Abstract
Eremostachys plants (Lamiaceae) are consumed by indigenous Iranian culture as medicinal plants, but experimental studies on their biological properties are lacking. In order to investigate the biological potentials, methanol extract of Eremostachys labiosa (ELM) was tested for toxicity on various cancer cell lines using MTT 3, (4, 5-dimethylthiazal-2-yl)-2,5-diphenyl tetrazolium bromide colorimetric assay. Anti-cancer effect of ELM was shown by its significant toxicity on different cancer cell lines especially on gastric (AGS) cancer cells. To evaluate the anti-inflammatory effect of ELM, the amount of produced nitric oxide (NO) was assessed in lipopolysaccharide-stimulated J774A.1 macrophages using Griess reagent, and a remarkable decline was observed. Additionally, ELM showed considerable suppression on the proliferation of L. major promastigotes. The present study provides some evidence on the remarkable biological activities of E. labiosa. These findings also afford the scientific basis for the use of E. labiosa as a candidate medicinal plant for further in-depth phytochemical investigations towards anti-leishmanial, anticancer and anti-inflammatory compounds.
Introduction
Iran has a rich resource of numerous medicinal plants, many of which have been applied by local inhabitants from ancient times who had knowledge on their health-beneficial properties (Mosaddegh, Naghibi, Moazzeni, Pirani, & Esmaeili, Citation2012; Modaressi et al., Citation2009). The genus Eremostachys (Lamiaceae) comprises about 60 species and has a wide distribution over large parts of Eurasia (Hooper & Field, Citation1937; Ghorbani, Citation2005). The Eremostachys has 16 species in Iran, of which E. glabra Boiss. ex Benth., E. laciniata Bunge, E. lanata Jamzad, E. labiosa Bunge, E. labiosiformis (Popov) Knorring and E. macrophylla Montbret & Aucher are endemic to the country (Kalvandi, Safikhani, & Najafi Gh Babakhanlo, Citation2007). Traditionally, some of the Eremostachys species have been used for treating different ailments. Previous phytochemical investigations revealed the presence of flavonoids, chrysoeriol glycosides and monoterpene glycosides in these plants (Gella & Vavilova, Citation1981; Jensen, Caliş, Gotfredsen, & Sotofte, Citation2007; Nazemiyeh et al., Citation2008).
Pharmacological researches suggested medicinal usages of Eremostachys species. E. macrophylla is used for the treatment of wounds, snake bites, rheumatism and joint pains (Nori-Shargh, Kiaei, & Deyhimi, Citation2007). E. glabra is found in the north-west of Iran and claimed to contain different iridoid and phenylethanoid glucosides. Moreover, the rhizomes of E. glabra which has been shown to contain ferulic acid derivatives are used as local analgesic and anti-inflammatory compounds in Iran. Additionally, it displays a wide range of biological activities including free radical scavenging, antioxidant and anti-bacterial effects (Delazar et al., Citation2004, Citation2005; Erdemoglu, Turan, Cakici, Sener, & Aydin, Citation2006). E. laciniata is one of the Iranian flora, and its roots as well as flower decoction have traditionally been used orally for the treatment of allergy, headache and liver diseases. Phytochemical analysis of E. laciniata showed the presence of various monoterpenes, sesquiterpenes, iridoid glucosides and flavonoids (Eftekharsadat et al., Citation2011; Navaei, & Mirza, Citation2006). In addition, different extracts prepared from E. laciniata were reported to possess free radical scavenging, anti-inflammatory and antioxidant properties (Khan, Nisar, Rehman, Khan, & Nasir, Citation2010; Modaressi et al., Citation2009). Essential oil of E. laevigata was showed considerable anti-bacterial and antioxidant activities (Esmaeili, Citation2012).
Eremostachys labiosa [Phlomoides labiosa (Bunge) Adylov, Kamelin & Makhm.] is growing in Iran and is simply recognised by large white flowers with a pale yellow lower lip and usually incised leaves. The plant is highly variable in habit, leaf dissection and pubescence (Hassanzadeh, Emami, Asili, & Tayarani-Najaran, Citation2011). Since E. labiosa is regarded as a medicinal plant in Iran, the present study was aimed at assessing the possible biological activities including anti-cancer, anti-inflammatory and leishmanicidal properties.
Materials and methods
Preparation of methanol extract
E. labiosa plant was collected from Tandoreh National park (Chelmir region, 25 km north-west of Dargaz, Razavi Khorasan Province, north-east of Iran) in November 2007. The dried and powdered roots (500 g) were macerated in methanol (3 L) for 24 h at room temperature. The maceration was repeated for three times at the same condition. The methanol was evaporated at reduced pressure, and the obtained gummy residue (62.72 g) was used as E. labiosa methanol extract (ELM).
Determining the growth-inhibitory effect of ELM
Growth-inhibitory potential of ELM on cancer cell lines was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) colorimetric assay (Emami, Zamanai Taghizadeh Rabe, Ahi, Mahmoudi, & Tabasi, Citation2009; Ghazanfari, Zamanai Taghizadeh Rabe, Tabasi, & Mahmoudi, Citation2011; Mahmoudi, Zamanai Taghizadeh Rabe, Ahi, & Emami, Citation2009; Mosmann, Citation1983; Zamanai Taghizadeh Rabe, Mahmoudi, Ahi, & Emami, Citation2011). For this purpose, various cancer cell lines including gastric (AGS), breast (MCF-7), cervix (Hela), prostate (LNCaP), kidney (ACHN) cancer cell lines and normal fibroblasts (L929) were obtained from National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco Laboratories, Detroit, MI, USA) in 5% CO2 humidified incubator at 37°C. Cells were treated (1×104 cell/well) with different concentrations of ELM (10–500 µg/ml) for 24 h. Then, MTT solution (5 mg/ml) was added to each well, and plates were incubated for further 3 h. Finally, dimethylsulfoxide (DMSO) was replaced by the medium to dissolve any Formosan crystals. The optical density (OD) was read using the microplate reader at 545 nm. The growth inhibitory activity of ELM was calculated by the following formula:
Measurement of nitric oxide production
The J774A.1 murine macrophages were cultured in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin in 5% CO2 incubator at 37°C. Cells (8×105 cell/well) were treated with non-toxic concentrations of ELM and stimulated for 24 h with or without LPS (1 µg/mL). Nitrite accumulation, as an indicator of NO synthesis, was measured in the culture medium using Griess method (Emami, Zamanai Taghizadeh Rabe, Iranshahi, Ahi, & Mahmoudi, Citation2010; Green, Glowski, Skipper, Wishnok, & Tannenbaum, Citation1982). Briefly, culture medium was mixed with equal amounts of Griess reagent [equal volumes of 1% (w/v) sulphanilamide in 5% (v/v) phosphoric acid and 0.1% (w/v) naphtylenediamine–HCl] and incubated at room temperature for 20 min. Finally, the absorbance at 545 nm was measured using a microplate reader. Nitrite concentration (in micromolar) was calculated from a sodium nitrite standard curve.
Assessment of in vitro leishmanicidal activity
Leishmania major (MRHO/IR/75/ER) promastigotes were cultured using RPMI 1640 supplemented with 10% heat-inactivated foetal calf serum, 2 mM L-glutamine and penicillin–streptomycin (Gibco Laboratories, Detroit, MI, USA) at 27°C, in 5% CO2 incubator. L. major promastigotes (4×105/well) were incubated with ELM (10–500 µg/ml) for 24 h. Then, MTT solution (10 mg/ml) was added to each well followed by incubation for further 4 h. The enzyme reaction was then stopped by an addition of 50% isopropanol–10% sodium dodecyl sulphate. The OD at 545 nm was measured using a microplate reader (Emami, Zamani Taghizadeh Rabe, Ahi, & Mahmoudi, Citation2012).
Statistical analysis
The significance of differences was evaluated by one-way analysis of variance (ANOVA) and Bonferroni's post hoc using SPSS 11.0 software. All the results were expressed as mean ± SD. A probability level of P ≤ 0.05 was considered statistically significant.
Results
In vitro cytotoxicity effect of ELM on cancer and normal cell lines
ELM was evaluated for its in vitro cytotoxic property on different cancer cell lines and normal fibroblasts using MTT assay. The results were demonstrated as growth inhibition percent of treated cells in comparison with untreated control (). For each cell line, there was a linear relationship between proliferation inhibition rate and the concentration of ELM (10–500 µg/ml) which indicates considerable concentration-dependent inhibitory effect on cell proliferation. The growth-inhibitory effect of ELM was significant (P ≤ 0.05) at concentrations more than 10 µg/ml.
Note: Data were expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control, tested by ANOVA test.
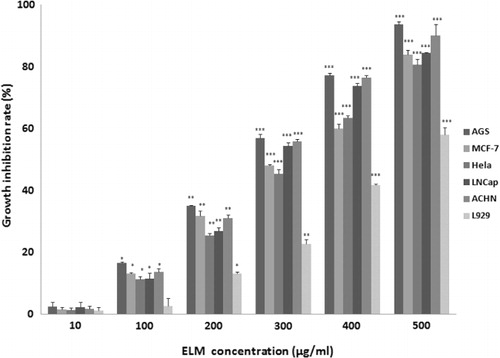
shows the concentrations producing 50% growth inhibition (IC50) of the ELM on tested cell lines, of which AGS cell proliferation was most potently suppressed with the lowest IC50 value (247 ± 0.2 mg/ml). Interestingly, ELM showed the lowest inhibitory effect on the growth of normal L929 cells (IC50: 450 ± 0.7 mg/ml). Therefore, ELM exhibited a selective toxicity on cancer cells rather than normal fibroblast-like cells. This finding is an evidence for the anti-cancer potential of ELM as well as its safety on normal cells.
Table 1. Concentrations producing 50% growth inhibition (IC50) of the ELM on different cancer cell lines.
Inhibitory effect of ELM on nitric oxide production
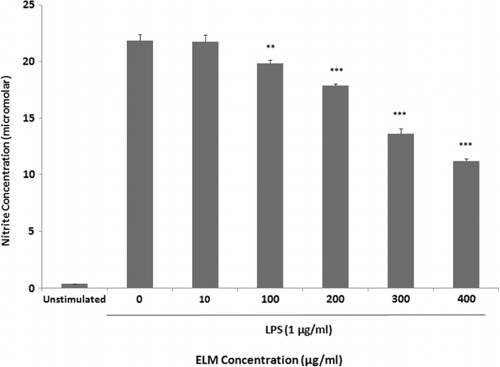
Supernatants of cultured macrophages were quantified for NO content using Griess reagent. A released basal level of NO in unstimulated J774.A1 macrophages was 0.34±0.01 µM, while LPS stimulation increased NO production to 21.83 ± 0.55 µM. As shown in , treatment with 10, 100, 200, 300 and 400 µg/ml of ELM caused 99.48 ± 2.9, 90.73 ± 1.47, 81.80 ± 0.75, 62.37 ± 2.10 and 51.30 ± 0.83 percent inhibition in NO production, respectively. The production of NO was significantly (P ≤ 0.05) decreased after the treatment of LPS-stimulated macrophages at the concentrations higher than 10 µg/ml.
Growth-inhibitory activity on L. major promastigotes
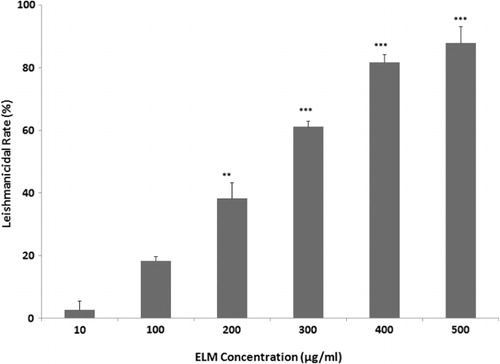
As shown in , the leishmanicidal activity of ELM against L. major was considerably increased by concentrations more than 200 µg/ml (P ≤ 0.05). ELM (10–500 µg/ml) considerably inhibited the proliferation of L. major promastigotes with IC50 value of 232.6 ± 2.1 µg/ml after 24 h of incubation in vitro.
Discussion
Some species of Eremostachys (Lamiaceae) are endemic to Iran. They have been used as traditional medicine plants for the treatment of wounds, snake bites, rheumatism and joint pains and also as a local analgesic and anti-inflammatory remedy from the ancient times by several Iranian nomadic tribes (Modaressi et al., Citation2009; Mosaddegh et al., Citation2012). Recent studies displayed significant free radical scavenging, antioxidant and antibacterial activities of Eremostachys genus (Delazar et al., Citation2004, Citation2005; Erdemoglu et al., Citation2006). Among various members, E. labiosa is endemic to Iran and has been traditionally used as a medicinal plant (Ghorbani, Citation2005; Hassanzadeh et al., Citation2011).
The present study investigated the biological activities of methanol extract from the root part of E. labiosa. As our results indicated, ELM possessed anti-cancer, anti-inflammatory and leishmanicidal properties. ELM showed toxicity on different cancer cell lines with less growth-inhibitory effect on normal fibroblasts. Since no previous report is available considering the anti-cancer property of E. labiosa, our finding is a new evidence for its anti-cancer effect and safety on normal cells.
Moreover, the present experiment assessed the anti-inflammatory effect of ELM using LPS-stimulated macrophages as an inflammatory model. ELM significantly inhibited the production of nitric oxide in a concentration-dependent manner.
These findings are coherent with the similar pharmacological profile of the extracts obtained from the other species of Eremostachys, such as E. laciniata, in which the methanol extract and different fractions exhibited in vivo anti-inflammatory properties against edoema formation (Khan et al., Citation2010).
Although E. glabra, E. laciniata and E. laevigata display antibacterial activities, the leishmanicidal effect of Eremostachys species has not been reported (Esmaeili, Citation2012). Our experiment shows the significant growth inhibitory effect of ELM against L. major promastigotes in vitro.
There is no doubt that several plants which are used for medicinal purposes by native Iranian do in fact contain biologically active extracts and compounds. Accordingly, the current experiment indicates several biological activities of ELM that has been used as a medicinal plant. ELM exerted significant anti-cancer, anti-inflammatory and leishmanicidal properties in vitro which are probably related to the presence of flavonoids, chrysoeriol glycosides and monoterpene glycosides. The plant may be an excellent source in the future for activity-guided isolation of valuable compounds. Moreover, more experiments are needed for enlightening the chemical structure of the components responsible for the biological properties of E. labiosa.
Conclusion
In conclusion, a few biological activities were detected in methanol extract from E. labiosa. This study supports the view that the ethno-directed bio-rational approach is the best search strategy for discovering bioactive extracts of medicinal plants. The present study outlines a good preliminary basis for the selection of candidate plant species for further pharmacological investigations to fight against the parasite-, cancer- and autoimmunity-related ailments. Further fractioning experiments are in progress to isolate the active compounds responsible for these biological activities.
Funding
This study was supported by a Grant [number 87310] from vice president for research, Mashhad University of Medical Sciences.
Additional information
Funding
References
- Delazar, A., Gibbons, S., Kumarasamy, Y., Nahar, L., Shoeb, M. D., & Sarker, S. (2005). Antioxidant phenylethanoid glycosides from the rhizomes of Eremostachys glabra (Lamiaceae). Biochemical Systematics and Ecology, 33(1), 87–90. doi:10.1016/j.bse.2004.05.011
- Delazar, A., Shoeb, M., Kumarasamy, Y., Byres, M., Nahar, L., Modarresi, M., & Sarker, S. D. (2004). Two bioactive ferulic acid derivatives from Eremostachys glabra. DARU, 12, 49–53.
- Eftekharsadat, B., Kazem Shakouri, S., Shimia, M., Rahbar, M., Ghojazadeh, M., Rashidi, M., & Hadi Faraji, M. (2011). Effect of E. laciniata (L) ointment on mild and moderate carpal tunnel syndrome: a double-blind, randomized clinical trial. Phytotherapy Research, 25, 290–295.
- Emami, A., Zamani Taghizadeh Rabe, S., Ahi, A., & Mahmoudi, M. (2012). Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iranian Journal of Basic Medical Sciences, 15, 807–811.
- Emami, S. A., Zamanai Taghizadeh Rabe, S., Ahi, A., Mahmoudi, M., & Tabasi, N. (2009). Study the cytotoxic and pro-apoptotic activity of Artemisia annua extracts. Pharmacologyonline, 3, 1062–1069.
- Emami, S. A., Zamanai Taghizadeh Rabe, S., Iranshahi, M., Ahi, A., & Mahmoudi, M. (2010). Sesquiterpene lactone fraction from Artemisia khorassanica inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression through the inactivation of NF-κB. Immunopharmacology and Immunotoxicology, 32, 688–695. doi:10.3109/08923971003677808
- Erdemoglu, N., Turan, N. N., Cakici, I., Sener, B., & Aydin, A. (2006). Antioxidant activities of some Lamiaceae plant extracts. Phytotherapy Research, 20(1), 9–13. doi:10.1002/ptr.1816
- Esmaeili, A. (2012). Biological activities of Eremostachys laevigata Bunge grown in Iran. Pakistan Journal of Pharmceutical Sciences, 25, 803–808.
- Gella, E. V., & Vavilova, N. K. (1981). Monoterpene glycosides of Eremostachys fetissovii. Khim Prir Soedin, 3, 390–391.
- Ghazanfari, T., Zamanai Taghizadeh Rabe, S., Tabasi, N., & Mahmoudi, M. (2011). Study of cytotoxicity and pro-apoptotic effect of medical mushroom Pleurotus florida in cancer cell lines. Pharmacologyonline, 3, 774–783.
- Ghorbani, A. (2005). Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran (Part 1): General results. Journal of Ethnopharmacology, 102(1), 58–68. doi:10.1016/j.jep.2005.05.035
- Green, L. C., Glowski, J., Skipper, P. L., Wishnok, J. S., & Tannenbaum, S. R. (1982). Analysis of nitrate, nitrite and [15N] in biological fluids. Analytical Biochemistry, 126(1), 131–138. doi:10.1016/0003-2697(82)90118-X
- Hassanzadeh, M. K., Emami, S. A., Asili, J., & Tayarani-Najaran, Z. (2011). Review of the essential oil composition of Iranian Lamiaceae. Journal of Essential Oil Research, 23(1), 35–74. doi:10.1080/10412905.2011.9700429
- Hooper, D., & Field, H. (1937). Useful plants and drugs of Iran and Iraq. Field museum of Natural History. Botanical Series, 9, 71–241.
- Jensen, S. R., Caliş, I., Gotfredsen, C. H., & Sotofte, I. (2007). Structural revision of some recently published iridoid glucosides. Journal of Natural Products, 70(1), 29–32. doi:10.1021/np060452a
- Kalvandi, R., Safikhani, K., & Najafi Gh Babakhanlo, P. (2007). Identification of medicinal plants of Hamedan province. Iranian Journal of Medicinal and Aromatic Plants, 23, 350–374.
- Khan, S., Nisar, M., Rehman, W., Khan, R., & Nasir, F. (2010). Anti-inflammatory study on crude methanol extract and different fractions of Eremostachys laciniata. Pharmaceutical Biology, 48, 1115–1118. doi:10.3109/13880200903517950
- Mahmoudi, M., Zamanai Taghizadeh Rabe, S., Ahi, A., & Emami, S. A. (2009). Evaluation of the cytotoxic activity of different Artemisia khorasanica samples on cancer cell lines. Pharmacologyonline, 2, 778–786.
- Modaressi, M., Delazar, A., Nazemiyeh, H., Fathi-Azad, F., Smith, E., Rahman, M., … Sarker, S. D. (2009). Antibacterial iridoid glucosides from Eremostachys laciniata. Phytotherapy Research, 23(1), 99–103. doi:10.1002/ptr.2568
- Mosaddegh, M., Naghibi, F., Moazzeni, H., Pirani, A., & Esmaeili, S. (2012). Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. Journal of Ethnopharmacology, 141(1), 80–95. doi:10.1016/j.jep.2012.02.004
- Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 983, 55–63. doi:10.1016/0022-1759(83)90303-4
- Navaei, M. N., & Mirza, M. (2006). Chemical composition of the oil of Eremostachys laciniata (L.) Bunge from Iran. Flavour and Fragrance Journal, 21, 645–646. doi:10.1002/ffj.1635
- Nazemiyeh, H., Delazar, A., Ghahramani, M. A., Talebpour, A. H., Nahar, L., & Sarker, S. D. (2008). Phenolic glycosides from Phlomis lanceolata (Lamiaceae). Natural Product Communications, 3, 53–56.
- Nori-Shargh, D., Kiaei, S. M., & Deyhimi, F. (2007). The volatile constituents analysis of Eremostachys macrophylla Montbr. & Auch. from Iran. Natural Product Research, 21, 733–735. doi:10.1080/14786410601083563
- Zamanai Taghizadeh Rabe, S., Mahmoudi, M., Ahi, A., & Emami, S. A. (2011). Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharmaceutical Biology, 49, 962–969. doi:10.3109/13880209.2011.559251