Abstract
An indirect chemiluminescent competitive ELISA (CL-ELISA) method was developed to detect residues of melamine in milk. A high-quality polyclonal antibody towards melamine cyanurate (MC) was prepared based on synthesis of a novel immunogen. The method is applicable over the range of 0.5–7.0 µg.mL−1 of MC, with an IC50 value of 1.7 µg.mL−1. The developed method was used in the detection of melamine residue in milk with the detection limit of 1 ng.mL−1. There was no cross-reactivity with commonly used veterinary drugs. The CL-ELISA method developed provides an alternative to chromatography spectrometry for regulatory analysis of melamine in milk and could be promisingly used to improve the sensitivity of the available ELISA test kits.
1. Introduction
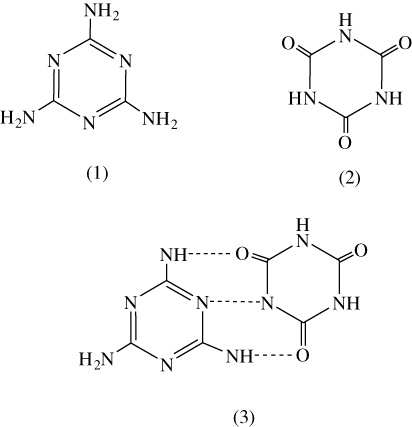
Melamine is one of the most important industrial chemicals and widely used to make plastics, fertilisers, intermediates of organic synthesis, flame retardant and other products. Melamine was not approved as an ingredient in foods and food products. In recent years, however, melamine was deliberately and illegally added in food products to increase the parent-protein content. In March 2007, imported pet food ingredients contaminated with melamine and its analogue cyanuric acid led to a major outbreak of renal disease and associated deaths in pets in the USA. In September 2008, the occurrences of “the infants suffering from kidney stones” have been found in many cities of China. Pharmacology studies in rats and in dogs showed that high doses of melamine would not produce renal toxicity (Lipschitz & Stokey, Citation1945). The similar conclusion was obtained in the study of cyanuric acid which showed that it did not lead to renal toxicity (Hammond et al., Citation1986). It was found that melamine could be hydrolysed to ammeline, ammelide and cyanuric acid and crystallised with cyanuric acid to form low soluble melamine cyanurate (MC) () in kidney tubules. The crystals, having a grid structure composed of melamine and cyanuric acid bound together by hydrogen bonds (), formed an insoluble precipitate in renal tubules, leading to progressive tubular blockage and degeneration (Dobson et al., Citation2008), causing illness and death (Puschner, Poppenga, Lowenstine, Filigenzi, & Pesavento, Citation2007). In most countries, the tolerance level for melamine is regulated at 1 µg.g−1 for baby formula and 2.5 µg.g−1 for food containing more than 15% milk.
Up to now, many techniques have been developed to detect melamine and its hydrolysates, including capillary zone electrophoresis (Yan, Zhou, Zhu, & Chen, Citation2009), zwitteronic hydrophilic interaction chromatography and tandem mass spectrometry (Heller & Nochetto, Citation2008), gas chromatography (Yokley, Mayer, Rezaaiyan, Manuli, & Cheung, Citation2000), liquid chromatography (Andersen et al., Citation2008; Baynes et al., Citation2008; Cai, Ouyang, Qian, & Peng, Citation2008; Ding et al., Citation2008; Filigenzi, Tor, Poppenga, Aston, & Puschner, Citation2007; Karbiwnyk et al., Citation2009; Patakioutas, Savvas, Matakoulis, Sakellarides, & Albanis, Citation2007; Sancho, Ibáñez, Grimalt, Pozo, & Hernández, Citation2005; Scheepers et al., Citation1995; Wu et al., Citation2009), and immunoassay (Garber, Citation2008). Compared with chromatography, enzyme-linked immunosorbant assay (ELISA) is simple, rapid and cost-efficient and, therefore, could be an alternative to the chromatography method in high-throughput field screening. Several commercial ELISA test kits to detect melamine are available, such as Abraxis Melamine Plate kit, Beacon Melamine Plate Kit, Cusabio Melamine ELISA Kit and Romer Labs® AgraQuant® Melamine Sensitive Assay. All these kits are based on traditional colorimetric detection system. It is well-known that the sensitivity of the ELISA is greatly affected by the detector used. The CL-ELISA employing chemiluminescence in the detection system can offer 1–2 orders of magnitude gain of sensitivity compared to traditional ELISA with colorimetric detection system. In this study, chemiluminescence was applied to develop a more sensitive ELISA method for detection of melamine in milk.
2. Materials and methods
2.1. Chemicals and materials
Melamine was obtained from a reagent factory (Jinan, China), and cyanuric acid was from J&K Scientific Ltd. (Beijing, China). Bovine serum albumin (BSA), ovalbumin (OVA), Freund's complete and incomplete adjuvants (cFA and iFA, respectively) and 1-ethyl-3-(dimethylaminopropyl) carbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich (St. Louis, MO). N-Hydroxysuccinimide (NHS) was from Cxbio Biotechnology Ltd. (Shanghai, China). Goat anti-rabbit IgG-horseradish peroxidase (GAR-IgG-HRP) conjugate was prepared in our laboratory (Slemon, Salvaterra, & Saito, Citation1980). 3-Carboxybenzaldehyde (CBA) was purchased from Dianyao Chemical Co. (Shanghai, China). p-Cresol (>99%) was provided by Alfa-Aesar (Tianjin, China). Luminol (>99%) was provided by Qingyun Chemical Co. (Hefei, China). Dimethyl sulfoxide (DMSO), methanol, hydrogen peroxide (30%), Tris, NaCl and other reagents were of chemical grade supplied by Guangmang Chemical Co. (Jinan, China).
2.2. Instrumentation
The CL-ELISA, carried out with high-binding white polystyrene 96 wells microtiter plates purchased from Greiner bio-one (Shanghai, China), was measured with a Microplate Luminometer pH checker (PHS-25, Lei-ci, Shanghai, China). Refrigerated centrifuge (Biofuge stratos, Heraeus) was used for buffer preparation.
2.3. Buffers and solutions
The following buffers were used in this study: phosphate-buffered saline (PBS, pH 7.4) containing 138 mM NaCl, 1.5 mM KH2PO4, 7 mM Na2HPO4 and 2.7 mM KCl; PBST: PBS containing 0.05% (v/v) Tween20; coating buffer: 0.05 M carbonate buffer (pH 9.6) consisted of 15 mM Na2CO3 and 35 mM NaHCO3; blocking buffer: PBS with 1% of OVA and 0.05% (v/v) Tween20; 0.1 M Tris-HCl buffer (pH 8.8); 0.5 M sodium borate buffer (pH 8.5); CL-substrate solution (10 mM luminol and 1 mM p-cresol dissolved in Tris-HCl buffer with pH 8.8).
2.4. Preparation of melamine conjugates
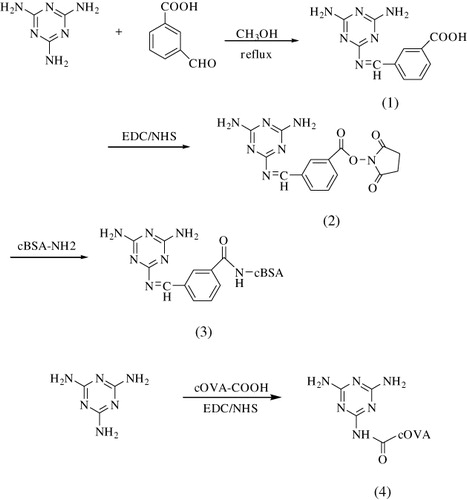
The immunogen melamine-cBSA and the coating antigen melamine-cOVA were prepared by the method previously described (Jung, Le, Wengenmayer, Wolf, & Kramer, Citation1985). First, BSA was modified with ethylenediamine to form cationized BSA (cBSA) (Liu et al., Citation2007). About 168 mg of melamine and 100 mg CBA with the ratio of 2:1 were added to 40 mL of methanol (). The mixture was stirred and refluxed for 24 h to get intermediate (1). Then, the solution was dried by a rotary evaporator under vacuum. The intermediate (1) was dissolved in 4 mL of DMSO, with an addition of 150 mg EDC and 100 mg NHS for activation. The mixture solution was stirred for 24 h at room temperature. Then the solution was added slowly with stirring to a solution of 123.3 mg of cBSA dissolved in 10 mL of PBS (0.1 M, pH 7.4) and stirred at room temperature for 3 h. Finally, the reaction mixture was dialysed (MWCO = 12,000–14,000 Da) against PBS (0.1 M, pH 7.4) for three days and dialysed against ultra-pure deionised water for another two days. The solution was then centrifuged, and the supernatant was lyophilised to get melamine-cBSA conjugate. The melamine-cOVA conjugate was prepared similarly. The solution containing 107 mg of cOVA was diluted in 10 mL of PBS, and then 13.67 mg EDC and 8.2 mg NHS was added. Finally, 15 mg of melamine dissolved in 1 mL of DMSO was added to the mixture and then reacted for 5 h at room temperature under continuous stirring. The reaction solution was dialysed as described above, and the melamine-cOVA conjugate was obtained and stored at −20°C until use.
2.5. Immunisation of rabbits
Three male New Zealand white rabbits (2 kg) were subcutaneously injected at multiple sites in the back with melamine-cBSA conjugate. The initial immunogen contained 0.5 mg of conjugate in 1 mL of a mixture of NaCl (0.9%) and cFA [1:1 (v: v)]. Subsequent booster immunogen contained a mixture of 0.5 mL NaCl (0.9%) and 0.5 mL of iFA containing 0.25 mg of the conjugate. It was injected 20 days later and then at 15 days intervals. One week after each immunisation, serum titers were determined by the CL-ELISA. The antiserum obtained after each booster was prepared by allowing the blood to clot overnight at 4°C, followed by centrifugation to remove particulate material. Seven days after the last booster, all rabbits were exsanguinated by heart puncture under general anaesthesia and euthanized by lethal injection before recovery. The serum was separated from blood cells by storage of the blood overnight at 4°C and centrifugation at 7000 g for 20 min. The crude serum was purified by saturated ammonium sulphate (SAS) precipitation (three times using 50, 33 and 33% (v/v) of SAS, respectively), and sodium azide was added as a preservative at a final concentration of 0.02% (w/w). The purified serum was aliquotted and stored at −20°C for use.
2.6. CL-ELISA checkerboard procedure
The 96-well-polystyrene plates were coated with the diluted melamine-cOVA solution in the range of 10, 5, 2.5, 1.25, 0.625, 0.3125, 0.15625 and 0.078125 µg.mL−1 (100 µL/well) overnight at 4°C. The plate was washed with 280 µL/well of PBST three times, blocked with 250 µL/well of blocking buffer and then incubated for 2 h at room temperature. After the removal of the blocking buffer and three washings of the plate, a series of diluted antiserum or antibody solution (1/200–1/248,000 dilution) was added (100 µL/well), then the plate was incubated for 1.5 h at 37°C. After three washings, 100 µL/well of 1:2000 GAR-IgG-HRP was added, followed by incubation at 37°C for 50 min. After the plates were washed, 100 µL/well of CL-substrate solution was added and chemiluminescence intensity value was immediately measured.
2.7. Development of indirect competitive CL-ELISA
The optimised concentration of the coating antigen and the primary antibody was determined by checkerboard experiment, as indicated above. The indirect competitive CL-ELISA procedure was conducted as follows: 96-well polystyrene plates were coated with 100 µL/well of 2.5 µg.mL−1 melamine-cOVA solution overnight at 4°C. The plate was then washed three times with 280 µL/well of PBST and blocked with 250 µL/well of blocking buffer by incubation for 2 h at room temperature. Blocking buffer was removed, and the plate was washed again. For the competition group, the concentration of antibody was set constant (1:8000 in PBST, 50 µL/well) and a series of diluted analytes solution (0.001 µg.mL−1—100 µg.mL−1, 50 µL/well) were added, followed by incubation for 1.5 h. Then the plate was washed and incubated with 100 µL/well of 1:2000 GAR-IgG-HRP at 37°C for 50 min. After washing, the CL-substrate solution was added and chemiluminescence was quantified. The chemiluminescence intensity value was corrected by the blank value. Reading of well with or without analytes was indicated by B and B0, respectively. The inhibition ratio was defined as B/B0, and IC50 was defined as the analyte concentration with chemiluminescence intensity half of B0.
2.8. Standard calibration curve
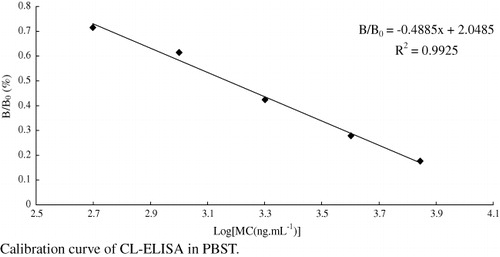
With the final MC concentrations of 0.5, 1.0, 2.0, 4.0 and 7.0 µg.mL−1 (equal to 0.247, 0.494, 0.989, 1.977 and 3.460 µg.mL−1 of melamine), melamine-OVA as coating antigen, MC as the competitor, the standard calibration curve was established as performed above. The relationship of MC concentration with the B/B0 values was quantified.
2.9. Antibody specificity
Specificity of the antiserum was assessed by detection of cross-reactivity towards melamine and cyanuric acid by the CL-ELISA method. Other commonly used food additives, including chloramphenicol, ractopamine, salbutamol, ciprofloxacin and terbutaline were also detected by the same method. Cross-reactivity (CR%) values was calculated as follows:
2.10. Matrix effects determination
The milk samples were defatted by centrifugation at 4°C (1000 g, 20 min) and spiked with melamine PBST solution, and the same concentration of cyanuric acid in PBST was added to form MC. Competition curves with final MC concentrations of 0.001, 0.01, 0.1, 0.4, 1, 2, 4, 10 and 100 µg.mL−1 were established in PBST and in various dilutions (1:5, 1:10 and 1:20) of milk to study the matrix effect of milk. The matrix sample with the most similar IC50 value to buffer group was selected for inter- and intra-assay determination.
2.11. Inter- and intra-assay variation determination
The milk samples were fortified by MC at final concentrations of 0.5, 1.0, 2.0, 4.0 and 7.0 µg.mL−1 in milk diluted 1:20. Inter-assay variation was calculated from results of five replicates of each dilution carried out on five different days. Intra-assay variation was measured by analysis of five replicates of each dilution on a single day. Sample recoveries were determined from the standard curve.
3. Results and discussion
3.1. Preparation of conjugates
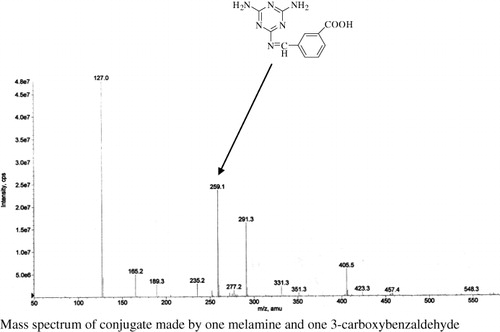
As a small molecule, melamine must be conjugated to a carrier protein to be immunogenic. In order to prepare a melamine immunogen, a series of synthetic methods were used to modify melamine. Only the approach described in provided an immunogen that elicited an antibody response with high-specificity and sensitivity in rabbits. In this procedure, melamine was treated with 3-CBA to bring in a carboxylic acid group to form an intermediate one (), which was then linked with carrier protein BSA through EDC/NHS reaction. There are three amine groups in the molecule of melamine, therefore, it is possible to obtain three different melamine–CBA conjugates which are corresponding to one, two or three of the amine groups in melamine reacting with aldehyde groups in CBA molecule. To prepare a qualified immunogen, it is preferred to form a conjugate composed of one melamine molecule and one CBA molecule. After testing different reactant ratios, the molar ratio of CBA: melamine = 1:2 was selected to prepare immunogen, as shown in . The mixture of products from the reaction was measured by mass spectrometry and only the conjugate made by one melamine and one CBA molecule was found in the mass spectrum (see ). Conjugates made by two or three molecules of CBA with one molecule of melamine were not detected in the mass spectrum, indicating the main product of preferred conjugate. To prepare the melamine immunogen, the carrier protein BSA was initially linked to melamine directly without any linkage reagent such as 3-CBA. The immunogen prepared in this way, however, did not elicit an appropriate antibody response in rabbits. The small melamine molecule might have been covered by the large carrier protein BSA and not recognised by the rabbit immune system. Ultra violet (UV) spectrometry was a common method to identify the conjugation between hapten and carrier protein. For this procedure, however, no strong evidence was found in UV spectrum for the existence of conjugate.
3.2. Characterisation of the antibody
The antiserum from each of the two rabbits were carefully screened by various coating antigens synthesised to determine the quality of antibody. Generally speaking, starting from the third immunisation, the titer of the antiserum increased gradually until reaching a level that remains constant after further immunisation. For the antiserum used in this research, the titer measured one week after the third immunisation was 102,400 by CL-ELISA and reached 409,600 at the fifth immunisation with no significant change afterwards.
Previous studies showed that heterogeneous assays can increase the immunoassay sensitivity (Beier, Creemer, Ziprin, & Nisbet, Citation2005; Gueguen et al., Citation2000; Lee, Ahn, Park, Ko, & Kim, Citation2002; Muldoon, Holtzapple, Deshpande, Beier, & Stanker, Citation2000) by reducing affinity of the antibody to the spacer arm (Beier, Ripley, Young, & Kaiser, Citation2001; Greirson, Allen, Gare, & Watson, Citation1991). In our study, five different immunogens and five corresponding coating antigens were synthesised. Each antiserum was screened using different coating antigens by CL-ELISA. Our results indicated that the homologous structure provided high titer but low inhibition and the heterogeneous structure provided low titer but high inhibition. Among all of the combinations of antibodies and coating antigens, the antiserum resulting from the use of the immunogen synthesised and antigen in was the combination which produced the best specificity and sensitivity.
During the screening procedure, both melamine and MC were used as competitors in the CL-ELISA system. For MC group, it is necessary to form MC first, and then measure MC by the CL-ELISA method. Although MC has limited solubility in water, it is sufficient for immunoassay. Melamine itself provided an IC50 result of 150 µg.mL−1, which was not adequate to satisfy the permitted level of 1 µg.mL−1. Among the compounds listed in , only MC had significant reactivity with the antiserum to provide an IC50 value of 1.7 µg.mL−1 (equivalent to 0.84 µg.mL−1 of melamine), as shown in . Limit of detection (LOD) for this assay was defined as IC10 value according to the competitive curve and found to be approximately 1 ng.mL−1 (). There are already several commercial ELISA kits for melamine determination, including Abraxis Melamine Plate kit, Cusabio Melamine ELISA Kit, Romer LaboratoriesAgraQuant Melamine Sensitive Assay and Beacon Melamine Plate Kit, in which Abraxis Melamine Plate kit are reported to be the most sensitive, with the LOD value of 9 ng.mL−1 for melamine detection in buffer. The results of our research show that the CL-ELISA not only meets the requirement of 1 ng.mL−1 in most countries but also displays a sensitivity more than commercially available test kits. The antibody also has good specificity, indicated by the cross-reaction test. As was shown in , other compounds like chloramphenicol, terbutaline, salbutamol, ciprofloxacin and ractopamine showed almost no reactivity towards the antiserum.
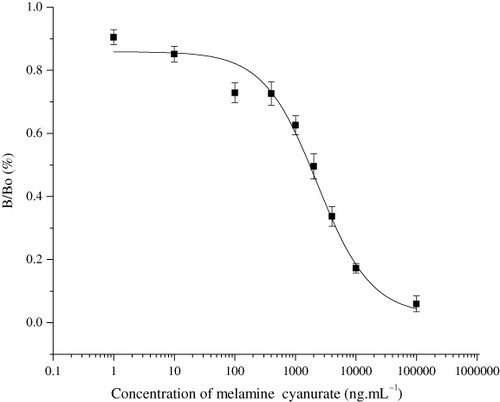
Table 1. IC50 values and cross-reactivity of selected compounds.
3.3. Application of the CL-ELISA to detect melamine in milk sample
To test the application of the CL-ELISA developed in this research, milk samples purchased from a local market were tested. The milk samples were spiked with different levels of melamine, and then the spiked melamine was linked with cyanuric acid. The milk samples were diluted at different levels to determine the matrix effect. When the samples were diluted gradually from 1:2 to 1:20, the corresponding inhibition curves were similar to the inhibition curve obtained in PBS buffer. At the dilution factor of 1:20, the inhibition curve almost overlapped with that in buffer group, indicating the minimal matrix effect at this dilution factor. Accordingly, the dilution of 1:20 was used throughout this study. shows that the CL-ELISA had intra-assay coefficient of variation below 19% and inter-assay coefficient of variation below 17%. The recovery ratios were calculated by the standard curve (see ) and inter- and intra-assay recovery ratio were in the range of 85–109% and 89–112%, respectively.
Table 2. Inter- and intra-assay variation of raw milk spiked with melamine cyanurate.
4. Conclusions
In summary, an indirect CL-ELISA method for detection of derived melamine was established. The detection limit for this method is approximately 1 ng.mL−1, which can satisfy the regulatory requirement of 1 µg.mL−1 in most of the countries and is more sensitive than most commercial ELISA kits for melamine control. In this research, immunogen of melamine-cBSA and coating antigen of melamine-cOVA were synthesised, and polyclonal antibody from immunised rabbits was used to develop the immunoassay. Milk spiked with melamine was detected, with satisfactory recovery (in the range of 85 − 112%) obtained. There is room for further improving the quality of anti-melamine antibody. We are currently conducting research to improve the assay to get more specific and sensitive antibody to melamine.
Supplemental data
Funding
This research was supported by National Natural Science Foundation of China [No. 20675048], National High-Tech Research and Development Program of China [863 Program, No. 07AA10Z435], [2007AA06A407], the Tianjin Science and Technology Program [No. 11ZCGHHZ01200], and the Fundamental Research Funds for the Central Universities [No. 65011121].
Additional information
Funding
References
- Andersen, W. C., Turnipseed, S. B., Karbiwnyk, C. M., Clark, S. B., Madson, M. R., Gieseker, C. M., … Reimschuessel, R. (2008). Determination and confirmation of melamine residues in catfish, trout, tilapia, salmon, and shrimp by liquid chromatography with tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 56, 4340–4347. doi:10.1021/jf800295z
- Baynes, R. E., Smith, G., Mason, S. E., Barrett, E., Barlow, B. M., & Riviere, J. E. (2008). Pharmacokinetics of melamine in pigs following intravenous administration. Food and Chemical Toxicology, 46, 1196–1200. doi:10.1016/j.fct.2007.11.013
- Beier, R. C., Creemer, L. C., Ziprin, R. L., & Nisbet, D. J. (2005). Production and characterization of monoclonal antibodies against the antibiotic tilmicosin. Journal of Agricultural and Food Chemistry, 53, 9679–9688. doi:10.1021/jf051987x
- Beier, R. C., Ripley, L. H., Young, C. R., & Kaiser, C. M. (2001). Production, characterization, and cross-reactivity studies of monoclonal antibodies against the coccidiostat nicarbazin. Journal of Agricultural and Food Chemistry, 49, 4542–4552. doi:10.1021/jf010208j
- Cai, Q., Ouyang, Y., Qian, Z., & Peng, Y. (2008). Determination of melamine residue in feeds by ultra performance liquid chromatography coupled with electrospray tandem mass spectrometry. Chinese Journal of Chromatography, 26, 339–342.
- Ding, T., Xu, J., Li, J., Shen, C., Wu, B., Chen, H., & Li, S. (2008). Determination of melamine residue in plant origin protein powder using high performance liquid chromatography-diode array detection and high performance liquid chromatography-electrospray ionization tandem mass spectrometry. Chinese Journal of Chromatography, 26, 6–9.
- Dobson, R. L. M., Motlagh, S., Quijano, M., Cambron, R. T., Baker, T. R., Pullen, A. M., … Daston, G. P. (2008). Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. Toxicological Sciences, 106, 251–262. doi:10.1093/toxsci/kfn160
- Filigenzi, S. M., Tor, R. E., Poppenga, H. R., Aston, A. L., & Puschner, B. (2007). The determination of melamine in muscle tissue by liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry, 21, 4027–4032. doi:10.1002/rcm.3289
- Garber, E. A. (2008). Detection of melamine using commercial enzyme-linked immunosorbent assay technology. Journal of Food Protection, 71, 590–594.
- Greirson, B. N., Allen, D. G., Gare, N. F., & Watson, I. M. (1991). Development and application of an enzyme-linked immunosorbent assay for lupin alkaloids. Journal of Agricultural and Food Chemistry, 39, 2327–2331. doi:10.1021/jf00012a047
- Gueguen, F., Boisde, F., Queffelec, A. L., Haelters, J. P., Thouvenot, D., Corbel, B., & Nodet, P. (2000). Hapten synthesis for the development of a competitive inhibition enzyme-immunoassay for thiram. Journal of Agricultural and Food Chemistry, 48, 4492–4499. doi:10.1021/jf000378g
- Hammond, B. G., Barbee, S. J., Inoue, T., Ishida, N., Levinskas, G. J., & Stevens, M. W. (1986). A review of toxicology studies on cyanurate and its chlorinated derivatives. Environmental Health Perspectives, 69, 287–292. doi:10.1289/ehp.8669287
- Heller, D. N., & Nochetto, C. B. (2008). Simultaneous determination and confirmation of melamine and cyanuric acid in animal feed by zwitterionic hydrophilic interaction chromatography and tandem mass spectrometry. Rapid Communications in Mass Spectrometry, 22, 3624–3632. doi:10.1002/rcm.3779
- Jung, R., Le, J. Y., Wengenmayer, F., Wolf, E., & Kramer, M. (1985). Mutagenicity studies of a carcinogenic nitrofuran and some analogues. Biomedica Biochimica Acta, 44, 485–492.
- Karbiwnyk, C. M., Andersen, W. C., Turnipseed, S. B., Storey, J. M., Madson, M. R., Miller, K. E., … Reimschuessel, R. (2009). Determination of cyanuric acid residues in catfish, trout, tilapia, salmon and shrimp by liquid chromatography-tandem mass spectrometry. Analytica Chimica Acta, 637, 101–111. doi:10.1016/j.aca.2008.08.037
- Lee, J. K., Ahn, K. C., Park, O. S., Ko, Y. K., & Kim, D.-W. (2002). Development of an immunoassay for the residues of the herbicide bensulfuron-methyl. Journal of Agricultural and Food Chemistry, 50, 1791–1803. doi:10.1021/jf011150b
- Lipschitz, W. L., & Stokey, E. (1945). The mode of action of three new diuretics: Melamine, adenine and formoguanamine. Journal of Pharmacology And Experimental Therapeutics, 83, 235–249.
- Liu, W, Zhao, C. B., Zhang, Y. L., Lu, S. X., Liu, J. T., & Xi, R. M. (2007). Preparation of polyclonal antibodies to a derivative of 1-aminohydantoin (AHD) and development of an indirect competitive ELISA for the detection of nitrofurantoin residue in water. Journal of Agricultural and Food Chemistry, 55, 6829–6834. doi:10.1021/jf070620k
- Muldoon, M. T., Holtzapple, C. K., Deshpande, S. S., Beier, R. C., & Stanker, L. H. (2000). Development of a monoclonal antibody-based cELISA for the analysis of sulfadimethoxine 1. Development and characterization of monoclonal antibodies and molecular modeling studies of antibody recognition. Journal of Agricultural and Food Chemistry, 48, 537–544.
- Patakioutas, G., Savvas, D., Matakoulis, C., Sakellarides, T., & Albanis, T. (2007). Application and fate of cyromazine in a closed-cycle hydroponic cultivation of bean (Phaseolus Vulgaris L.). Journal of Agricultural and Food Chemistry, 55, 9928–9935. doi:10.1021/jf071726i
- Puschner, B., Poppenga, R. H., Lowenstine, L. J., Filigenzi, M. S., & Pesavento, P. A. (2007). Assessment of melamine and cyanuric acid toxicity in cats. Journal of Veterinary Diagnostic Investigation, 19, 616–624. doi:10.1177/104063870701900602
- Sancho, J. V., Ibáñez, M., Grimalt, S., Pozo, Ó. J., & Hernández, F. (2005). Residue determination of cyromazine and its metabolite melamine in chard samples by ion-pair liquid chromatography coupled to electrospray tandem mass spectrometry. Analytica Chimica Acta, 530, 237–243. doi:10.1016/j.aca.2004.09.038
- Scheepers, M. L., Meier, R. J., Markwort, L., Gelan, J. M., Vanderzande, D. J., & Kip, B. J. (1995). Determination of free melamine content in melamine-formaldehyde resins by Raman spectroscopy. Vibrational Spectroscopy, 9, 139–146. doi:10.1016/0924‘2031(94)00091-T
- Slemon, J. R., Salvaterra, P. M., & Saito, K. (1980). Preparation and characterization of peroxidase: Antiperoxidase-Fab complex. Journal of Histochemistry and Cytochemistry, 28, 10–15. doi:10.1177/28.1.6766153
- Wu, Q. Q., Fan, K. X., Sha, W., Ruan, H. Q., Zeng, R., & Shieh, C. H. (2009). Highly sensitive detection of melamine based on reversed phase liquid chromatography mass spectrometry. Chinese Science Bulletin, 54, 732–737. doi:10.1007/s11434-009-0114-6
- Yan, N., Zhou, L., Zhu, Z. F., & Chen, X. G. (2009). Determination of melamine in dairy products, fish feed, and fish by capillary zone electrophoresis with diode array detection. Journal of Agricultural and Food Chemistry, 57, 807–811. doi:10.1021/jf803429e
- Yokley, R. A., Mayer, L. C., Rezaaiyan, R., Manuli, M. E., & Cheung, M. W. (2000). Analytical method for the determination of cyromazine and melamine residues in soil using LC-UV and GC-MSD. Journal of Agricultural and Food Chemistry, 48, 3352–3358. doi:10.1021/jf991231w