Abstract
Impacts of the Maillard reaction conditions (maltose/parvalbumin weight ratio, reaction temperature and reaction time) on the antigenicity of parvalbumin (PV) were explored using response surface methodology. The model to predict the antigenicity of PVand an optimal reaction condition were obtained. In addition, the antigenicity, immunoglobulin E (IgE) binding ability and amino acid composition of PV-maltose were analysed. The reaction temperature showed a significant effect on the antigenicity of PV. The optimal reaction condition was: maltose/PV weight ratio was 2.04, reaction temperature was 67.15°C and reaction time was 74.25 h. The predicted antigenicity was 0.276 µg mL−1 under these conditions, and the test antigenicity was 0.388 µg mL−1. The IgE binding ability of PV-maltose was significantly suppressed after Maillard reaction. Therefore, Maillard reaction was an effective way to control the main allergen of grass carp.
1. Introduction
Fish provides an abundant source of highly digestible proteins and plays an important role in human nutrition. However, fish is one of the major causes of food allergy (Sampson, Citation2004). The major allergen of fish has been found to be parvalbumin (PV). Among different fish species, PVs share sequence similarities between 61% and 93%, which support cross-reactivity (Perez-Gordo et al., Citation2011; Swoboda, Bugajska-Schretter, Valenta, & Spitzauer, Citation2002). PV of fish muscle is a stable acidic Ca2 + binding protein with a molecular weight of 12 kDa. PV is present in high amounts in white muscles (Kobayashi et al., Citation2006).
PV in many different species of fish, such as red stingray (dasyatis akajei) (Cai et al., Citation2010), codfish (Van Do, Hordvik, Endresen, & Elsayed, Citation2003), carp (Ma et al., Citation2008) and salmo salar (Hildebrandt & Garber, Citation2010), has been comprehensively studied. Previous studies focused on the structural and immunological properties, for instance, purification, characterisation and determination (Fæste & Plassen, Citation2008) of PV, cross-reactivity of allergens from different fish (Griesmeier et al., Citation2010; Vando, Elsayed, Florvaag, Hordvik, Endersen, Citation2005) and quantification of PV level in different kinds of fish (Kuehn, Scheuermann, Hilger, & Hentges, Citation2010). PV is resistant to heat treatment, extreme pH and denaturing agents. However, Chatterjee, Mondal, Chakraborti, Patra, and Chatterjee (Citation2006) reported that boiling and frying could affect the allergenic reactivity of fish. Many processing methods have been used to control the protein allergen, such as heat treatment, irradiation (Seo et al., Citation2004), high pressure (Peñas et al., Citation2006), enzymatic hydrolysis and Maillard reaction, but studies on the control of allergenic reactivity of PV are still limited. Maillard reaction has been proposed as a useful way to control allergen proteins. Slütte et al. (Citation2010) reported that the immunogenicity of ovalbumin could be reduced by glycation with trimethyl. Van de Lagemaat, Manuel Silván, Javier Moreno, Olano, and Dolores del Castillo (Citation2007) reported that the antigenicity of a soy protein isolate could be reduced by conjugation with fructose and fructooligosaccharides. The allergenicity of buckwheat could be reduced by glycosylation with polysaccharides, arabinogalactan or xyloglucan (Nakamura et al., Citation2008). Maillard reaction between hazelnut and glucose lead to decreasing immunoreactivity and allergenicity (Iwan et al., Citation2011). Our previous studies have found that conjugation of whey protein with glucose or maltose could reduce the antigenicity of α-lactalbumin and β-lactoglobulin (Bu, Lu, Zheng, & Luo, Citation2009; Bu, Luo, Lu, & Zhang, Citation2010; Li, Luo, & Feng, Citation2011). However, there is limited information on the impacts of Maillard reaction on PV, the major allergen of fish.
Grass carp is a popular species of freshwater fish in China. Maltose is a common raw material in food industry and it can be easily digested and absorbed. The purpose of this study is to use maltose as the glycosyl donors and investigate the effects of Maillard reaction conditions on the antigenicity of PV. Response surface methodology was applied to establish a model to predict the antigenicity of PV-maltose conjugates and to determine an optimal condition on which the lowest antigenicity of PV could be obtained. The effect of Maillard reaction was validated by the determination of immunoglobulin E (IgE) binding ability to PV and PV-maltose. Amino acid composition of PV after Maillard reaction was also analysed.
2. Materials and methods
2.1. Materials
Grass carps were purchased from a fish market in Xiaoyuehe of Beijing, China and transported to the laboratory alive. They were killed by a blow to the head, scaled, gutted and rinsed with tap water. The white muscle was collected and used for the experiment immediately.
Sephadex G-75 was purchased from Amresco (USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and HRP-conjugated goat anti-human IgE antibody were purchased from Sigma (St Louis, MO, USA). The 19 fish-allergic patients’ sera were provided by the Children’ Hospital Zhejiang University School of Medicine. Maltose was purchased from Beijing Aoboxing Biotech Company Ltd (China). All other reagents were analytical grade.
2.2. Purification of PV
The white muscle samples were homogenised with five volumes of 0.02 M Tris-HCl buffer (pH 7.5) containing 0.005 M ethylene diamine tetraacetic acid (EDTA). The homogenised samples were centrifuged at 10,000 g for 20 min (4°C). The supernatant was collected after centrifugation and then heated in a water bath at 95°C for 30 min, after which it was centrifuged at 11,000 g for 30 min. The supernatant was dialysed and freeze-dried as the PV crude extract. The PV crude extract (10 mg mL−1, 5 mL) was subjected to gel filtration on a Sephadex G-75 column (1.6 × 100 cm), which was eluted with a linear flow rate of 0.5 mL min−1. The eluent was 0.01 M phosphate buffer (pH 7.0) containing 0.15 M NaCl. Elution was collected for every 4 mL and detected by UV absorbance at 220 nm. The fractions containing protein from the same peak were pooled.
2.3. SDS − PAGE analysis
SDS-PAGE was carried out to analyse the purified PV and Maillard reaction conjugates as Li et al. (Citation2011) described, using a 12.5% acrylamide separating gel and a 4% acrylamide stacking gel. 15 µL sample (10mg mL−1) was loaded onto the gel.
2.4. Rabbit sera and human sera pool
A polyclonal anti-sera was raised against the purified grass carp PV by injecting New Zealand white rabbits with PV as described in the previous study (Bu, Lu et al., Citation2009). In the first immunisation, rabbits were injected with 1 mL of diluted (1:1 Freund's complete adjuvant) 1 mg mL−1 PV solution and the injection site was hind leg muscles. After two weeks, subcutaneous injection was carried out to the rabbits with 1 mL of diluted (1:1 incomplete Freund's adjuvant) 1 mg mL−1 PV. Two weeks later, the rabbits were injected with 1 mL solution containing 2 mg PV via ear vein. After detecting the titer of antibodies by the indirect ELISA (enzyme-linked immunosorbent assay) method, the rabbits were finally bled in weeksix, and anti-PV sera were obtained by centrifuging at 3000 g for 20 min. The sera were stored at −80°C until being used.
The human sera were obtained from 19 fish-allergic patients and were provided by the Children’ Hospital Zhejiang University School of Medicine. All the patients’ sera had been detected by ELISA experiments in advance. Compared with the sera from six healthy volunteers, all the patients’ sera had been shown to elevate IgE specific for fish PV. Sera from the patients were pooled and sera from the healthy volunteers were pooled as a control. All sera were stored at −80°C until used.
2.5. Maillard reaction conjugates preparation
The protein and sugar used in this study were PV crude extract and maltose, respectively. Maillard reaction conditions were based on the experimental design. In order to evenly mix PV and maltose, they were mixed in demineralised water first and then freeze-dried to powder. The relative humidity of desiccator for Maillard reaction was 79% by exposed to a saturated KBr solution. The mixed powder was incubated in desiccators at different reaction conditions according to the experimental design. The samples were carried out after the reaction ended and dissolved and then freeze-dried before analysis.
2.6. Colour analysis
The samples were dissolved in demineralised water and the protein concentration was 1 mg mL−1. The absorbance at 420 nm was determined to evaluate the brown colour development of the Maillard reaction conjugates.
2.7. Amino acid content analysis
The samples were hydrolysed in 10 mL HCl (6 M L−1) contenting 0.1% phenol in evacuated and sealed tubes at 110°C for 24 h. Hydrolysate was dissolved in deionised water and the volume was adjusted to 25 mL. 20 µL norleucine was mixed with 200 µL hydrolysed sample solution as the interior label and then freeze-dried. The freeze-dried powder was dissolved in 220 µL HCl (0.1 M L−1) and mixed with 100 µL triethylamine and 100 µL phenyl isothiocyanate and derived for 1 h. The triethylamine and phenyl isothiocyanate were dissolved in acetonitrile before used. After derivatisation, 400 µL n-hexane was added into the mixture and the lower solution was used for acid content analysis after 10 min. The standard amino acid solution was derived as the same as the samples. The analytical column was Venusil-AA amino acid analysis column (100 Å, 4.6 mm × 250 mm, 5 µm). The liquid chromatography conditions were as follows. Solvent A was 0.01 M L−1 sodium acetate, solvent B was 80% (V/V) acetonitrile. A gradient elution was used. The flow rate was 1 mL min−1. The injection volume was 2 µL and detected at 254 nm.
2.8. Indirect competitive ELISA
Indirect competitive ELISA was carried out as described by Bu, Luo, Zheng, and Zheng (Citation2009), to measure the antigenicity and allergenicity of PV in the samples. After preliminary experiments, the determined ELISA test conditions were as follows: coating concentration of PV was 2 µg mL−1, rabbit anti-PV serum was diluted 160,000 times and sample was dissolved in phosphate buffer saline (PBS) at a protein concentration of 0.2 mg mL−1. The antigenicity was calculated from standard curve, and a linear logarithmic correlation was observed in the range from 0.25 to 32 µg mL−1. For the allergenicity test, the coating concentration of PV was 0.1 mg mL−1, the patients sera was diluted two times and the protein concentration of samples were 0.01, 0.1, 1, 10, 100 and 1000 µg mL−1. HRP-conjugated goat anti-human IgE antibody was diluted 200 times. The inhibition rate of PV allergenicity was calculated as follows: inhibition rate (%) = (1 − B/B0) × 100. In the formula, B is the absorbance measured in the samples and B0 is the absorbance measured in the absence of the samples.
2.9. Experimental design and statistical analysis
Maltose/PV weight ratio (X1), reaction temperature (X2) and reaction time (X3) were chosen as independent variables (k = 3) in the experimental design. The dependent variable was the antigenicity (Y) of PV. The independent variables were optimised using a central composite rotatable design which contained five levels coded as −1.682, −1, 0, 1 and 1.682 for each independent variable. shows the coded values and the corresponding actual values of the three independent variables. The actual values ranges of three independent variables were from 0 to 4 (X1), 45°C to 85°C (X2) and 24 h to 120 h (X3), respectively. The complete central composite design consisted of 23 experiments, and the 23 experiments included a full factorial design plus 2 × 3 star experiments and 9 centre experiments. The centre experiments were performed to measure the accuracy and to verify changes in the estimation procedure. In addition, all of the 23 experiments were run in an identical environment. Experimental data were analysed by SAS 8.2 (SAS Institute Inc, Cary, NC, USA) and carried out as our pervious study (Bu, Lu et al., Citation2009).
Table 1. Coded and uncoded settings of independent variables for Maillard reaction conditions according to central composite rotatable design.
3. Results and discussion
3.1. Purification of PV
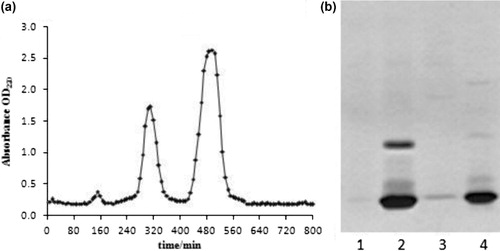
PV from grass carp was isolated and partially purified by gel chromatography. Three fractions were collected and analysed by SDS–PAGE. As shown in , Grass carp PV was eluted between approximately 264 and 360 min which was the second peak. There was no band of the third fraction in SDS-PAGE (), because it contained some proteins of small molecular weight.
3.2. Colour changes and SDS-PAGE analysis of PV-maltose conjugates
As shown in , when the reaction temperatures were 45°C and 53.1°C, the colour of the conjugates did not change significantly. However, when the reaction temperatures were 65°C and 76.9°C, the brown colour became darker with the increase of reaction time and maltose/PV weight ratio. The colour of PV-maltose changed because of the complex melanoidins which formed at the end of the Maillard reaction.
Table 2. Full factorial central composite design matrix and the antigenicity and allergenicity of the grass carp parvalbumin in parvalbumin-maltose conjugates.
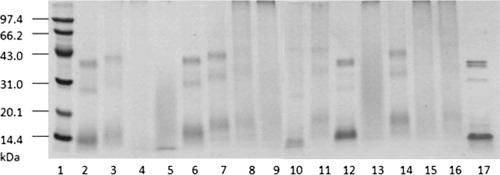
When Maillard reaction proceeded between reducing sugar and protein, the reducing end of the sugar and the ε-amino residue of the protein formed a covalent bond, leading to an increase of the protein molecular weight (Kato, Minaki, & Kobayashi, Citation1993). In , the bands going up meant that PV and maltose had reacted. Compared with original PV extract (line 17 of ), the bands of line 2, 3, 6, 7 and 14 showed changes at different degrees. There was no obvious difference between line 12 and 17. It demonstrated that the reaction under 45°C was mild. Similar results have been obtained in the research of pea albumins glycated at 37°C for 7 days (Mierzejewska, Mitrowska, Rudnicka, Kubicka, & Kostyra, Citation2008). There was no clear protein band in line 5, 8, 9, 11, 13, 15 and 16. It might be because of the different molecular weights of Maillard reaction products formed under long reaction time (100.5 h and 120 h) or high reaction temperature (76.9°C and 85°C).
3.3. Assessment of model of antigenicity for three independent variables
shows the experimental design and the antigenicity of PV after reaction with maltose under different reaction conditions. Through regression analysis on 23 experiments, the results indicated that several terms were not significant (P > 0.05). After fitting the full second-order model, non-significant terms were eliminated from the regression model and there were six regression terms in the second-order model for PV. The model was: Y1 = 8.55 − 3.82X1 − 23.32X2 − 10.90X3 + 13.88X2X2 + 16.43X2X3.
The P-value of fitted model for PV was 0.0001 and the adjusted R 2 was 0.9298. The P-value was very small and the R 2 value was very high. So the second-order model was highly significant and it could show the factual relationship between responses and independent variables.
3.4. Effect of Maillard reaction condition on antigenicity of PV in PV-maltose conjugates
shows the analysis of regression coefficients for the regression prediction model. The highly significant (P < 0.01) effects on the antigenicity were the linear effects of temperature and time, the quadratic effects of temperature and the interactive effect between temperature and time. The significant (P < 0.05) effect on the antigenicity was the linear effect of maltose/PV weight ratio. The value of regression coefficient revealed the effects of Maillard reaction condition on the antigenicity of PV, so temperature was the most effective factor, followed by the time. The optimal reaction conditions were as follows: maltose/PV weight ratio was 2.04, reaction temperature was 67.15°C and reaction time was 74.25 h. The predicted minimum antigenicity of PV was 0.276 µg mL−1, and the antigenicity of original PV extract was 26.54 µg mL−1. The experimental antigenicity of PV-maltose under the optimal reaction conditions was 0.388 µg mL−1. The experimental value was close to the predicted value of the antigenicity. Obviously, the antigenicity of PV was significantly reduced.
Table 3. Regression coefficients for regression prediction model of the antigenicity of parvalbumin of grass carp.
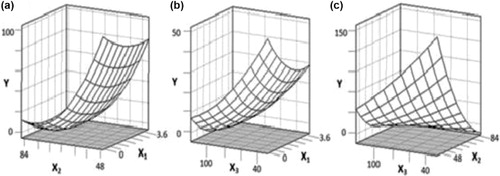
The antigenicity of PV-maltose conjugates and the reaction conditions are listed in . Compared with original PV, not all of the Maillard reaction products had a lower antigenicity. Jiménez-Saiz, Belloque, Molina, and López-Fandiño (Citation2011) reported that, Maillard reaction with glucose reduced the binding of IgG to ovomucoid but increasing the binding of IgG to ovalbumin. The effects of maltose/PV weight ratio and temperature at an aptotic time of 72 h are shown by the response surface plot in . In this research, the antigenicity of PV decreased with the increase of maltose/PV weight ratio. However, increasing maltose/PV weight ratio could not affect the antigenicity of PV when the Maillard reaction between PV and maltose was sufficient. The antigenicity of PV decreased first and then became steady as the temperature increased from 45 to 85°C. It demonstrated that the increase of the temperature could not unlimitedly reduce the antigenicity. When the reaction temperature was lower than 60–70°C, the antigenicity of PV was higher than the original PV no matter how to increase maltose/PV weight ratio. This indicated that the effect of temperature was more important than that of maltose/PV weight ratio. shows the effect of maltose/PV weight ratio and time at the temperature of 65°C. The antigenicity of PV decreased with the addition of maltose first and then changed slowly. The increase in reaction time resulted in the decrease of antigenicity. These results are similar to those observed by Nakamura et al. (Citation2006), who reported that the allergenicity of squid tropomyosin decreased with the increased Maillard reaction time. The response surface plot in revealed the effect of temperature and time at the maltose/PV weight ratio of 2:1. The reaction time and temperature had an interaction effect on the antigenicity of PV. Generally, the changes of epitopes are crucial to the changes of antigenicity. During the reaction, heat and glycation were two effective factors that would affect the antigenicity of PV. Heat treatment could affect the protein structure, such as exposure of hidden SH-groups, polymerisation and cross-linking, which resulted in the changes of antigenic epitopes (Oldfield, Singh, Taylor, & Pearce, Citation1998). Glycation could cause reduction of antigenic epitopes. So when the reaction temperature was low, the antigenicity of PV decreased with the increasing time, which meant the conjugation with maltose played a leading role in the changes of antigenicity. When the reaction temperature was higher than about 70°C, the antigenicity increased with the increasing reaction time, which meant the heat treatment played a leading role in the changes of antigenicity.
3.5. Effect of Maillard reaction on the IgE binding ability of PV
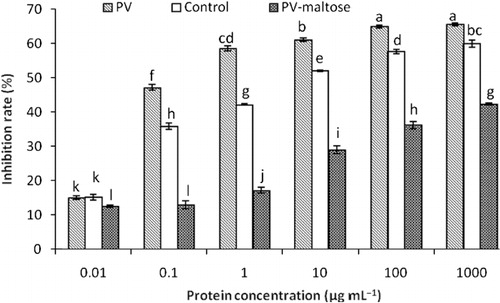
In order to evaluate the optimised result, PV-maltose was prepared under the optimal condition of Maillard reaction, and the IgE binding ability of PV-maltose was measured using the sera of fish-allergic patients. PV control was prepared under the same condition as the optimal condition of Maillard reaction without maltose. As shown in , the inhibited rate of PV, PV control and PV-maltose at the same protein concentration were significantly different when the protein concentration changed from 0.01 to 1000 µg mL−1. It indicated that the IgE binding ability of PV was suppressed after reacting with maltose. Similarly, Nakamura et al. (Citation2006) reported that the specific IgE binding ability of tropomyosin was suppressed when tropomyosin conjugated with ribose. Compared with PV, the IgE binding ability of control sample was suppressed, but it was higher than that of PV-maltose. The result suggested that the structural change of PV caused by the heat treatment was one of the factors leading to the reduction of the IgE binding ability. The other factor was the change of allergen epitopes resulted from Maillard reaction.
3.6. Amino acid composition of PV and PV-maltose
The amino acid compositions were shown in . After Maillard reaction, the essential amino acid content of PV decreased to 14.50% and the non-essential amino acid content of PV decreased to 23.21%. Among the essential amino acids, lysine was the most affected by the Maillard reaction, decreasing by 58.10%. The contents of Methionine, threonine and valine were also decreased significantly. Among the non-essential amino acids, arginine showed the largest reduction (75.47%). In addition, the contents of histidine, serine, tyrosine and alanine were also deducted by Maillard reaction. Maillard reaction between PV and maltose only decreased to 18.48% of total amino acid of PV, but it decreased almost all antigenicity of PV. Nakamura et al. (Citation2006) reported that the available lysine and IgE-binding ability of squid tropomysosin decreased markedly after Maillard reaction. Bu et al. (Citation2010) indicated that the free amino groups of whey protein decreased during the Maillard reaction between whey protein and glucose, leading to decreased antigenicity. These results were similar to our study.
Table 4 Amino acid compositions of Parvalbumin and Parvalbumin-maltose.
4. Conclusion
A model for predicting the interaction between the antigenicity of PV and Maillard reaction products was established. In this study, temperature had the most important effect on the antigenicity of PV. The predicted antigenicity of PV was reduced from 26.54 µg mL−1 to 0.276 µg mL−1 under the optimal condition of Maillard reaction, which was close to the validated value (0.388 µg mL−1). Maillard reaction can significantly suppress the IgE binding to PV, which is an effective method to control the major allergen of grass carp.
Funding
This study was supported by the earmarked fund for China Agriculture Research System (CARS-46) and National Natural Science Foundation of China (award nr 30871946).
Additional information
Funding
References
- Bu, G., Lu, J., Zheng, Z., & Luo, Y. (2009). Influence of Maillard reaction conditions on the antigenicity of bovine α-lactalbumin using response surface methodology. Journal of the Science of Food and Agriculture, 89, 2428–2434. doi:10.1002/jsfa.3741
- Bu, G., Luo, Y., Lu, J., & Zhang, Y. (2010). Reduced antigenicity of β-lactoglobulin by conjugation with glucose through controlled Maillard reaction conditions. Food and Agricultural Immunology, 21, 143–156. doi:10.1080/09540100903452122
- Bu, G., Luo, Y., Zheng, Z., & Zheng, H. (2009). Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin lactoglobulin in whey protein isolate. Food and Agricultural Immunology, 20, 195–206. doi:10.1080/09540100903026116
- Cai, Q. F., Liu, G. M., Li, T., Hara, K., Wang, X. C., Su, W. J., & Cao, M. J. (2010). Purification and characterization of parvalbumins, the major allergens in red stingray (Dasyatis akajei). Journal of Agricultural and Food Chemistry, 58, 12964–12969. doi:10.1021/jf103316h
- Chatterjee, U., Mondal, G., Chakraborti, P., Patra, H. K., & Chatterjee, B. P. (2006). Changes in the allergenicity during different preparations of pomfret, hilsa, bhetki and mackerel fish as illustrated by enzyme-linked immunosorbent assay and immunoblotting. International Archives of Allergy and Immunology, 141, 1–10. doi:10.1159/000094176
- Fæste, C. K., & Plassen, C. (2008). Quantitative sandwich ELISA for the determination of fish in foods. Journal of Immunological Methods, 329(1–2), 45–55. doi:10.1111/j.1398-9995.2009.02162.x
- Griesmeier, U., Vázquez-Cortés, S., Bublin, M., Radauer, C., Ma, Y., Briza, P., … Breiteneder, H. (2010). Expression levels of parvalbumis determine allergenicity of fish species. Allergy: European Journal of Allergy and Clinical Immunology, 65, 191–198. doi:10.1111/j.1398-9995.2009.02162.x
- Hildebrandt, S., & Garber, E. A. E. (2010). Effects of processing on detection and quantification of the parvalbumin gene in Atlantic salmon (Salmo salar). Food Chemistry, 119(1), 75–80. doi:10.1016/j.foodchem.2009.05.074
- Iwan, M., Vissers, Y. M., Fiedorowicz, E., Kostyra, H., Kostyra, E., Savelkoul, H. F. J., & Wichers, H. J. (2011). Impact of Maillard Reaction on Immunoreactivity and Allergenicity of the Hazelnut Allergen Cor a 11. Journal of Agricultural and Food Chemistry, 59, 7163–7171. doi:10.1021/jf2007375
- Jiménez-Saiz, R., Belloque, J., Molina, E., & López-Fandiño, R. (2011). Human immunoglobulin e (IgE) binding to heated and glycated ovalbumin and ovomucoid before and after in vitro digestion. Journal of Agricultural and Food Chemistry, 59, 10044–10051. doi:10.1021/jf2014638
- Kato, A., Minaki, K., & Kobayashi, K. (1993). Improvement of emulsifying properties of egg white proteins by the attachment of polysaccharide of egg white proteins by the attachment of polysaccharide through Maillard reaction in a dry state. Journal of Agricultural and Food Chemistry, 41, 540–543. doi:10.1021/jf00028a006
- Kobayashi, A., Tanaka, H., Hamada, Y., Ishizaki, S., Nagashima, Y., & Shiomi, K. (2006). Comparison of allergenicity and allergens between fish white and dark muscles. Allergy: European Journal of Allergy and Clinical Immunology, 61, 357–363. doi:10.1111/j.1398-9995.2006.00966.x
- Kuehn, A., Scheuermann, T., Hilger, C., & Hentges, F. (2010). Important variations in PV content in common fish species: A factor possibly contributing to variable allergenicity. International Archives of Allergy and Immunology, 153, 359–366. doi:10.1159/000316346
- Li, Z., Luo, Y., & Feng, L. (2011). Effects of Maillard reaction conditions on the antigenicity of α-lactalbumin and β-lactoglobulin in whey protein conjugated with maltose. European Food Research and Technology, 233, 387–394. doi:10.1007/s00217-011-1532-7
- Ma, Y., Griesmeier, U., Susani, M., Radauer, C., Briza, P., Erler, A., … Breiteneder, H. (2008). Comparison of natural and recombinant forms of the major fish allergen parvalbumin from cod and carp. Molecular Nutrition and Food Research, 52, S196–S207. doi:10.1002/mnfr.200700284
- Mierzejewska, D., Mitrowska, P., Rudnicka, B., Kubicka, E., & Kostyra, H. (2008). Effect of non-enzymatic glycosylation of pea albumins on their immunoreactive properties. Food Chemistry, 111, 127–131. doi:10.1016/j.foodchem.2008.03.046
- Nakamura, A., Sasaki, F., Watanabe, K., Ojima, T., Ahn, D.-H., & Saeki, H. (2006). Changes in allergenicity and digestibility of squid tropomyosin during the Maillard reaction with ribose. Journal of Agricultural and Food Chemistry, 54, 9529–9534. doi:10.1021/jf061070d
- Nakamura, S., Suzuki, Y., Ishikawa, E., Yakushi, T., Jing, H., Miyamoto, T., & Hashizume, K. (2008). Reduction of in vitro allergenicity of buckwheat Fag e 1 through the Maillard-type glycosylation with polysaccharides. Food Chemistry, 109, 538–545. doi:10.1016/j.foodchem.2007.12.075
- Oldfield, D. J., Singh, H., Taylor, M. W., & Pearce, K. N. (1998). Kinetics of denaturation and aggregation of whey proteins in skim milk heated in an ultra-high temperature (UHT) pilot plant. International Dairy Journal, 8(4), 311–318. doi:10.1016/S0958-6946(98)00089-2
- Peñas, E., Restani, P., Ballabio, C., Préstamo, G., Fiocchi, A., & Gomez, R. (2006). Evaluation of the residual antigenicity of dairy whey hydrolysates obtained by combination of enzymatic hydrolysis and high-pressure treatment. Journal of Food Protection, 69, 1707–1712. Retrieved from http://docserver.ingentaconnect.com.login.ezproxy.library.ualberta.ca/deliver/connect/iafp/0362028x/v69n7/s30.pdf?expires=1378769022&id=75427545&titleid=5200021&accname=University+of+Alberta&checksum=4E671AC2F7E33EB40B7CD2D522EA787C
- Perez-Gordo, M., Cuesta-Herranz, J., Maroto, A. S., Cases, B., Ibáñez, M. D., Vivanco, F., & Pastor-Vargas C. (2011). Identification of sole parvalbumin as a major allergen: Study of cross-reactivity between parvalbumins in aSpanish fish-allergic population. Clinical and Experimental Allergy, 41, 750–758. doi:10.1111/j.1365-2222.2011.03721.x
- Sampson, H. A. (2004). Update on food allergy. Journal of Allergy and Clinical Immunology, 113, 805–819. doi:10.1016/j.jaci.2004.03.014
- Seo, J. H., Lee, J. W., Lee, Y. S., Lee, S. Y., Kim, M. R., Yook, H. S., & Byuni, M. W. (2004). Change of an egg allergen in a white layer cake containing gamma-irradiated egg white. Journal of Food Protection, 67, 1725–1730. Retrieved from http://www.scopus.com/record/display.url?eid=2-s2.0-4043140787&origin=resultslist&sort=plf-f&src=s&st1=Change+of+an+egg+allergen+in+a+white+layer+cake+containing+gamma-irradiated+egg+white&sid=9198D86B25B8A22F164C0D05BA5E4EEB.aXczxbyuHHiXgaIW6Ho7g%3a20&sot=b&sdt=b&sl=100&s=TITLE-ABS-KEY%28Change+of+an+egg+allergen+in+a+white+layer+cake+containing+gamma-irradiated+egg+white%29&relpos=0&relpos=0&citeCnt=8&searchTerm=TITLE-ABS-KEY%28Change+of+an+egg+allergen+in+a+white+layer+cake+containing+gamma-irradiated+egg+white%29
- Slütte, B., Soema, P. C., Ding, Z., Verheul, R., Hennink, W., & Jiskoot, W. (2010). Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. Journal of Controlled Release, 143, 207–214. doi:10.1016/j.jconrel.2010.01.007
- Swoboda, I., Bugajska-Schretter, A., Valenta, R., & Spitzauer, S. (2002). Recombinant fish parvalbumins: Candidates for diagnosis and treatment of fish allergy. Allergy: European Journal of Allergy and Clinical Immunology, 57(s72), 94–96. doi:10.1034/j.1398-9995.57.s72.21.x
- van de Lagemaat, J., Manuel Silván, J., Javier Moreno, F., Olano, A., Dolores del Castillo, M. (2007). In vitro glycation and antigenicity of soy proteins. Food Research International, 40, 153–160. doi:10.1016/j.foodres.2006.09.006
- Van Do, T., Elsayed, S., Florvaag, E., Hordvik, I., & Endresen, C. (2005). Allergy to fish parvalbumins: Studies on the cross-reactivity of allergens from 9 commonly consumed fish. Journal of Allergy and Clinical Immunology, 116, 1314–1320. doi:10.1016/j.jaci.2005.07.033
- Van Do, T., Hordvik, I., Endresen, C., & Elsayed, S. (2003). The major allergen (parvalbumin) of codfish is encoded by at least two isotypic genes: cDNA cloning, expression and antibody binding of the recombinant allergens. Molecular Immunology, 39, 595–602. doi:10.1016/S0161-5890(02)00200-6