Abstract
In this work, we tried to investigate the impact of treatment with instant controlled pressure drop technology (DIC) on the allergenicity of milk proteins. In DIC treatment, milk proteins are exposed to a high-pressure saturated steam (0.4 MPa, 144 °C) during 25 s and ended with an abrupt pressure drop towards a vacuum (5 kPa, 32 °C). Immunoglobulin E (IgE)-binding ability is determined with indirect enzyme linked immuno spectrophotometric analysis and western blot test. Results indicate significant changes in the IgE binding of treated proteins with DIC. Treated caseins showed increase in the IgE-binding ability; this indicates that DIC enhance the antigenicity of treated caseins. In the opposite, treated whey proteins (ß-lactoglobulin and α-lactalbumin) showed decrease in the IgE binding of these proteins. DIC treatment at 0.4 MPa during 25 s causes a greater reduction of the allergenicity of whey proteins than treatment with 0.6 MPa for 25 s.
1. Introduction
Allergy to cow's milk proteins (CMA) is a complex disorder, mainly Immunoglobulin E (IgE) mediated; it can also use other antibodies or cellular mechanism (Vandenplas et al., Citation2007). Milk is the first food with which the child comes into contact after breastfeeding or from the first day of life when breastfeeding is not possible, during early childhood, CMA occupies the third place in frequency after the allergy to eggs and fish (De Boissieu & Dupont, Citation2006).
The data provided by the literature on the prevalence of CMA vary widely because of differences in diagnostic methods and ages studied, CMA affects approximately 2–3% (Host, Citation2002), and its incidence appears to be increasing (Host & Halken, Citation1998).
Different treatments can reduce the allergenicity of these proteins; conventional hydrolysis with gastric proteases associated or not with physical treatments such as heat treatment, high pressure or microwave is used. These treatments can alter the allergenic properties of proteins by hiding, destroying or disclosing allergenic epitopes through, sequential and conformational changes (Belloque, Alonso, Lo, & Chico, Citation2009; Bonomi et al., Citation2003; Chico, Lopez-Fandino, Alonso, & Belloque, Citation2008; El Mecherfi et al., Citation2011; Izquierdoa, Penas, Baeza, & Gomez, Citation2008; Taheri-Kafrani et al., Citation2009).
The effect of instant controlled pressure drop technology (DIC) treatment on milk proteins has never been previously studied, however, Cuadrado et al. (Citation2011) and Takacs et al. (Citation2013) have observed a reduction in the binding of IgE to soybeans, peanuts, chick peas and lentils proteins.
The aim of our study is to determine the effect of DIC treatment on IgE-binding ability of CMA.
2. Materials and methods
2.1. Material
2.1.1. Whey proteins
The whey protein isolate used in this study was purchased from Prolacta 90, supplied in powder form by Lactalis (Laval, France). The content of the different proteins was 70% ß-lactoglobulin (β-Lag), 20% of α-lactalbumin (α-LA), 6% of immunoglobulin and bovine serum albumin and 4% of aggregates formed during the atomisation process.
2.1.2. Caseins
Bovine caseins were precipitated from commercial skimmed milk, at pH 4.6 with 1 N HCl and separated from whey by centrifugation (8000g, 60 min, 4°C), then washed three times with distilled water. Caseins were dialysed against distilled water (cut-off 10,000). After dialysis, the solutions were lyophilised and stored at −20°C until use.
2.2. Sera
Sera from 10 atopic children (aged 1–9 months) were diagnosed with cow's milk allergy IgE mediated. They showed immediate reactions related to ingestion of cow's milk (vomiting, diarrhoea, dermatitis and urticaria) with positive skin prick tests to cow's milk protein.
All sera were obtained from the Pediatric Hospital El Mansourah Constantine Algeria.
2.3. DIC treatment
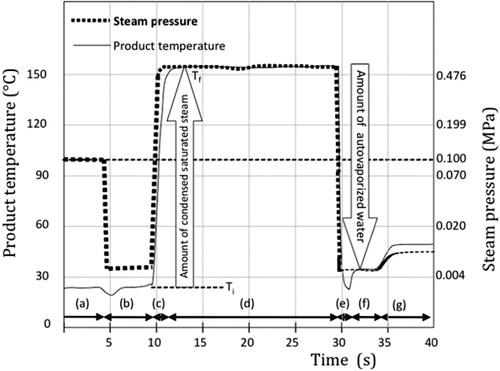
DIC was defined in 1989, as a high temperature–short time stage usually carried out by establishing high-pressure saturated steam–high temperature (0.1–0.7 MPa relating with 100–165 °C during 5–60 s). This is followed by an instant pressure drop towards a vacuum at about 5 kPa and an instant cooling, at a rate ΔP/Δt higher than 0.5 MPa s−1. Depending on the severity of the treatment, the protein's structure can be slightly or greatly modified (Allaf & Vidal, Citation1989).
The procedure is controlled at different scales for temperature (±2°C), pressure (±0.01 MPa), drop (±100 kPa) and operation time (±2 s).
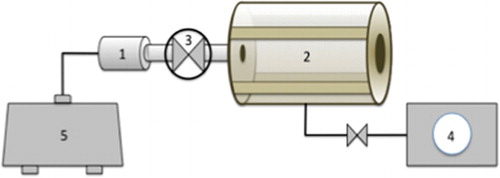
In the present work, the reactor used was a DIC apparatus (Micro-DIC provided from ABCAR-DIC Process, La Rochelle, France) (). It is a 30 cc processing vessel, a 7-litre vacuum tank connected with a water ring vacuum pump allowing the pressure to reach 5 kPa. A pneumatic valve ensures an ‘instant’ connection between the vacuum tank and the processing vessel; it can open in less than 50 ms. Other valves control the flow of steam and compressed air within the processing vessel. shows the different stages of a DIC treatment.
Preliminary experiments were performed to define the range of various operating parameters such as saturated steam pressure P and total thermal treatment time t.
In this study, bovine caseins are treated with a pressure of 0.4 MPa (144 °C) for 25 s, followed with instantaneously drop (5 kPa, 32 °C). The drop is carried out in less than 0.5 s.
Whey proteins were treated at two different conditions, 0.4 MPa (144 °C) and 0.6 MPa (159 °C) during 25 s, followed with instantaneously drop (5 kPa, 32 °C) in less than 0.5 s.
2.4. Gel electrophoresis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out to evaluate the protein profiles of native and treated proteins (caseins and whey proteins). Electrophoresis was performed using the Bio-Rad minigel system Miniprotean II. A separating gel of 12% and a stacking gel of 6% were used.
Proteins were dissolved in a 50 mM phosphate buffer (pH 7.8) to a final concentration of 15 mg/ml.
2.4.1. SDS-PAGE in reduced conditions
The samples were prepared in sample buffer (1:3, v/v) consisting of 4% SDS, 3% ß-mercaptoethanol, 10% glycerol, 50 mM Tris pH 6.8, and the final concentration of proteins is 5 mg/ml. The samples were boiled at 100°C for 5 min before loading the gel.
The migration was performed at 10 mA in the stacking gel and 20 mA in the running gel, protein bands were detected after staining with Coomassie blue R-250 and destaining in a solution of ethanol/water/acetic acid (30/65/5).
2.4.2. SDS-PAGE without reducing agent
ß-Mercaptoethanol denatures proteins by reducing disulphide bonds, thus overcoming some forms of tertiary protein folding, and breaks the quaternary protein structure (oligomeric subunits).
Electrophoresis was performed as described above, only the samples were dissolved in a sample buffer without reducing agent (ß-mercaptoethanol).
2.5. Indirect ELISA
Enzyme linked immuno spectrophotometric analysis (ELISA) was performed using sandwich ELISA. The polystyrene microplates MaxiSorp U-96 and Nunc (VWR, 735-0002) were coated with 100 µl/well of allergen solution at a concentration of 5µg ml−1 in phosphate buffered saline solution (PBS) pH 7.4, and incubated for 3 hours at 30°C. The wells were washed with PBS/Tween-20 (PBS/T) 0.1% three times. After washing, wells were saturated by loading 250µl/well with PBS/Tween-20 0.1% polyvinyl alcohol 1% (PBS/T/PVA) and incubated for 1 hour at 37°C to block sites of non-specific interactions.
After washing three times with PBS/T 0.1%, 100 µl/well of 1/5 diluted sera of allergic children was added and incubated overnight at 4°C. After removing the solution, the wells were washed three times with PBS/T 0.1%.
About 100 µl of anti-human IgE (Epsilon chain specific) conjugated to alkaline phosphatase developed in goat (A-3525, Sigma) diluted 1/1000 in PBS/T/PVA was added to each well and was incubated for 2 hours at 37°C. After a final washing step, 150 µl of fluorescent substrate 4-methylumbelliferyl phosphate (4-MUP) diluted 1/5 in 1 M Tris/HCl, pH 9.8, was loaded in each well and incubated for 90 min at 37°C. Fluorescence intensity was measured with the FLX 800 plate reader (BIO-TEK Instruments) equipped with a 360 nm excitation filter and a 440 nm emission filter. All samples were performed in duplicates.
2.6. Western blot
After separation of proteins by SDS-PAGE, the gel was soaked in a Tris–glycine buffer containing 25 mM Tris, 192 mM glycine, 0.1% SDS, 20% methanol, pH 8.3. The gel was electrotransferred onto a nitrocellulose membrane (Hybond-C extra, Amersham, UK) at 150 mA for 45 min in a semi-dry transfer cell (Bio-Rad).
Membranes with immobilised proteins were incubated in blocking solution of PBS containing 0.1% Tween-20 and 2% polyvinyl alcohol (v/v) for 1 hour at 4°C. After removal of the blocking solution, pool of 10 sera from allergic patients (1:10 diluted in PBS containing 0.1% Tween-20) was added to each membrane and incubated overnight at 4 °C.
The membranes were washed three times with PBS/T (0.1%) at 20°C to remove excess non-bounded serum, and incubated with anti-human IgE monoclonal mouse (IgG) antibody (Fitzgerald, Concord, MA, USA) diluted 1:1000 (4 mg/ml) for 1 hour. After three washing for 5 min in PBS/T (0.1%), membranes were incubated with Alexa Fluor 680 2BS conjugated goat anti-mouse IgG (Invitrogen, Breda, Netherlands) diluted 1/10,000 (200 ng/mL) for 1 hour. After three washes of 5 min in PBS/T (0.1%), membranes were scanned at 700 nm with an infrared Odyssey imaging system (LI-COR Biosciences, Lincoln, NE, USA).
3. Results and discussion
DIC is a new technology that combines the effect of pressure and high temperature in a short time, followed by an instant pressure drop to vacuum.
Physical treatments (heat, high pressure) induce changes in protein's allergenicity by altering their structure leading to loss of conformational epitopes or revelation of hidden epitopes. Changes in protein's structure are caused by rearrangement and/or destruction of non-covalent bonds such as hydrogen bonds, hydrophobic and electrostatic interactions stabilising their tertiary and quaternary structures (Havea, Carr, & Creamer, Citation2004). Pressure leads to protein unfolding, which favours sulphydryl (−SH) and disulphide (−SS−) groups exchange interactions, leading to the formation of disulphide-bonded aggregates, unfolding also appears to unmask buried hydrophobic groups witch induce changes in the conformation of the proteins (Considine, Patel, Anema, Singh, & Creamer, Citation2007; Schokker, Singh, & Creamer, Citation2000).
3.1. Impact of DIC on casein
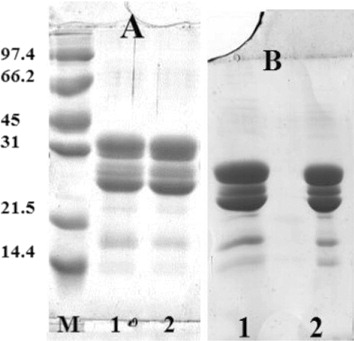
shows the results of SDS-PAGE with and without reducing agent ( and ), lanes 1 and 2 correspond to native and DIC-treated caseins respectively, the pattern is identical, both caseins migrate at the same speed. DIC treatment does not appear to induce any changes on the structure of caseins.
IgE binding of sera of patients with confirmed cow's milk allergy (CMA) to caseins has been studied by indirect ELISA ().
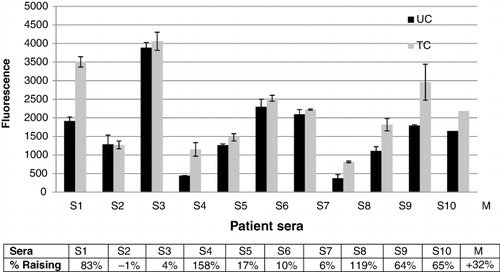
The rate of decrease or increase of the antigenic activity was calculated using the following formula: ((AH/AN) –1) .100) where AH is the fluorescence value obtained from treated proteins and AN is the value of fluorescence of the native proteins (Cabanillas et al., Citation2012).
The results show an increase in IgE binding of DIC-treated caseins to all sera, except for sera numbers 2, 3 and 7. Increase of the responses varies between 10% and 158%. The average response of 10 sera showed an increase of 32% for DIC-treated caseins.
Results obtained by indirect ELISA were confirmed by western blot experiments using pool sera described previously ().
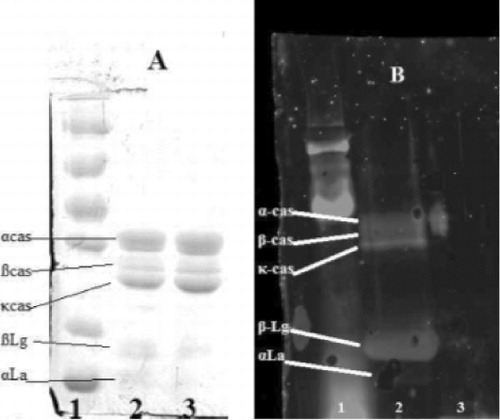
shows the eletrophoretic pattern of native and DIC-treated caseins, while shows the corresponding immunoblotting pattern. It indicates clearly that pool sera recognise both caseins, the treated caseins show increased response to sera of allergic children, the spots corresponding to α and β casein appears to be more IgE reactive.
The literature reports different effects of high pressure and/or heat treatment on the structure of casein as a function of temperature, pressure and duration of treatment (Huppertz, Fox, de Kruif, & Kelly, Citation2006; Orlien, Knudsen, Colon, & Skibsted, Citation2006; Regnault, Thiebaud, Dumay, & Cheftel, Citation2004). Physical treatment can induce aggregation or dissociation of the casein micelles. Hydrophobic and electrostatic interactions responsible for the stabilisation of casein micelles decrease with increasing pressure, which usually leads to dissociation of the casein micelles (Anema, Lowe, & Stockmann, Citation2005; Bernard, Créminon, Yvon, & Wal, Citation1998; Gaucheron et al., Citation1997).
Caseins have barely a clear three-dimensional structure; this structure suggests that the caseins have mostly linear epitopes (Huppertz, Fox, & Kelly, Citation2004; Johansson et al., Citation2009). Increased allergenicity of caseins induced by DIC treatment could be due to the dissociation of the casein micelles or aggregation of casein's monomers. Indeed Wal (Citation2001) observed that the sum of IgE titers against the different caseins greatly exceeded the IgE titer to the whole casein, while Docena, Fernandez, Chirdo, and Fossati (Citation1996) reported that the aggregation of casein monomers reveals new determinants that seems to be absent in the monomoric forms, since some sera show IgE reactivity only against aggregates but not against their individual components.
3.2. Impact of DIC on whey protein
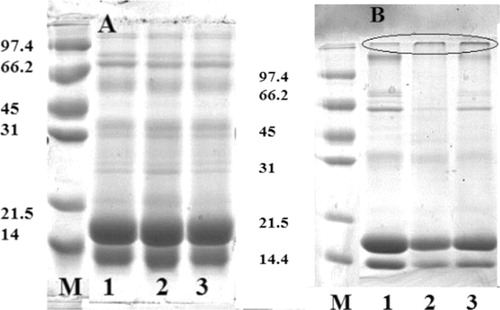
and shows the SDS-PAGE patterns of native and treated whey under reducing conditions and non-reducing conditions respectively. shows that both β-Lag and α-LA are present in a monomeric form. After DIC treatment (0.4 and 0.6 MPa) and high temperature (144 and 159°C) for 25 s (lanes 2 and 3), the two proteins showed the same profile. In non-reducing conditions (), both proteins showed the same profile, treated whey proteins seems to be partially aggregated.
shows the IgE binding of sera to native and treated whey proteins (β-Lag and α-LA); significant difference is noted.
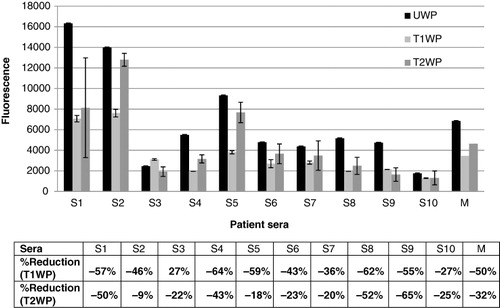
DIC treatment of whey proteins at 0.4 MPa for 25 s reduces considerably the allergenic potential of these proteins, 9/10 sera showed a decrease of the allergenic response, the reduction varies between 27% and 59%.
Treatment with DIC at 0.6 MPa also induced a decrease in the allergenicity of whey protein, the reduction is significantly lower than the decrease induced by treatment at 0.4 MPa, all sera showed reduced IgE reactivity, the reduction varies between 9% and 65%.
The average response of whey proteins treated at 0.4 MPa is reduced by half (50%), while the reduction in those treated with 0.6 MPa is 32%.
Immunoblot profile of whey protein is shown in , the allergenic response of whey protein (represented by the fluorescence intensity of the spot) appears to be mainly due to β-Lag and α-LA did not show a significant response to the pool of sera (lane 2, ). After treatment at 0.4 MPa, the fluorescence intensity is significantly reduced (lane 3, ); treatment at 0.6 MPa (lane 4) also showed a reduction in the intensity of the fluorescence but it is significantly less than the reduction after treatment at 0.4 MPa.
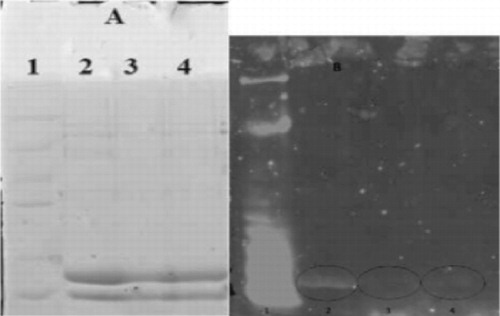
Effect of high pressure and heat treatment on the structure and allergenicity of whey proteins are widely investigated, especially the effect on β-Lag considered as the most allergenic protein and more resistant to hydrolysis (Natale et al., Citation2004).
The decrease in the allergenicity of whey proteins observed in this study is probably due to the modification of the structure of whey protein. Heat treatment and high pressure induce large changes in tertiary and secondary molecular structures of these proteins (Hayakawa, Linko, & Linko, Citation1996). Structural changes include dissociation of the dimer and irreversible aggregation (Aouzelleg, Bull, Price, & Kelly, Citation2004; Iametti et al., Citation1997).
4. Conclusion
The impact of DIC treatment on the immunoreactivity of milk proteins (caseins and whey proteins) is presented in this study. The allergenicity of caseins is improved after treatment while the allergenicity of whey protein is greatly reduced.
The increase or decrease in allergenicity of milk proteins observed in this study could be related to changes in the structure of these proteins. Treatment with DIC is a combined effect of the pressure (0.4 MPa) and heat treatment (144°C) for 25 s, followed by an instantaneous pressure drop (5 kPa) and cooling to 32°C within 0.5 s. The impact of this treatment on the structure of proteins is still unknown.
This study is the first study on the effect of DIC treatment on the allergenicity of milk proteins. Further studies on the effect of DIC on the protein structure and the impact of treatment at different conditions of pressure and time on the allergenicity of these proteins are required.
References
- Allaf, K., & Vidal, P. (1989, June). Feasibility study of a new process of drying/swelling by instantaneous decompression toward vacuum of in pieces vegetables in view of a rapid rehydration. Compiègne: Gradient activity Plotting University of Technology of Compiegne UTC N°CR/89/103, industrial SILVA-LAON partner.
- Anema, S. G., Lowe, E. K., & Stockmann, R. (2005). Particle size changes and casein solubilisation in high-pressure-treated skim milk. Food Hydrocolloids, 19, 257–267. doi:10.1016/j.foodhyd.2004.04.025
- Aouzelleg, A., Bull, L. A., Price, N. C., & Kelly, S. M. (2004). Molecular studies of pressure/temperature-induced structural changes in bovine β-lactoglobulin. Journal of the Science of Food and Agriculture, 84, 398–404. doi:10.1002/jsfa.1669
- Belloque, J., Alonso, E., Lo, R., & Chico, R. (2009). Antibody binding and functional properties of whey protein hydrolysates obtained under high pressure. Food Hydrocolloids, 23, 593–599. doi:10.1016/j.foodhyd.2008.04.001
- Bernard, H., Créminon, C., Yvon, M., & Wal, J. M. (1998). Specificity of the human IgE response to the different purified caseins in allergy to cow's milk proteins. International Archives of Allergy and Immunology, 115, 235–244. doi:10.1159/000023906
- Bonomi, F., Fiocchi, A., Frøkiær, H., Gaiaschi, A., Iametti, S., Poiesi, C., … Rovere, P. (2003). Reduction of immunoreactivity of bovine β-lactoglobulin upon combined physical and proteolytic treatment. Journal of Dairy Research, 70(1), 51–59. doi:10.1017/S0022029902005678
- Cabanillas, B., Maleki, S. J., Rodríguez, J., Burbano, C., Muzquiz, M., Aránzazu Jiménez, M., … Crespo, J. F. (2012). Heat and pressure treatments effects on peanut allergenicity. Food Chemistry, 132, 360–366. doi:10.1016/j.foodchem.2011.10.093
- Chico, R., Lopez-Fandino, R., Alonso, E., & Belloque, J. (2008). Proteolytic pattern, antigenicity, and serum Immunoglobulin E binding of β-lactoglobulin hydrolysates obtained by pepsin and high-pressure treatments. Journal of Dairy Science, 91, 928–938. doi:10.3168/jds.2007-0657
- Considine, T., Patel, H. A., Anema, S. G., Singh, H., & Creamer, L. K. (2007). Interactions of milk proteins during heat and high hydrostatic pressure treatments – A review. Innovative Food Science and Emerging Technologies, 8(1), 1–23. doi:10.1016/j.ifset.2006.08.003
- Cuadrado, C., Cabanillas, B., Pedrosa, M. M., Muzquiz, M., Haddad, J., Allaf, K., & Burbano, C. (2011). Effect of instant controlled pressure drop on IgE antibody reactivity to peanut, lentil, chickpea and soybean proteins. International Archives of Allergy Immunology, 156, 391–404.
- De Boissieu, D., & Dupont, C. (2006). Allergie au lait de vache IgE-médiée. Archives de pédiatrie, 13, 1283–1284. doi:10.1016/j.arcped.2006.06.013
- Docena, G. H., Fernandez, R., Chirdo, F. G., & Fossati, C. A. (1996). Identification of casein as the major allergenic and antigenic protein of cow's milk. Allergy, 51, 412–416.
- El Mecherfi, K., Saidi, D., Kheroua, O., Boudraa, G., Touhami, M., Rouaud, O., … Haertlé, T. (2011). Combined microwave and enzymatic treatments for β-lactoglobulin and bovine whey proteins and their effect on the IgE immunoreactivity . European Food Research and Technology, 233, 859–867. doi:10.1007/s00217-011-1581-y
- Gaucheron, F., Famelart, M., Mariette, F., Raulot, K., Michel, F., & Le Graet, Y. (1997). Combined effects of temperature and high pressure on physicochemical characteristics of skim milk. Food Chemistry, 59, 439–447. doi:10.1016/S0308-8146(96)00301-9
- Havea, P., Carr, A. J., & Creamer, L. K. (2004). The roles of disulphide and non-covalent bonding in the functional properties of heat-induced whey protein gels. Journal of Dairy Research, 71, 330–339. doi:10.1017/S002202990400024X
- Hayakawa, I., Linko, Y. Y., & Linko, P. (1996). Mechanism of high pressure denaturation of proteins. Food Science Technology, 29, 756–762.
- Host, A. (2002). Frequency of cow's milk allergy in childhood. Annals of Allergy, Asthma and Immunology, 89(6), 33–37. doi:10.1016/S1081-1206(10)62120-5
- Host, A., & Halken, S. (1998). Epidemiology and prevention of cow's milk allergy. Allergy, 53(Suppl. 46), 111–113. doi:10.1111/j.1398-9995.1998.tb04978.x
- Huppertz, T., Fox, P. F., & Kelly, A. L. (2004). Dissociation of caseins in high pressure-treated bovine milk. International Dairy Journal, 14, 675–680. doi:10.1016/j.idairyj.2003.11.009
- Huppertz, T., Fox, P. F., de Kruif, K. G., & Kelly, A. L. (2006). High pressure-induced changes in bovine milk proteins: A review. Biochimica et Biophysica Acta, 1764, 593–598. doi:10.1016/j.bbapap.2005.11.010
- Iametti, S., Transidico, P., Bonomi, F., Vecchio, G., Pittia, P., Rovere, P., & DallAglio, G. (1997). Molecular modifications of β-lactoglobulin upon exposure to high pressure. Journal of Agricultural and Food Chemistry, 45(1), 23–29. doi:10.1021/jf960330w
- Izquierdoa, F. J., Penas, E., Baeza, M. L., & Gomez, R. (2008). Effects of combined microwave and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins. International Dairy Journal, 18, 918–922. doi:10.1016/j.idairyj.2008.01.005
- Johansson, A., Lugand, A., Rolet-Répécaud, O., Delage, M. M., Peltre, G., Marchesseau, S., … Dupont, D. (2009). Epitope characterization of a supramolecular protein assembly with a collection of monoclonal antibodies: The case of casein micelle. Molecular Immunology, 46, 1058–1066. doi:10.1016/j.molimm.2008.09.028
- Natale, M., Bisson, C., Monti, G., Peltran, A., Garoffo, L. P., Valentini, S., … Conti, A. (2004). Cow's milk allergens identification by two-dimensional immunoblotting and mass spectrometry. Molecular Nutrition & Food Research, 48, 363–369. doi:10.1002/mnfr.200400011
- Orlien, V., Knudsen, J. C., Colon, M., & Skibsted, L. H. (2006). Dynamics of casein micelles in skim milk during and after high pressure treatment. Food Chemistry, 98, 513–521. doi:10.1016/j.foodchem.2005.05.082
- Regnault, S., Thiebaud, M., Dumay, E., & Cheftel, J. C. (2004). Pressurisation of raw skim milk and of a dispersion of phosphocaseinate at 9 °C or 20 °C: Effects on casein micelle size distribution. International Dairy Journal, 14(1), 55–68. doi:10.1016/S0958-6946(03)00144-4
- Schokker, E. P., Singh, H., & Creamer, L. K. (2000). Heat-induced aggregation of β-lactoglobulin A and B with α-lactalbumin. International Dairy Journal, 10, 843–853. doi:10.1016/S0958-6946(01)00022-X
- Taheri-Kafrani, A., Gaudin, J. C., Rabesona, H., Nioi, C., Agarwal, D., Drouet, M., …Haertle, T. (2009). Effects of heating and glycation of β-lactoglobulin on its recognition by IgE of sera from cow milk allergy patients. Journal of Agricultural and Food Chemistry, 57, 4974–4982. doi:10.1021/jf804038t
- Takacs, K., Guillamon, E., Pedrosa, M. M., Cuadrado, C., Burbano, C., Muzquiz, M., … Gelencsér, E. (2013). Study of the effect of instant controlled pressure drop (DIC) treatment on IgE-reactive legume-protein patterns by electrophoresis and immunoblot. Food and Agricultural Immunology, 24, 1–13. doi:10.1080/09540105.2012.759539
- Vandenplas, Y., Brueton, M., Dupont, C., Hill, D., Isolauri, E., & Koletzko, S. M. (2007). Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Archives of Disease in Childhood, 92, 902–908. doi:10.1136/adc.2006.110999
- Wal, J. M. (2001). Structure and function of milk allergens. Allergy, 56(Suppl. 67), 35–38. doi:10.1111/j.1398-9995.2001.00911.x