2 h. After these procedures, serial diluents of the OP standard (50 µL/well) which were diluted in PBS containing 10% methanol were distributed onto the plates, followed by the monoclonal antibodies (50 µL/well), and then the plates were incubated for 30 min at 37°C. Finally, the plates were measured as shown in Section 2.5.
2.8. Determination of CR
The sensitivity and specificity of the OP antibody were characterised by the IC50 and CRs. The CR values were calculated using the following formula: CR (%)=(IC50 of octylphenol/IC50 of related substances)×100%.
2.9. Preparation and detection of samples
In this study, the developed immunoassay was used to analyse water samples from Tai Lake in China. Taihu Lake is a large lake in eastern China and is polluted due to the presence of many factories in the area. Raw water samples spiked with OP were diluted appropriately with 10% MeOH–PBST solution (Estévez et al., Citation2006) and measured using the optimised ELISA. The recoveries of the fortified water samples were calculated based on the standard curves and the measured values of the samples. The coefficient of variation of intra-assay was determined based on five replicates and the inter-assay variation was tested for three consecutive days.
3. Results and discussion
3.1. Hapten derivative design and synthesis
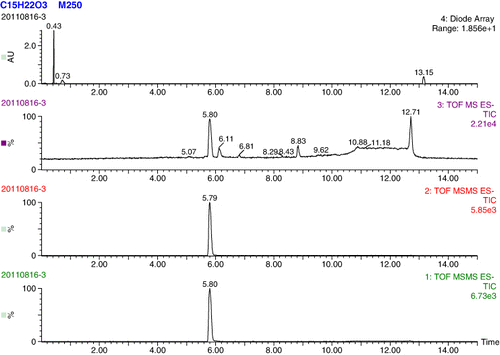
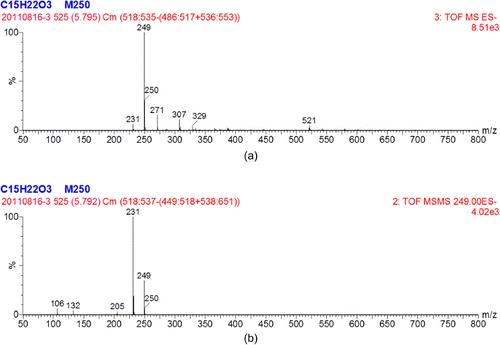
Choosing an appropriate immunising hapten is very important for ELISA detection (Estévez et al., Citation2006) and should have the ability to induce an immune response and obtain a highly specific antibody. Therefore, we synthesised an analogue which had a very similar structure to NP and retained the phenol group and alkyl chain structure with the exception of the carboxyl chain end. The NP analogue was dissolved in deuterated chloroform for characterisation by NMR. The NMR analysis was based on the chemical shift values and the integral values with tetramethylsilane (TMS) as the internal standard. 1H NMR (CDCl3, 400 MHz): δ 0.071 (s, 3H), 1.255–1.301 (m, 8H), 1.560–1.643 (m, 4H), 2.324–2.362 (m, 2H), 2.504–1.542 (m, 2H), 6.731–6.753 (m, 2H), 7.023–7.024 (m, 2H), 7.260 (s, 1H). δ 0.071 (single, 3H) represented the 3H atoms of the methyl group in TMS, and δ 7.260 (single, 1H) represented the deuterium atom in deuterated chloroform. The integral values of 4.062 (δ 1.560–1.643) and 9.131 (δ 1.255–1.301) indicated that the 12 hydrogen atoms of the six methylenes in the alkyl chain split into two large group peaks due to the suction electronic effect between the benzene ring and carboxyl. The integral value of 2.000 (δ 2.324–2.362) indicated the presence of two hydrogen atoms of the methylene nearest to the carboxyl. The integral value of 2.043 (δ 2.504–1.542) indicated the presence of two hydrogen atoms of the methylene nearest to the benzene ring. Integral values of 1.717 (δ 6.731–6.753) and 1.765 (δ 7.023–7.024) represented the hydrogen atoms of the two methylenes in the benzene ring, which were close to the alkyl chain and the hydroxyl, respectively. The NMR information indicated that the NP analogue was very pure and had been synthesised successfully. The NP analogue was also identified by UPLC-TOF-MS/MS ( and ). The molecular weight of the NP analogue was 250.33 and its chemical formula was C15H22O3. As shown in , a strong single peak was found within 5.80 min which indicated the target compound of the NP analogue. The blank experiment showed that the peaks at 6.11, 6.81 and 8.83 min were due to impurities inside the chromatographic column. The first order mass spectrum at 5.80 min is shown in , where the ions (m/z) 249 and 250 represented the [M–1]− and M parent ions of the NP analogue, respectively. shows the second-order mass spectrum at 5.80 min, and the daughter ions 231, 205, 131 and 106 represented the molecular fragments of the NP analogue. These results demonstrated that the synthesis procedure of the NP analogue was very successful.
3.2. Preparation of hapten–protein conjugates
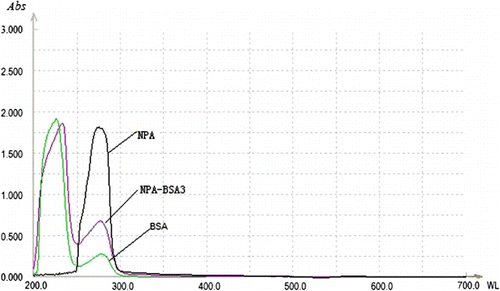
The conjugation ratio characterised the molar ratio of the two conjugated molecules and was established by the principle of testing the two molecular contents or relative contents. In this study, the hapten derivative (NPA) was conjugated to BSA as an immunogen and the conjugation ratio was obtained using an ultraviolet (UV) scanner (UNICO, UV-2802pcs). The protein content of the hapten–protein conjugates was measured by Coomassie brilliant blue staining. The carrier protein and hapten–BSA conjugate were diluted to 500 µg/mL with ultrapure water and their UV absorption spectra are illustrated in . The hapten showed one specific UV absorption peak at 272 nm, while the two UV absorption peaks of the carrier protein were observed at 220 and 280 nm. It was obvious that the complete antigen had the characteristic UV peaks of the carrier protein and hapten and showed superposition properties. In addition, a little UV peak shift was also observed for the immunogen. These results indicated that the hapten–protein conjugates were successfully synthesised. The absorption values at 272 and 280 nm were measured and the conjugation ratio was calculated from molar absorptivity and was found to be approximately 17.
3.3. Screening of antibodies and specificity of the icELISA
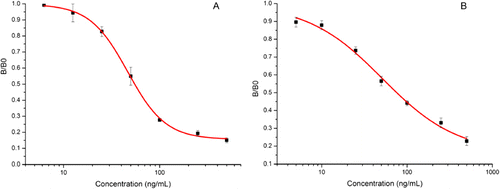
The icELISA was employed to analyse the titre and the specificity of antibodies. The NPA–OVA was used as the coating antigen and the standard substances were first dissolved in methanol and diluted in 10% MeOH–PBS (Zeravik et al., Citation2004). Because OP and NP have a phenolic hydroxy and a long alkyl chain, they were considered to be hydrophobic compounds due to their slight solubility in water and adsorption activity on solid materials (Brix, Hvidt, & Carlsen, Citation2001). The icELISA method was carried out to assess the polyclonal antibody. An IC50 value of 315 ng/mL, a LOD (10% inhibitory concentration) of 24 ng/mL and a linear range of quantitation (20–80% inhibitory concentration) from 46 to 2147 ng/mL were observed for 4-NP technical mixture (). The polyclonal antibody was found to exhibit higher specificity for OP, with an IC50 value of 51 ng/mL, a LOD of 14 ng/mL and a linear range of quantitation from 23 to 196 ng/mL (). The reason for this may be that the 4-NP technical mixture has many isomers with branched alkyl chains which the antibody could not recognise; however, the OP was very pure with purity up to 98%.
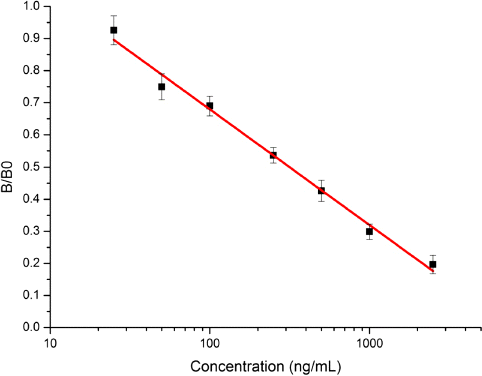
An icELISA was also developed to characterise the monoclonal antibody, reaching an IC50 value of 76 ng/mL and a linear range of quantitation from 16 to 451 ng/mL for OP (). The specificity of the monoclonal antibody was tested using a set of compounds structurally related to OP, and the results are shown in . The CR results indicated that the monoclonal antibody was very sensitive to alkyl chains. As a result, the monoclonal antibody was shown to be highly specific for OP.
Table 1. CR of the monoclonal antibody with OP and other compounds.
3.4. Recovery studies
The developed immunoassay based on the monoclonal antibody was used to analyse water samples from Tai Lake in China. Three fortified levels (100, 250 and 500 ng/mL) in the water samples were measured, and the data are shown in .
Table 2. Recovery of OP from the fortified water samples by icELISA.
The intra- and inter-assay recoveries for fortified water samples ranged from 84.38 to 96.47% and from 79.53 to 94.51%, respectively, with intra-assay coefficients of variation of 4.79–8.66% and an inter-assay CV value of 7.63–12.31%. The obtained results showed that the proposed ELISA technique could be applied to the direct detection of OP in water samples with a simple dilution treatment.
4. Conclusions
A polyclonal antibody was generated from the immunogen NPA–BSA, which used a linear carboxylated analogue of NP as the hapten. The polyclonal antibody had good recognition for OP, but exhibited poor specificity for NP technical mixture. As commercial 4-NP products have a large number of isomers with branched alkyl chains and show structural diversity, it is difficult to select monoclonal antibodies using the 4-NP mixture as the standard substance. Therefore, a monoclonal antibody was screened using OP as the standard substance, and showed good specificity for OP, with no CR with other phenol derivatives. An icELISA method based on a monoclonal antibody was successfully established and used to detect fortified water samples from Tai Lake with a satisfactory recovery rate at different spiked levels. The developed immunoassay was very simple, rapid and sensitive, and could be applied to monitor OP in other environments and in foods.
Acknowledgement
This work is financially supported by the National Natural Science Foundation of China (21071066, 91027038, 21101079 and 21175034).
References
- Abdulla Bin-Dohaish el, J. (2012). The effects of 4-nonylphenol contamination on livers of Tilapia fish (Oreochromus spilurs) in Jeddah. Biological Research, 45(1), 15–20. doi:10.1590/S0716-97602012000100002
- Andreu, V., Ferrer, E., Rubio, J. L., Font, G., & Pico, Y. (2007). Quantitative determination of octylphenol, nonylphenol, alkylphenol ethoxylates and alcohol ethoxylates by pressurized liquid extraction and liquid chromatography–mass spectrometry in soils treated with sewage sludges. Science of the Total Environment, 378(1–2), 124–129. doi:10.1016/j.scitotenv.2007.01.024
- Brix, R., Hvidt, S., & Carlsen, L. (2001). Solubility of nonylphenol and nonylphenol ethoxylates. On the possible role of micelles. Chemosphere, 44(4), 759–763. doi:10.1016/S0045-6535(00)00366-0
- Cespedes, R., Skryjova, K., Rakova, M., Zeravik, J., Franek, M., Lacorte, S., & Barcelo, D. (2006). Validation of an enzyme-linked immunosorbent assay (ELISA) for the determination of 4-nonylphenol and octylphenol in surface water samples by LC-ESI–MS. Talanta, 70(4), 745–751. doi:10.1016/j.talanta.2006.07.001
- Chaube, R., Gautam, G. J., & Joy, K. P. (2013). Teratogenic effects of 4-nonylphenol on early embryonic and larval development of the catfish Heteropneustes fossilis. Archives of Environmental Contamination and Toxicology, 64(4), 554–561. doi:10.1007/s00244-012-9851-7
- Chen, C. C., Lin, Y. P., Wang, C. W., Tzeng, H. C., Wu, C. H., Chen, Y. C., … Wu, Y. C. (2006). DNA-gold nanorod conjugates for remote control of localized gene expression by near infrared irradiation. Journal of the American Chemical Society, 128(11), 3709–3715. doi:10.1021/ja0570180
- Estévez, M. C., Kreuzer, M., Sánchez-Baeza, F., & Marco, M. P. (2006). Analysis of nonylphenol: Advances and improvements in the immunochemical determination using antibodies raised against the technical mixture and hydrophilic immunoreagents. Environmental Science & Technology, 40(2), 559–568. doi:10.1021/es050984y
- Ferrara, F., Fabietti, F., Delise, M., & Funari, E. (2005). Alkylphenols and alkylphenol ethoxylates contamination of crustaceans and fishes from the Adriatic Sea (Italy). Chemosphere, 59(8), 1145–1150. doi:10.1016/j.chemosphere.2004.11.085
- Ferrer, E., Santoni, E., Vittori, S., Font, G., Mañes, J., & Sagratini, G. (2011). Simultaneous determination of bisphenol A, octylphenol, and nonylphenol by pressurised liquid extraction and liquid chromatography–tandem mass spectrometry in powdered milk and infant formulas. Food Chemistry, 126(1), 360–367. doi:10.1016/j.foodchem.2010.10.098
- Franek, M., & Hruska, K. (2005). Antibody based methods for environmental and food analysis: A review. Veterinarni Medicina, 50(1), 1–10. Retrieved from http://www.vri.cz/docs/vetmed/50-1-1.pdf
- Franek, M., Zeravik, J., Eremin, S. A., Yakovleva, J., Badea, M., Danet, A., … Emneus, J. (2001). Antibody-based methods for surfactant screening. Fresenius Journal of Analytical Chemistry, 371(4), 456–466. doi:10.1007/s002160101079
- Ghekiere, A., Verslycke, T., & Janssen, C. (2006). Effects of methoprene, nonylphenol, and estrone on the vitellogenesis of the mysid Neomysis integer. General and Comparative Endocrinology, 147(2), 190–195. doi:10.1016/j.ygcen.2005.12.021
- Goda, Y., Kobayashi, A., Fujimoto, S., Toyoda, Y., Miyagawa, K., Ike, M., & Fujita, M. (2004). Development of enzyme-linked immunosorbent assay for detection of alkylphenol polyethoxylates and their biodegradation products. Water Research, 38(20), 4323–4330. doi:10.1016/j.watres.2004.07.030
- Hotta, Y., Hosoda, A., Sano, F., Wakayama, M., Niwa, K., Yoshikawa, H., & Tamura, H. (2010). Ecotoxicity by the biodegradation of alkylphenol polyethoxylates depends on the effect of trace elements. Journal of Agricultural and Food Chemistry, 58(2), 1062–1067. doi:10.1021/jf902772m
- Koh, Y. K., Chiu, T. Y., Paterakis, N., Boobis, A., Scrimshawe, M. D., Lester, J. N., & Cartmell, E. (2009). Fate and occurrence of alkylphenolic compounds in sewage sludges determined by liquid chromatography tandem mass spectrometry. Environmental Technology, 30(13), 1415–1424. doi:10.1080/09593330903179765
- Lu, C., Chen, X., & Liu, H. (2009). Determination of alkylphenols and alkylphenol polyethoxylates in textiles by normal-phase high performance liquid chromatography and solid-phase extraction. Se pu =Chinese Journal of Chromatography/Zhongguo hua xue hui, 27(4), 458–462. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19938503
- Mart'ianov, A. A., Dzantiev, B. B., Zherdev, A. V., Eremin, S. A., Cespedes, R., Petrovic, M., & Barcelo, D. (2005). Immunoenzyme assay of nonylphenol: Study of selectivity and detection of alkylphenolic non-ionic surfactants in water samples. Talanta, 65(2), 367–374. doi:10.1016/j.talanta.2004.07.004
- Mart'ianov, A. A., Zherdev, A. V., Eremin, S. A., & Dzantiev, B. B. (2004). Preparation of antibodies and development of enzyme-linked immunosorbent assay for nonylphenol. International Journal of Environmental Analytical Chemistry, 84(13), 965–978. doi:10.1080/03067310410001729024
- Matsui, K., Kawaji, I., Utsumi, Y., Ukita, Y., Asano, T., Takeo, M., … Negoro, S. (2007). Enzyme-linked immunosorbent assay for nonylphenol using antibody-bound microfluid filters in vertical fluidic operation. Journal of Bioscience and Bioengineering, 104(4), 347–350. doi:10.1263/jbb.104.347
- Meucci, V., & Arukwe, A. (2005). Detection of vitellogenin and zona radiata protein expressions in surface mucus of immature juvenile Atlantic salmon (Salmo salar) exposed to waterborne nonylphenol. Aquatic Toxicology (Amsterdam, Netherlands), 73(1), 1–10. doi:10.1016/j.aquatox.2005.03.021
- Meulenberg, E. P. (2009). Phenolics: Occurrence and immunochemical detection in environment and food. Molecules (Basel, Switzerland), 14(1), 439–473. doi:10.3390/molecules14010439
- Mikhura, I. V., Formanovsky, A. A., Nikitin, A. O., Yakovleva, J. N., & Eremin, S. A. (2000). Synthesis of ω-(4-hydroxyphenyl)alkanecarboxylic acids. Mendeleev Communications, 10(5), 193–194. doi:10.1070/MC2000v010n05ABEH001336
- Norton, D., & Shamsi, S. A. (2007). Capillary electrochromatography–mass spectrometry of nonionic surfactants. Analytical Chemistry, 79(24), 9459–9470. doi:10.1021/ac071124y
- Pachura-Bouchet, S., Blaise, C., & Vasseur, P. (2006). Toxicity of nonylphenol on the cnidarian Hydra attenuata and environmental risk assessment. Environmental Toxicology, 21(4), 388–394. doi:10.1002/tox.20201
- Sabik, H., Gagne, F., Blaise, C., Marcogliese, D. J., & Jeannot, R. (2003). Occurrence of alkylphenol polyethoxylates in the St. Lawrence River and their bioconcentration by mussels (Elliptio complanata). Chemosphere, 51(5), 349–356. doi:10.1016/S0045-6535(02)00862-7
- Samsonova, J. V., Rubtsova, M. Y., & Franek, M. (2003). Determination of 4-n-nonylphenol in water by enzyme immunoassay. Analytical and Bioanalytical Chemistry, 375(8), 1017–1019. doi:10.1007/s00216-003-1815-3
- Samsonova, J. V., Uskova, N. A., Andresyuk, A. N., Franek, M., & Elliott, C. T. (2004). Biacore biosensor immunoassay for 4-nonylphenols: Assay optimization and applicability for shellfish analysis. Chemosphere, 57(8), 975–985. doi:10.1016/j.chemosphere.2004.07.028
- Servos, M. R. (1999). Review of the aquatic toxicity, estrogenic responses and bioaccumulation of alkylphenols and alkylphenol polyethoxylates. Water Quality Research Journal of Canada, 34(1), 123–177. Retrieved from http://digital.library.mcgill.ca/wqrj/pdfs/wqrj_vol_34_no_1_art_04.pdf
- Soto, A. M., Justicia, H., Wray, J. W., & Sonnenschein, C. (1991). p-Nonyl-phenol: An estrogenic xenobiotic released from “modified” polystyrene. Environmental Health Perspectives, 92, 167–173. doi:10.1289/ehp.9192167
- Stenholm, Å., Holmström, S., Hjärthag, S., & Lind, O. (2012). Strategies for selecting optimal sampling and work-up procedures for analysing alkylphenol polyethoxylates in effluents from non-activated sludge biofilm reactors. Environmental Technology, 33(2), 129–141. doi:10.1080/09593330.2011.551843
- Takasu, T., Iles, A., & Hasebe, K. (2002). Determination of alkylphenols and alkylphenol polyethoxylates by reversed-phase high-performance liquid chromatography and solid-phase extraction. Analytical and Bioanalytical Chemistry, 372(4), 554–561. doi:10.1007/s00216-001-1135-4
- Temponi, M., Kageshita, T., Perosa, F., Ono, R., Okada, H., & Ferrone, S. (1989). Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: Comparison with other methods of purification. Hybridoma, 8(1), 85–95. doi:10.1089/hyb.1989.8.85
- Uguz, C., Iscan, M., Ergüven, A., Isgor, B., & Togan, I. (2003). The bioaccumulation of nonyphenol and its adverse effect on the liver of rainbow trout (Onchorynchus mykiss). Environmental Research, 92(3), 262–270. doi:10.1016/S0013-9351(03)00033-1
- Vaccaro, E., Meucci, V., Intorre, L., Soldani, G., Di Bello, D., Longo, V., … Pretti, C. (2005). Effects of 17beta-estradiol, 4-nonylphenol and PCB 126 on the estrogenic activity and phase 1 and 2 biotransformation enzymes in male sea bass (Dicentrarchus labrax). Aquatic Toxicology (Amsterdam, Netherlands), 75(4), 293–305. doi:10.1016/j.aquatox.2005.08.009
- Vega-Morales, T., Sosa-Ferrera, Z., & Santana-Rodriguez, J. J. (2010). Determination of alkylphenol polyethoxylates, bisphenol-A, 17alpha-ethynylestradiol and 17beta-estradiol and its metabolites in sewage samples by SPE and LC/MS/MS. Journal of Hazardous Materials, 183(1–3), 701–711. doi:10.1016/j.jhazmat.2010.07.083
- Wu, Z. Y., & Marriott, P. J. (2011). One- and comprehensive two-dimensional high-performance liquid chromatography analysis of alkylphenol polyethoxylates. Journal of Separation Science, 34(23), 3322–3329. doi:10.1002/jssc.201100701
- Zeravik, J., Skryjová, K., Nevoranková, Z., & Fránek, M. (2004). Development of direct ELISA for the determination of 4-nonylphenol and octylphenol. Analytical Chemistry, 76(4), 1021–1027. doi:10.1021/ac030217m