Abstract
A survey of aflatoxin M1 (AFM1) contamination in packaged milk and infant formula milk samples in the Goan market, India, was conducted using high performance liquid chromatography, association of analytical communities approved commercial kit and a sensitive chemiluminescent sandwich enzyme-linked immunosorbent assay (ELISA). A total of 72 samples of infant formula milk food (18) and packaged milk samples (54) was analysed. One hundred per cent of the analysed samples exceeded the European Communities recommended limits (50 ng/L) and 75% of the samples exceeded Codex Alimentarius, Food Safety and Standards Authority of India (FSSAI) and US Food and Drug Administration recommended limits (500 ng/L). The range of contamination of AFM1 was found lower in infant milk formula (501–713 ng/L) than liquid milk (511–809 ng/L). The methods were also compared for their performance, and ELISA was found to be most suitable for analysis of low-level AFM1 contamination in milk.
Introduction
Milk and milk products in several countries have been widely surveyed for the natural occurrence of aflatoxin M1 (AFM1). AFM1 is the metabolite of aflatoxin B1 (AFB1) and is found in milk when lactating animals are fed with contaminated feedstuffs. When animals consume AFB1-contaminated feedstuffs, the toxin is metabolised in the liver and excreted as AFM1 via milk and urination (Henry et al., Citation2001). AFM1 is bound to milk proteins, especially casein, which leads to its presence in dairy products (Prandini et al., Citation2009). AFM1 is known for its hepatotoxic and carcinogenic effects. The presence of AFM1 in milk possess a major risk for humans especially infants as it can have immunosuppressive, mutagenic and teratogenic effects. Studies show that, AFM1 is relatively stable during milk pasteurisation, storage as well as during the preparation of various dairy products (Badea et al., Citation2004; Codex Committee on Food Additives and Contaminants, Citation2001). The toxic and carcinogenic effects of AFM1 led WHO-International Agency for Research on Cancer (IARC) to change its classification from group 2 to group 1 (IARC, vol. 82, Citation2002). Occurrence of AFM1 in milk and milk products is a worldwide concern since these products are consumed by all groups of the population (Fallah, Citation2010). Due to the carcinogenic and toxic nature of the AFM1 in milk and milk derivatives (Oveisi, Jannat, Sadeghi, Hajimahmoodi, & Nikzad, Citation2007; Sassahara, Netto, & Yanaka, Citation2005), several countries have set or proposed legal regulations for AFM1 levels in milk and dairy products. These regulations vary in different countries and are often based on economic considerations (Stoloff, Van Egmond, & Parks, Citation1991). The European Community legislation imposes maximum permissible limit for AFM1 concentration as 50 ng/L for milk and 25 ng/L for infant formulae (Henry et al., Citation2001). Recently the Food Safety and Standards Authority of India (FSSAI) have set the permissible limit for AFM1 concentration in milk and milk products at 0.5 µg/kg (FSSAI, Citation2011).
The literature describes the various methods in which milk may be analysed directly or after simple and limited pretreatment (Badea et al., Citation2004; Farjam, De Vries, Lingeman, & Brinkman, Citation1991; Micheli, Grecco, Badea, Moscone, & Palleschi, Citation2005; Parker & Tothill, Citation2009). The analysis of dairy products still involves time-consuming extraction of AFM1 which, in addition, requires organic solvents. Among other established methods, immunochemical techniques are very popular for mycotoxins analysis with many literatures reporting the use of a commercially developed enzyme-linked immunosorbent assay (ELISA) (Devi et al., Citation2002; Lopez, Ramos, Ramadan, & Bulacio, Citation2003; Rastogi, Dwivedi, Khanna, & Das, Citation2004; Rodriguez, Delso, & Escudero, Citation2003). ELISA is not only a suitable tool for quick and sensitive analysis with high sample throughput but also cost-effective and requires only a little sample volume for analysis (Parker & Tothill, Citation2009; Pei, Zhang, Eremin, & Lee, Citation2009; Sawaf, Abdullah, & Sheet, Citation2012). Among the established ELISA techniques, sandwich-type immunoassay is an effective bioassay due to the high specificity and sensitivity (Knopp, Citation2006). Recently, we have reported a novel approach where a highly sensitive microplate sandwich ELISA was developed and integrated with magnetic nano particles (MNPs) which could detect ultra trace amount of AFM1 in milk (Kanungo, Pal, & Bhand, Citation2011).
There are many reports available in the view of detection of AFM1 residues in milk samples (Bakırdere, Yaroğlu, Tırık, Demiröz, & Karaca, Citation2012; Iqbal, Asi, & Ariño, Citation2011; Kawamura et al., Citation1994; Rohani, Aminaee, & Kianfar, Citation2011). In India, a survey found that 87.3% of the milk-based samples analysed were contaminated with AFM1; of these 99% were much above European permissible limits (Rastogi et al., Citation2004). This is a major concern considering that India is the largest producer of milk in the world (Devi et al., Citation2002; Parker & Tothill, Citation2009; Rastogi et al., Citation2004), and there are very scarce reports of AFM1 analysis. One recent report describes about the occurrence of AFM1 in raw, pasteurised and ultra-high temperature treatment of milk of the major brands prevalent in the Karnataka and Tamil Nadu region of India (Siddappa, Nanjegowda, & Viswanath, Citation2012). It has been surveyed that varieties of packaged milk samples are available in the Indian market for consumption without any food safety certification. There is a need for routine screening of such packaged samples which projects a vital need for simple, robust, low-cost bioanalytical methods for AFM1 detection in milk and milk products that can be used in the laboratories of developing countries.
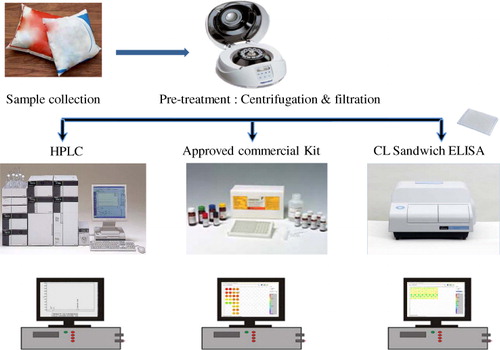
In the present work, a survey was conducted to check the occurrence of AFM1 in commercial milk samples and infant formula milk samples of Goa, India. Herein, we analysed 15 milk brands and 3 infant formula milk brands (total 72 samples) to quantify the AFM1 level as recommended by the FSSAI, Codex, US Food and Drug Administration (USFDA) and European Union (EU) guidelines. One of the milk samples was artificially contaminated with known concentrations of AFM1, and these were detected and validated by high performance liquid chromatography (HPLC) for confirmation of the presence of the toxin. This AFM1 analysis using HPLC was carried out in SGS India Pvt. LTD., Chennai, India. The commercial milk and infant formula samples were tested by two commercial kits (Art. No.: RCitation1121 & RCitation5802) bought from Ridascreen® (approved by association of analytical communities [AOAC]) and a sensitive chemiluminescent (CL) technique based sandwich ELISA ().
Experimental procedure
Materials and methods
Chemicals and instrumentation
AFM1, bovine serum albumin (BSA), tween 20, luminol, certified reference material (CRM) ERM-BD282 (AFM1 in whole milk powder, <0.02 µg/kg) were purchased from Sigma-Aldrich (USA). Hydrogen peroxide (H2O2) 30% (w/v), acetonitrile (ACN) HPLC grade, sodium chloride (NaCl), methanol (99% pure) was purchased from Merck (Germany). Rat monoclonal [1C6] primary antibody (1°Ab) of AFM1 and HRP conjugated secondary antibody (2°Ab) raised from rabbit were purchased from Abcam (UK). Sodium hypochlorite (4%) solution was purchased from Fisher Scientific (India). Milk samples were centrifuged by minispin plus centrifuge purchased from Eppendorf (Germany) and shaking of the samples were done by Spinix shaker, purchased from Tarsons (India). White 384 well polystyrene microtiter plates were purchased from Nunc (Denmark). Multichannel automatic pipette from Eppendorf was used for multi plate assay for AFM1 analysis in real sample. For CL measurement, VictorX4 2030 optiplate reader from Perkin Elmer (USA) was used. Glove box, Cole Parmer (USA), was used for the handling of AFM1 standard solution. Water produced in a Milli-Q system (Millipore, Beford, MA, USA) was used for preparing all the solutions. pH metre from Seven Multi Mettler Toledo, 8603, Switzerland, was used to prepare buffers. The Ridascreen® AFM1 30/15 test kit with dynamic range 0–80 ng/L (Kit-1) and Ridascreen® Fast AFM1 test kit with dynamic range 0–2000 ng/L (Kit-2) were purchased from R-Biopharm AG, Darmstadt, Germany. Milk samples and infant formula milk powders were purchased from the local markets of Goa, India. For HPLC analysis, CRM milk sample was spiked with AFM1, and the HPLC was carried out in an accredited Lab.
Preparation of buffers, AFM1 standard solutions and antibody solutions
Carbonate buffer (CB; pH 9.6), Phosphate buffered saline (PBS; pH 7.4), PBST buffer and blocking solution (BSA in PBS) were made by the same protocol as described in our earlier paper (Kanungo et al., Citation2011). All buffer solutions were stored at 4°C when not in use. All the AFM1 solutions were prepared in a Glove box under inert (N2) atmosphere. Working standard solutions in the range of 2.5–10,000 ng/L were prepared by diluting the stock with 5% ACN. [Safety Note: Aflatoxins are highly carcinogenic and should be handled with extreme care. Aflatoxin contaminated lab wares should be decontaminated with an aqueous solution of sodium hypochlorite (4%)]. From the stock solution of rat monoclonal [1C6] 1°Ab, working 1°Ab solution was prepared prior to the experiment. The working Ab dilutions were prepared by serial dilution in double distilled water as 1:1000, 1:2000, etc., and then added with equal volume of CB (1:1). Similarly, from the stock solution of 1 mg (2 mg/mL) rabbit polyclonal to rat IgG-H and L (HRP) 2°Ab, working 2°Ab solution was prepared prior to the experiment by serial dilution in PBS.
Milk sample collection and pretreatment
Both milk and infant formula milk powders were randomly collected from the markets of Goa. Totally, 15 popular liquid milk and 3 infant formula milk brands were analysed comprising 54 and 18 samples, respectively. Powder-based samples (formula milk food) were suspended in warm deionised water as per the instructions written on the packets. The packaged milk samples as well as the formula milk samples were centrifuged at 6000 g for about 10 min. After centrifugation, the upper fat layer was completely removed. The decanted aqueous layer was filtered through a syringe filter using 0.22 µ filter paper and used for the analysis.
Immunoassay procedure
To investigate the presence of AFM1, the milk samples were analysed by ELISA. First, the samples were analysed by sandwich ELISA. Subsequently, they were also tested by AOAC-approved commercial kits from Ridascreen® where competitive ELISA was performed as per the protocol provided in the literature (RCitation1121 and RCitation5802).
CL sandwich ELISA
Sandwich ELISA was performed in 384 microwell plate. We followed the same protocol as described in our earlier paper (Kanungo et al., Citation2011) with reduced incubation time of 1 h.
Competitive ELISA (using commercial Kit-1, dynamic range 0–80 ng/L)
The quantitative analysis of AFM1 in the samples was first performed by competitive ELISA using Ridascreen AFM1 30/15 test kit (Kit-1) as per the instructions. The absorbance was measured after addition of stop solution at 450 nm by the plate reader.
Competitive ELISA (using commercial Kit-2, dynamic range 0–2000 ng/L)
We have also analysed AFM1 in milk samples by Ridascreen® Fast AFM1 test, which has two types of antibodies and a wider dynamic range of antigens (0–2000 ng/L) as per the instructions. The absorbance was measured by the plate reader at 450 nm. The intensity of absorbance was inversely proportional to the concentration of AFM1 in samples. The AFM1 concentration results from the ELISA assay were then analysed.
Results and discussion
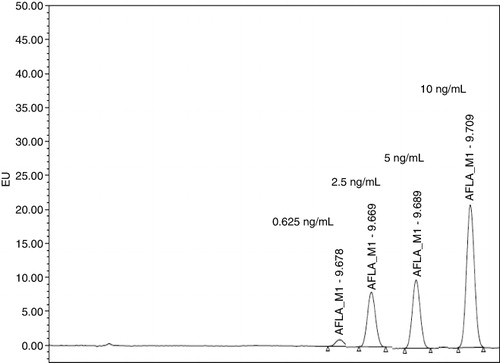
In HPLC analysis, the peaks were studied for the confirmatory test of AFM1 (). In , the peaks are shown for the concentrations such as 0.625, 2.5, 5 and 10 ng/mL, respectively, that were spiked in CRM milk samples and were analysed by an accredited laboratory.
AFM1 standard calibration curve
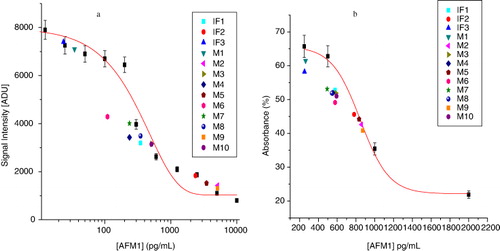
The calculations for CL sandwich ELISA were made by simple observations of signal intensities or photon counts plotted against various AFM1 concentrations. The signal intensity or photon counts were generated from the luminol/peroxidase reaction. The standard curve for AFM1 detection by CL sandwich ELISA is shown in . A concentration-dependent decrease in signal intensity was observed for AFM1. CRM (ERM-BD282) was reconstituted to a liquid form, which was later spiked with known amounts of AFM1 solutions in different concentrations. The assay was performed for three times, and the error values were plotted as a standard calibration curve. The dose–response curve showed the photon count in terms of signal intensity. From the standard calibration curve, we obtained the [AFM1] in tested infant formula milk powders as well as milk samples. The error bar indicates the standard deviation (n = 3), where ‘n’ is an independent assay by the proposed method. The SD and R2 were calculated to be 1.69 and 0.89, respectively. The calculations used for quantitative analysis by Ridascreen test kits were followed from the kit protocol. For the same, the percentage absorbance was plotted against the standard concentrations of AFM1 provided in the kit. The absorption intensity was found to be inversely proportional to AFM1 concentration in the sample. The calibration curve was then used to analyse several milk samples to detect AFM1 contamination levels.
The standard curve for competitive assay by commercial kit was obtained and is depicted in . A concentration-dependent decrease in percentage absorbance was observed for AFM1. The standard curve was used for calibration of unknown milk samples. The error bar indicates the standard deviation (n = 6), where n is an independent assay by the method. The IC50 was 817.8 pg/mL. The SD and R2 were calculated to be 2.24 and 0.91, respectively. From all the measurements, it was observed that all samples were contaminated with AFM1.
Recovery of AFM1 from spiked and CRM milk samples
The sandwich ELISA was validated with CRM (ERM-BD 282, zero level of AFM1). The milk powder was reconstituted as indicated in the certification report supplied by the IRMM, Belgium. To test the accuracy of the assay, AFM1 concentrations ranging from 2.5 to 10,000 ng/L were added to the CRM milk sample and assayed by both CL ELISA and Ridascreen Fast kit. CRM milk sample containing zero-level AFM1 was compared with a sample deliberately contaminated with known amounts of AFM1. Recovery was assessed by spiking AFM1 with the BD282 reconstituted material and presented as in . The fortified (2.5, 6.25, 12.5, 25, 50, 100, 200, 300, 600, 1250, 5000, 10,000 ng/L of AFM1) milk samples were interpolated from the calibration curve performed using reconstituted CRM. The precision and reliability of the CL ELISA are notable from the data presented in . The resultant data showed an excellent percentage of recovery, close to 100% for CRM. The precision was determined by calculating the relative standard deviation (RSD%) for the replicate measurements and the accuracy (RE%) was calculated by assessing the agreement between measured and nominal concentration of the fortified samples.
Table 1. Recovery studies of AFM1 using CL sandwich method and commercial kit.
AFM1 contamination level in commercial milk samples
The comparison of AFM1 contamination levels in real samples with EU, Codex, FSSAI and USFDA standard is summarised in . It was observed that 100% of all the samples exceeded EU standard, and around 75% of the samples exceeded Codex, FSSAI and USFDA standards. By CL sandwich ELISA method as well as by commercial kit, it was found that, out of 18 samples of infant formula milk analysed, 100% would not pass the EU regulations, while this number is reduced to 66.6% and 33.3%, respectively, if the USFDA, Codex or FSSAI regulations are applied. On the other hand, out of 54 liquid milk samples 100% exceeded EC regulations and 77.7% surpassed USFDA, Codex and FSSAI regulations.
Table 2. Comparison of AFM1 contamination levels in packaged milk samples with EU, Codex, FSSAI and USFDA standards.
The figures of merit of the CL sandwich ELISA were compared with Ridascreen kit and presented in . The parameters such as detection limit, analysis time, sensitivity, sample throughput and cost per sample were compared. The dynamic range and the upper limit of detection for the milk samples using the Ridascreen kit were found inferior when compared against the sandwich assay. Although the analysis time of the kit was less than our assay, the detection limit and sensitivity of the CL sandwich assay was found to be more promising than the kit. The higher analysis time of CL sandwich assay can be compensated against the number of real samples per assay (n = 128) in triplicate as against the commercial kit wherein 48 samples (Kit-1) and 24 samples (Kit-2) per assay can be analysed. Moreover, the use of 384 microwell plate facilitates reduction in reagent consumption and volume of toxic waste. The comparison result suggests that the sandwich assay is better suited for ultra-sensitive analysis of AFM1 contamination in milk samples and can be easily adapted for routine analysis.
Table 3. Comparison of CL sandwich method vs Ridascreen Kits.
Conclusions
Based on the random sampling and analysis of commercial milk samples and infant formula milk samples, it is evident that the all the analysed samples were found to contain AFM1 concentrations exceeding permissible limits of EU standard. These observations strongly suggest that it is necessary to pay attention to this subject. In our report, both CL sandwich and competitive ELISA have been shown to be simple and useful analytical techniques that can be used to monitor low-level AFM1 contamination in milk. Moreover the sandwich assay could detect AFM1 contaminaiton as low as 60 pg/mL, whereas commercial ELISA could detect 110 pg/mL. From the survey, it was found that all milk samples were contaminated with AFM1, but 75% of the samples exceeded the Codex, USFDA and FSSAI regulation level. The detected levels of AFM1 in the conducted survey of samples show a serious health alarm in regards to the safety limits for AFM1 levels in infant formula and milk samples of Indian market.
Acknowledgement
L.K. acknowledges NAIP for the award of Research Associate Fellowship.
Funding
This work is funded by National Agriculture Innovation Project (NAIP) No. C4/C10125, 2008–14, Indian Council of Agriculture and Research and the World Bank.
Additional information
Funding
References
- Badea, M., Micheli, L., Messia, M. C., Candigliota, T., Marconi, E., Mottram, T., … Palleschi, G. (2004). Aflatoxin M1 determination in raw milk using a flow-injection immunoassay system. Analytica Chimica Acta, 520(1–2), 141–148. doi:10.1016/j.aca.2004.05.068
- Bakırdere, S., Yaroğlu, T., Tırık, N., Demiröz, M., & Karaca, A. (2012). Determination of trace aflatoxin M1 levels in milk and milk products consumed in Turkey by using enzyme-linked immunosorbent assay. Food and Agricultural Immunology, 23(1), 1–9. doi:10.1080/09540105.2012.733354
- Codex Committee on Food Additives and Contaminants. (2001). CL CX/FAC 01/20, Comments Submitted on the Draft Maximum Level for Aflatoxin M1 in Milk. 33rd session. Hague: FAO/WHO.
- Devi, K. T., Mayo, M. A., Hall, A. J., Craufurd, P. Q., Wheeler, T. R., Waliyar, F., … Reddy, D. V. R. (2002). Development and application of an indirect competitive enzyme-linked immunoassay for aflatoxin M1 in milk and milk-based confectionery. Journal of Agricultural & Food Chemistry, 50(4), 933–937. doi:10.1021/jf011139b
- Fallah, A. (2010). Assessment of aflatoxin M1 contamination in pasteurized and UHT milk marketed in central part of Iran. Food and Chemical Toxicology, 48(3), 988–991. doi:10.1016/j.fct.2010.01.014
- Farjam, A., De Vries, R., Lingeman, H., & Brinkman, U. A. Th. (1991). Immuno precolumns for selective on-line sample pretreatment of aflatoxins in milk prior to column liquid chromatography. International Journal of Environmental Analytical Chemistry, 44(3), 175–184. doi:10.1080/03067319108027549
- Food Safety and Standards Authority of India (FSSAI). (2011). Food Safety and Standards (Contaminants, toxins and residues) Regulations. F.No. 2-15015/30/2010. Retrieved from http://www.fssai.gov.in/Portals/0/Pdf/Food%20safety%20and%20standards%20%28contaminats,%20toxins%20and%20residues%29%20regulation,%202011.pdf
- Henry, S. H., Whitaker, T., Rabbani, I., Bowers, J., Park, D., Price, W., … Coker, R. (2001). JECFA, “World Health Organization, safety evaluation of certain mycotoxins in food”. In Proceedings of the 56th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), WHO Food Additives Series No. 47, International Programme on Chemical Safety, Geneva, Switzerland.
- International Agency for Research on Cancer. (2002). IARC Monographs on the evaluations of carcinogenic risks to humans (vol. 82, p. 171). Lyon: Author.
- Iqbal, S. Z., Asi, M. R., & Ariño, A. (2011). Aflatoxin M1 contamination in cow and buffalo milk samples from the North West Frontier Province (NWFP) and Punjab provinces of Pakistan. Food Additives and Contaminants: Part B: Surveillance, 4(4), 282–288. doi:10.1080/19393210.2011.637237
- Kanungo, L., Pal, S., & Bhand, S. (2011). Miniaturised hybrid immunoassay for high sensitivity analysis of aflatoxin M1 in milk. Biosensors & Bioelectronics, 26(5), 2601–2606. doi:10.1016/j.bios.2010.11.014
- Kawamura, O., Wang, D.-S., Liang, Y.-X., Hasegawa, A., Saga, C., Visconti, A., Ueno, Y. (1994). Further survey of aflatoxin M1 in milk powders by ELISA. Food and Agricultural Immunology, 6(4), 465–467. doi:10.1080/09540109409354858
- Knopp, D. (2006). Immunoassay development for environmental analysis. Analytical and Bioanalytical Chemistry, 385(3), 425–427. doi:10.1007/s00216-006-0465-7
- Lopez, C. E., Ramos, L. L., Ramadan, S. S., & Bulacio, L. C. (2003). Presence of aflatoxin M1 in milk for human consumption in Argentina. Food Control, 14(1), 31–34. doi:10.1016/S0956-7135(02)00049-X
- Micheli, L., Grecco, R., Badea, M., Moscone, D., & Palleschi, G. (2005). An electrochemical immunosensor for aflatoxin M1 determination in milk using screen-printed electrodes. Biosensors and Bioelectronics, 21(4), 588–596. doi:10.1016/j.bios.2004.12.017
- Oveisi, M.-R., Jannat, B., Sadeghi, N., Hajimahmoodi, M., & Nikzad, A. (2007). Presence of aflatoxin M1 in milk and infant milk products in Tehran, Iran. Food Control, 18(10), 1216–1218. doi:10.1016/j.foodcont.2006.07.021
- Parker, C. O., & Tothill, I. E. (2009). Development of an electrochemical immunosensor for aflatoxin M1 in milk with focus on matrix interference. Biosensors & Bioelectronics, 24(8), 2452–2457. doi:10.1016/j.bios.2008.12.021
- Pei, S. C., Zhang, Y. Y., Eremin, A. S., & Lee, W. J. (2009). Detection of aflatoxin M1 in milk products from China by ELISA using monoclonal antibodies. Food Control, 20(12), 1080–1085. doi:10.1016/j.foodcont.2009.02.004
- Prandini, A., Tansini, G., Sigolo, S., Filippi, L., Laporta, M., Piva, G. (2009). On the occurrence of aflatoxin M1 in milk and dairy products. Food and Chemical Toxicology, 47, 984–991. doi:10.1016/j.fct.2007.10.005
- R1121 Aflatoxin M1 11-10-21k (1).pdf from RIDASCREEN® Aflatoxin M1. Art. No.: R1121. Retrieved from http://www.r-biopharm.com/products/food-feed-analysis/mycotoxins/aflatoxin/item/ridascreen-aflatoxin-m1
- R5802 FAST Aflatoxin M1 11-01-10k.pdf from RIDASCREEN® Aflatoxin M1. Art. No.: R5802. Retrieved from http://www.r-biopharm.com/products/food-feed-analysis/mycotoxins/aflatoxin/item/ridascreenfast-aflatoxin-m1-2
- Rastogi, S., Dwivedi, P. D., Khanna, S. K., & Das, M. (2004). Detection of aflatoxin M1 contamination in milk and infant milk products from Indian markets by ELISA. Food Control, 15(4), 287–290. doi:10.1016/S0956-7135(03)00078-1
- Rodriguez, V. M. L., Delso, C. M. M., & Escudero, O. D. (2003). ELISA and HPLC determination of the occurrence of aflatoxin M1 in raw cow's milk. Food Additives and Contaminants, 20(3), 276–280. doi:10.1080/0265203021000045208
- Rohani, F. G., Aminaee, M. M., & Kianfar, M. (2011). Survey of aflatoxin M1 in cow's milk for human consumption in Kerman Province of Iran. Food Additives and Contaminants: Part B, 4(3), 191–194. doi:10.1080/19393210.2011.599866
- Sassahara, M., Netto, D., & Yanaka, E. (2005). Aflatoxin occurrence in foodstuff supplied to dairy cattle and aflatoxin M1 in raw milk in the North of Parana state. Food and Chemical Toxicology, 43(6), 981–984. doi:10.1016/j.fct.2005.02.003
- Sawaf, Al. S. D., Abdullah, O. A., & Sheet, O. H. (2012). Use of enzyme linked immunosorbent assay for detection of aflatoxin M1 in milk powder. Iraqi Journal of Veterinary Sciences, 26, 39–42. Retrieved from http://www.vetmedmosul.org/ijvs/media/12-1-9e.pdf
- Siddappa, V., Nanjegowda, D. K., & Viswanath, P. (2012). Occurrence of aflatoxin M1 in some samples of UHT, raw & pasteurized milk from Indian states of Karnataka and Tamilnadu. Food and Chemical Toxicology, 50(11), 4158–4162. doi:10.1016/j.fct.2012.08.034
- Stoloff, L., Van Egmond, H., & Parks, D. (1991). Rationales for the establishment of limits and regulations for mycotoxins. Food Additives and Contaminants, 8(2), 213–221. doi:10.1080/02652039109373971