Abstract
Rabbit antisera were generated against four preparations of teliospores of Tilletia indica the fungus responsible for Karnal bunt in wheat having titre ranging from 1:2500 to 1:10,000. The suitability of four solubilising agents (I–IV) used for extraction of teliospores/spore proteins was assessed, highest protein extractability was observed with solubilising agent I (0.5% sodium dodecyl sulphate). The maximum immunoreactivity in enzyme linked immunosorbent assay (ELISA) was observed with the teliosporic protein(s) extracted using solubilising agent I followed by agent II, IV and III. Further, the results of western blotting with proteins from different fungal pathogens using anti-intact teliospores antibody showed one unique immunoreactive band of 28 kDa with teliosporic protein of T. indica only. The potential diagnostic antibody was generated against the characterised protein (28 kDa) of teliospore wall's antigen of T. indica. The developed diagnostic antibody and solubilising agent I could therefore be employed for development of specific and rapid immunodiagnostic format for detection of Karnal bunt.
Introduction
Wheat (Triticum aestivum) is one of the most important cereal crops and ubiquitous in the food culture of India as well as many other regions of the world. In India, it is the second important food crop being next only to rice and contributes about 82 million tonnes to the total food grain production of the country (The Hindu Survey of Indian Agriculture, Citation2010). The productivity of wheat is seriously hampered by the disease, Karnal bunt (KB) which is caused by the sporadic fungus, Tilletia indica (syn. Neovossia indica). The disease has frequent occurrence in the north-western plains of Himachal Pradesh, Punjab, Uttar Pradesh and in Tarai regions of Uttarakhand. The disease has also been reported in Pakistan, Iraq, Afghanistan, Nepal (Joshi, Singh, Srivastava, & Wilcoxon, Citation1983; Singh, Citation1994), Mexico (Warham, Citation1986), geographically isolated areas in the USA (Ykema, Floyd, Palm, & Peterson, Citation1996) and South Africa (Crous et al., Citation2001). It significantly impairs the grain quality, seed vigour and reduces market acceptability of the wheat grain produce. T. indica reduces grain quality by discolouring and imparting an objectionable odour to the grain and products made from it. Generally Tr. aestivum containing more than 3% bunted kernels is considered unsatisfactory for human consumption (Holmes, Jackson, & Perring, Citation1997). There are other Tilletia species that can be confused with T. indica and are commonly found in harvested grain or seeds. These include Tilletia walkeri (a pathogen of Lolium perenne and L. multiflorum), Tilletia horrida, Tilletia barclayana (a pathogen of Oryza spp.) and Tilletia ehrhartae (a pathogen of Ehrharta calycina). In Australia, T. walkeri and T. ehrhartae are found to contaminate harvested seed of Tr. aestivum. T. walkeri and T. horrida are present in the USA while T. barclayana in India and are detected in harvested seed of Tr. aestivum especially where Oryza spp. and Lolium spp. are grown in rotation with Tr. aestivum (Castlebury & Carris, Citation1999; Pascoe, Priest, Shivas, & Cunnington, Citation2005). Microscopic method of detection lacks consistency, accuracy and sensitivity, which results in frequent misidentifications of KB (Barry & Castlebury, Citation1999). Because of the morphological similarity of these pathogens, accurate identification of the different pathogens is important.
After first report of KB at Arizona State in March, 1996, United States Department of Agriculture (USDA) has designated T. indica as a quarantine pest. It is the target of strict quarantine regulations by most wheat-growing countries and, therefore, acts as barrier to wheat trade. Recently, it has also been categorised as one of biological weapons (Schaad, Shaw, Vidaver, Leach, & Erlick, Citation1999). Hence, wheat movement into USA and other KB-free countries is regulated and subjected to quarantine (Ykema et al., Citation1996). As it hinders the international trade of wheat, many countries are insisting for zero tolerance for KB. The current international diagnostic protocol (Inman, Hughes, & Bowyer, Citation2003; Wright, Murray, & Tan, Citation2003) involves the tentative identification of the spores based on morphology followed by germination of the spores and a molecular protocol to confirm the identity (Frederick et al., Citation2000). Microscopic identification and spore germination are slow, involve labour-intensive activities and require specialised training.
For successful management of KB, there is a strong need towards developing rapid and specific immunodiagnostic test procedure to check its further spread to disease-free areas through the use of disease-free seeds (Kumar, Singh, Kumar, & Garg, Citation2008). Further, the sensitivity and specificity of any immunological procedure are solely dictated by the quality of the immunological reagents employed for the development of an immunodiagnostic assay system. Earlier, attempts were made in our lab for extraction of teliospore wall protein(s) from T. indica using phenol extraction method in which three unique immunoreactive bands were observed (Gupta, Narwal, Umapathy, Mall, & Kumar, Citation2009). But the phenol extraction method was found to be tedious, time-consuming process and not fit for rapid diagnostic tests.
In this context, attempts were made in the present study for the optimisation of suitability of solubilising agents for the extraction of solubilised teliospores/spore proteins from different fungal pathogens. Four different types of solubilising agent were used for extraction of teliospores proteins and their suitability was assessed by estimation of extracted protein, extractability studies using sodium dodecyl sulphate-polyacrylamide gel electrophroesis (SDS-PAGE) analysis followed by immunoreactivity assay by western blotting and indirect enzyme linked immunosorbent assay (ELISA) using four different polyclonal antisera generated against different antigenic preparations of teliospores of T. indica. Further, proteins from teliospores/spores of different fungal pathogens were extracted using most optimum solubilising agent and cross-reactive studies were conducted by western blotting using anti-intact teliospores (IT) antibody for characterisation of potential diagnostic antigen. The characterisation of diagnostic antigen (Singh, Lakhera, Singh, Taj, & Kumar, Citation2013) and further characterisation in the present study led to development of monospecific polyclonal antibodies against the characterised diagnostic antigen of 28 kDa.
Materials and methods
Source of KB-infected wheat samples
The KB-infected wheat grains were collected from Punjab Agricultural University, Punjab.
Collection of Spores/teliospores
The teliospores of T. indica were extracted from infected wheat seeds with the help of surgical blades. The seeds were incised and the spore masses were loosened with the help of a needle. By shaking the incised seeds in glass vials, the teliospores were released and collected in 1.5-ml centrifuge tubes to be used as a source of immunogen.
Extraction of solubilised proteins from teliospores of T. indica
Proteins from teliospores were isolated by solubilisation of IT using four solubilising agents: I[0.5% sodium dodecyl sulphate (SDS)], II(20 mM Tris, 50 mM ethylenediaminetetraacetic acid (EDTA), pH 8.0), III-[50 mM NaHCO3, phosphate buffered saline (PBS), 50 mM Tris, 1 mM EDTA, 1% polyvinylpyrrolidone (PVP)] and IV (2% SDS, 8 M Urea, 10 mM Tris). The teliospores (20 mg) were crushed in centrifuge tube with pestle in 1 ml of respective solubilising agent followed by mixing it properly by vortexing. Fragments were pelleted from the extract by centrifugation at 10,000 rpm for 10 min. The resulting protein in supernatant was transferred to a clean tube and concentration was determined by Bradford methods (Citation1976).
SDS-PAGE analysis of solubilised teliospores/spore proteins
SDS-PAGE was performed for analysing solubilised teliospore proteins of T. indica extracted by different solubilising agents for optimisation of extractability of proteins from related fungal teliosporic/sporic proteins. After electrophoresis, the gel was fixed and stained with Coomassie brilliant blue for 4–6 hours. The gel was destained thoroughly by soaking it in the methanol/acetic acid solution, changing the destaining solution three or four times.
Preparation of immunogen
Four types of immunogens namely IT, sonicated teliospores (ST), pellet (P), and supernatant (S) were used. IT were obtained as such from the infected seed samples and suspended in phosphate buffered saline for using it as IT immunogen. For the preparation of ST immunogen, the teliospores were sonicated under ice six times, with a pulse of 90 seconds each. The extract was centrifuged at 10,000 rpm to separate out the sonicated spores. Spores (10mg) were also crushed using mortar–pestle with liquid nitrogen and 1.5 ml of protein extraction buffer. Approximately, 100% breakages of teliospores were seen under Nikon microscope. Broken spores were pelleted at 10,000 rpm at 4°C for 10 minutes and both pellet and supernatant were separated to use as P and S immunogen.
Preparation of immunoprobes
New Zealand white rabbits used for the generation of anti-teliospore antibodies (ATA) were procured from Indian Veterinary Research Institute (IVRI), Bareilly. The antigen preparation (250 µg) was emulsified with an equal volume of Freund's complete adjuvant (FCA) and was administered at multiple sites. The antigens (100–150 µg) emulsified in equal volume of Freund's incomplete adjuvant (FIA) were used for booster injections. The three booster doses were administered to get hyper-immune sera by following an immunization schedule.
Preparation of antisera
The blood from rabbits immunised with four different types of antigens was collected in separate oakridge tubes and clotted at room temperature by keeping it undisturbed for 2 hours. After keeping it overnight at 4°C, the clear serum was decanted into 1.5-ml centrifuge tube followed by centrifugation at 10,000 rpm for 10 minutes at 4°C to get rid of any remaining red blood cells. The straw-coloured clear supernatant was transferred into capped cryo vials and stored at –20°C.
Microtitre ELISA
The indirect ELISA was developed by slight modification of the technique initially described by Engvall and Perlman (Citation1971) and modified in our lab. The microtitre plates were coated with fixed concentration of antigen (1000 spores per well) in coating buffer (0.05 M sodium carbonate buffer, pH 9.6). The teliospore count was determined by using a haemocytometer. The plates were incubated for 1 hour at room temperature and then kept overnight at 4°C. They were then washed with PBS and blocked with 2% BSA [Bovine serum albumin, Sisco Research Laboratories, Mumbai (SRL)]. After non-specific blocking and extensive washing with PBS + 0.5% BSA, the microtitre plates were incubated with appropriate dilution of antisera of each immunogen for 1 hour. Washing was done thrice with PBS followed by incubation with 1:1000 diluted alkaline phosphatase (ALP)-conjugated goat anti-rabbit gamma globulin (Bangalore Genei, Bangalore) for 2 hours. After extensive washing, ALP activity was detected by addition of 100 µl of substrate (p-nitrophenyl phosphate sodium salt dissolved in ethanolamine buffer with concentration of 1 mg/ml). The plate was incubated for 30 minutes in dark and the reaction was stopped with 100 µl of 1.5 M NaOH solution. The colour intensity was measured in ELISA reader at 405 nm.
Western blotting
Teliosporic/sporic proteins (20 µg) extracted from different fungal pathogens along with pre-stained protein molecular weight marker were resolved and the proteins were transferred to a nitrocellulose (NC) membrane (0.45 mm) using semi-dried system a transfer apparatus (SCIE-PLAS, Cambridge, UK) at 2 mA/square cm for 90 min. The NC membrane was incubated in 5% skimmed milk in PBS solution at 37°C for 2 hours for blocking. The blocked membrane was probed with rabbit anti-teliospores antibody, diluted 1:250 in PBS for 2 hours at 37°C with constant shaking. Washing was performed four times with PBS allowing five minutes per wash, followed by incubation in goat anti-rabbit ALP conjugate for 45 min at 37°C with constant shaking. Washing was performed at least four times with 10 min at each wash, after which 5-bromo-4-chloro-3′-indolyphosphate/nitro-blue tetrazolium chloride (BCIP/NBT) substrate was added for colour development. Thereafter, the reaction was stopped by washing the membrane in double distilled water and the results were observed.
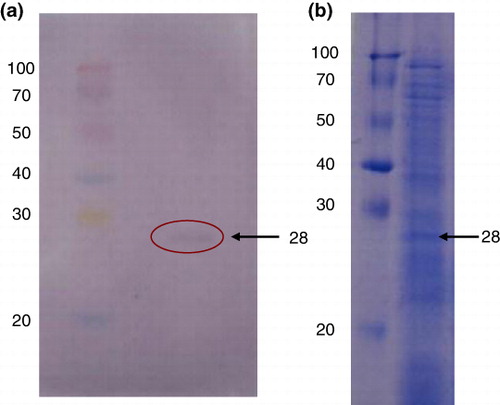
Elution of prominent band of protein of 28 kDa for generation of monospecific diagnostic antibody against T. indica
In order to generate monospecific antibody against unique, prominent immunoreactive band of protein (i.e. molecular weight of 28 kDa) of T. indica, the target protein was eluted in bulk from the gel after its extraction using solubilising agent I. Isolated protein was used as immunogen for raising monospecific diagnostic anti-teliospore antibody in rat. The immunogen was prepared using isolated 28 kDa of protein in PBS (pH 7.4) and emulsified with an equal volume of either FCA or FIA. The rats were immunised, by administering five injections through intraperitoneal, intramuscular and foot-pad routes.
Results
Determination of extractability of teliospore proteins using different solubilising agents
In order to evaluate the efficacy of four different solubilising agents for extraction of teliospore antigen(s), highest protein extractability was observed for solubilising agent I (0.5% SDS) followed by IV (2% SDS, 8 M Urea, 10 mM Tris), II (20 mM Tris, 50 mM EDTA (pH 8.0) and III (50 mM NaHCO3, PBS, 50 mM Tris, 1 mM EDTA, 1% PVP). It was estimated that around 149 µg/mg of teliospores was extracted with solubilising agent I followed by solubilising agent IV (99 µg/mg), II (75 µg/mg) and III (60 µg/mg) of teliospores (). Further, SDS-PAGE analysis of solubilised proteins revealed that quality of extracted proteins was also good in solubilising agent I as compared to II, III and IV which could be due to better recovery of solubilised proteins. The numbers of protein bands were also more in extracted proteins using solubilising agent I followed by solubilising agent IV, II and III ().
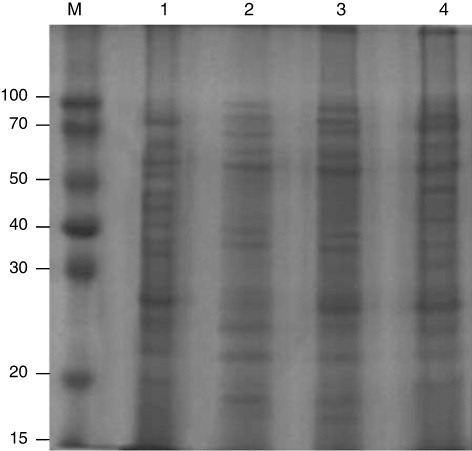
Table 1. Determination of extractability of teliosporic proteins using different solubilising agents.
Development of high-titre antibodies against teliosporic antigens of KB
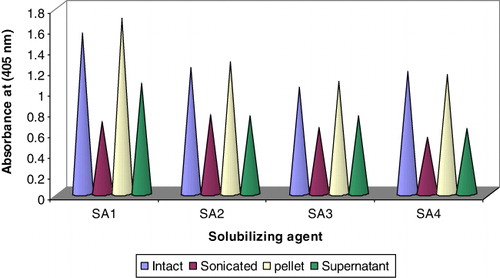
The four different polyclonal antibodies were generated against different antigenic preparations (intact, sonicated, pellet and supernatant) of teliospores of T. indica. Immunogenicity of teliospores antigens was studied from immunoreactivity studies of different rabbit anti-teliospore sera with teliospore as antigen. To determine the maximal antigen reactivity and antibody sensitivity range, the micro-titer plates were coated with optimal amount of antigen that falls within the detection range of indirect ELISA i.e. 1000 spores per well. Antibody dilutions for each of the antibody raised was used ranging from 1:125 to 1:20,000. The binding curve of antibodies indicated that the reactivity of antibodies raised against teliosporic pellet was as high as 3.5 (100%) at optical density (OD405) followed by 3.2, 3.1 and 2.9 for intact, supernatant and sonicated, respectively (). At dilution, 1:10,000, reactivity corresponding to OD405 was observed 0.4 and 0.46 for anti-pellet and anti-intact teliospore, respectively ( and ). However, the saturation of reactivity of antigen with anti-supernatant (0.47) and anti-sonicated (0.48) corresponding to OD405 was 1:5000 and 1:2500, respectively. The straight line was obtained in the antibody dilution range of 1:2500 to 1: 20,000 with anti-ST (), while almost similar immunoreactivity in the antibody dilution range of 1:5000 to 1:20,000 was observed with anti-supernatant teliospores (), indicating that dilutions higher than this will not improve the binding activity. The titre of antibodies is defined as maximal dilution of antibodies at which reactivity was visible. It was found to be 1:10000; 1:2500; 1:10,000 and 1:5000 for anti-IT, anti-sonicated, anti-pellet and anti-supernatant teliospore antibodies, respectively (). The reactivity of antibodies raised against teliosporic pellet as compared to those raised against IT may be related to the greater exposure of the antigenic epitopes on breakage of teliosporic cell wall. However, it revealed that titre of anti-pellet and anti-intact teliospore antibodies was similar and higher as compared to anti-supernatant and anti-sonicated teliospore antibodies. Therefore, anti-IT antibodies were chosen for further study considering ease in generation of antibodies and maximum reactivity.
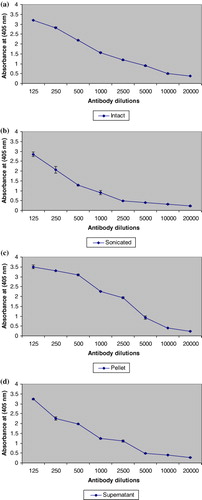
Table 2. Characteristics of ATA.
Evaluation of influence of solubilising agents on immunoreactivity of solubilised teliosporic proteins by indirect ELISA
Indirect ELISA was performed to find out the immunoreactivity of solubilised teliosporic proteins obtained as described above using four solubilising agents. The wells of microtitre plate were coated with solubilised teliosporic proteins isolated from four solubilising agents with concentration of 5 µg per well in triplicates. As evident from , the maximum immunoreactivity OD405 was observed in the teliosporic proteins extracted using solubilising agent I followed by agent II, IV and least with solubilising agent III and could be used for development of efficient diagnostic format due to its better efficacy of extractability of teliospores wall's antigen.
SDS-PAGE analysis of solubilised teliosporic/sporic proteins of T. indica and related fungal pathogens
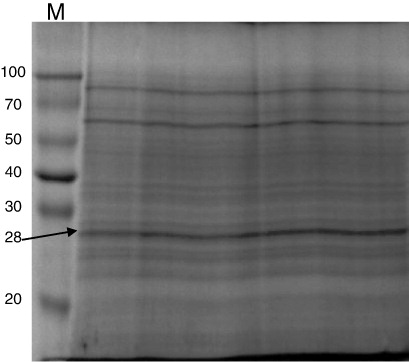
The SDS-PAGE analysis of teliospores/spores proteins showed distinct banding pattern for each species. A 12% SDS-PAGE gel was used which is capable of resolving polypeptides and proteins ranging from 10 kDa to 100 kDa. Only proteins in the range of 14–90 kDa were detected in the spore and teliospore walls of T. indica, Tilletia foetida, T. barclayana, Puccinia. recondita, Ustilago hordei and Ustilago virens () by Coomassie brilliant blue staining. One unique band was detected in T. indica by SDS-PAGE analysis having molecular weight of 28 kDa which was considered to be useful in making differential diagnosis with spores/teliospores of other fungal pathogens.
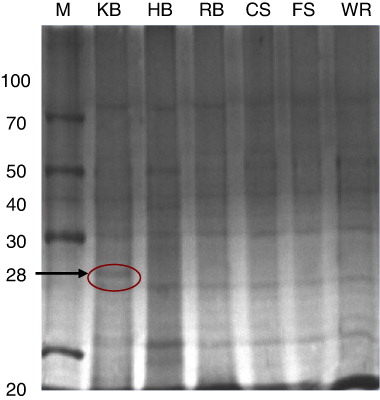
Many common bands were observed in bunt and rust pathogens in low-, medium- and high-molecular weight range i.e. 20–35 kDa, 38–50 kDa and 60–80 kDa, respectively. The teliospore/spore wall protein extracts of T. indica, T. barclayana, T. foetida, P. recondita and U. hordei and U. virens showed seven bands in low-molecular weight range, four in medium-molecular weight range and three in high-molecular weight range.
Western blot analysis of solubilised spore's/teliopore's proteins
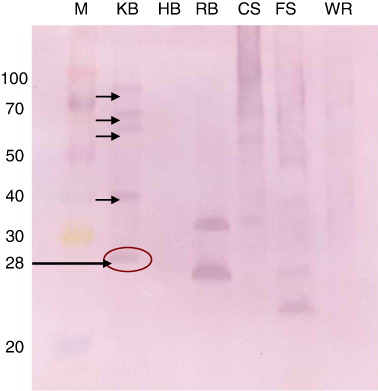
For immunodetection of teliospores of T. indica and making differential diagnosis with other seed-borne and related fungal pathogens, cross-reactivity of ATA was done by western blotting using the teliosporic/sporic proteins of T. indica, T. foetida, T. barclayana, U. hordei, U. virens and P. recondita extracted with solubilising agent I. Teliosporic/sporic proteins of different fungal pathogens were separated by SDS-PAGE, transferred to NC membranes and incubated with anti-teliospore antibody. Several proteins with different molecular weights were detected in SDS-PAGE of teliosporic proteins; however, after incubation of these proteins with ATA, less number of bands was observed. Five bands of approximately molecular weight 28, 40, 66, 68 and 83 kDa were detected with extracted proteins of T. indica. The anti-intact teliospore antibodies to T. indica recognised unique immunoreactive band having molecular weight of 28 kDa; however, anti-intact teliospore antibodies also reacted with extracted proteins from other fungal pathogens such as T. barclayana, U. hordei and U. virens. The four immunoreactive bands were observed with U. hordei and U. virens while two highly immunodominant bands were observed with T. barclayana but no band was observed with T. foetida and P. recondita. Two unique immunoreactive bands were present in U. virens having molecular weights of 14 and 50 kDa but no unique immunoreactive band was observed in T. barclayana and U. hordei (). The immunoreactivity of antisera raised against IT of T. indica was not observed with teliosporic/sporic proteins from T. foetida and P. recondita.
Development of monospecific antibodies against 28 kDa protein of teliospore of T. indica
Monospecific antibody against unique, prominent immunoreactive protein band of molecular weight of 28 kDa recognised as diagnostic antigen of T. indica was generated for development of a specific immunodiagnostic assay for detection of KB. A preparative gel was used to elute out the protein band of 28 kDa from polyacrylamide gel as shown in the . The immunisation with four booster doses of eluted specific protein band of 28 kDa from teliosporic proteins of T. indica resulted into the production of monospecific polyclonal antibodies. It was observed that SDS-PAGE followed by commassaie brilliant blue staining revealed multiple protein bands (approximately 20) while western blotting results using ATA against IT showed five immunoreactive bands. However, western blot analysis using developed monospecific anti-teliospore antibody recognises only a single 28 kDa protein as antigen indicating generation of diagnostic antibodies ().
Discussion
Disease diagnosis and pathogen detection are paramount importance to protect crops and natural plant systems, and are the crucial prelude to undertake prevention and management measures. Failures in pathogen detection and disease diagnosis lead directly to inadequate disease control and reductions in crop production and quality, and hence trade.
It is well established that immunodiagnostic method is strongly dictated by the quality of two important immunological reagents which are antigen and antibody. Immunodiagnostic tests are specific, sensitive and quick to detect small number of disease-causing entities. The success of any newer diagnostic method developed for seed-borne fungal pathogens is solely dependent on the effective removal of pathogen from wheat lots. The detergent washing test developed in our laboratory using ionic and non-ionic detergents was efficient for extraction of KB teliospores present as surface contaminants (Gupta, Kumar, Joshi, Agarwal, & Garg, Citation2003). In the present study, efforts were made for the designing of efficient solubilising agent for extraction of solubilised teliosporic proteins and high-titre antibodies for development of specific immunodiagnostic format for detection of teliospores of KB, a seed-borne fungal pathogen of wheat.
Immunodiagnostic procedure is not successful until there is efficient solubilising/extraction agent for target antigen available. In this context, the teliosporic proteins were solubilised employing different solubilising agents. The highest extractability of protein in solubilising agent I might be due to strong solubilising property of SDS detergent. Botelho et al. (Citation2010) also recognised the benefits of protein sample preparation, including solubilisation by SDS. Solubilising agent II also yielded better protein due to the presence of Tris buffer saline which washed spore wall fraction consisted of >90% dark, melanin-containing fragments. But the protein recovery is lower in solubilising agent III and IV. In solubilising agent IV it may be due to higher concentration of detergent. Further the quality of solubilised proteins was analysed by SDS-PAGE (12%). It was found that quality of protein is better in solubilising agent I as compared to II, III and IV that could be due to better recovery of solubilised protein. In earlier studies, extractions of protein from whole teliospores were analysed by Weber and Schauz (Citation1985) and Kawchuk, Kim, and Nielsen (Citation1988). The protein extracts were characterised that contained cellular proteins. However, it is worth to note that Banowetz, Trione, and Krygier (Citation1984) were unable to extract detectable amounts of protein antigens from IT of either Tilletia controversa or Tilletia tritici with a variety of solvents. However, in the present study good extractability of solubilised proteins was observed with solubilising agent I (0.5% SDS) and characterised further as best and efficient solubilising agent for extraction of teliosporic protein(s).
In order to develop good quality of immunological reagents, four different polyclonal ATA against different antigenic preparations of teliospores of T. indica were developed which can be employed for immunological characterisation of teliospores/spores of fungal pathogens. To determine the titre of these antibodies, indirect ELISA was performed (Gupta et al., Citation2009). Since, ELISA is employed for routine detection of fungal pathogens, (Broggio & Bertocci, Citation1995) there is need of high titred and specific antibodies for detection. The higher titre and reactivity of antibodies generated against teliosporic pellet and IT were observed as compared to whole sonicated and supernatant fractions of teliospores. Holland, Denny, and Bonde (Citation1990) also produced polyclonal antisera in rabbits against fungal cell wall antigens of T. indica and T. barclayana and differences were observed between the two in their reactivity patterns. The titre of anti-pellet and anti-IT was almost similar in present study; however, the reactivity of anti-pellet teliospore sera is slightly higher as compared to those raised against IT that may be related to the greater exposure of the antigenic epitopes on breakage of teliosporic cell wall. Further immunoreactivity analysis of four different ATA with different solubilised teliosporic antigens was performed. It reveals that maximum OD405 was observed in combination with antigen extracted from solubilising agent I with anti-pellet teliospore antibodies followed by combination of solubilising agent I with anti-IT antibodies. However, the anti-intact teliospore antibodies has been chosen for immunological characterisation due to two reasons: first, the titre of anti-pellet and anti-intact was almost similar, second complete antigenic entities of teliospores of surface available to elicit immune response. Therefore, for further characterisation, intact teliospore representing teliospore surface was taken for identification of potential diagnostic antigen(s) for generation of diagnostic antibodies.
The detection of plant pathogenic fungi by immunodiagnosis has been slow due to the problems experienced in raising specific antisera against infectious entities. Most such antisera cross-react widely with both related and unrelated fungi and host tissues or extracts when tested by ELISA or related techniques yet appear species-specific when tested by immunodiffusion. Documented result of western blotting in the present investigation using the teliosporic/sporic proteins of T. indica, T. foetida, T. barclayana, U. hordei, U. virens and P. recondita extracted by solubilising agent I, showed five immunoreactive proteins with teliosporic protein of T. indica using anti-intact teliospore antibodies. However, anti-intact teliospore antibodies also reacted with extracted proteins from other fungal pathogens such as T. barclayana, U. hordei and U. virens. However, the protein band of 28 kDa was uniquely present in teliosporic protein of T. indica only. This teliosporic protein of 28 kDa was already characterised in our lab (Singh et al., Citation2013). The protein band of 28 kDa was recognised as potential diagnostic antigen of teliospore of T. indica.
The success of any newer diagnostic method developed remains incomplete, without discussion of development of monospecific antibody against target pathogen. Attempts have been made to develop the monospecific antibody against unique, prominent immunoreactive band of protein of molecular weight of 28 kDa recognised as diagnostic antigen of T. indica. Antisera raised against specific fungal fractions such as species specific band on SDS-PAGE gel generally have a higher degree of specificity (Poupard, Grare, & Cavalier, Citation2010; Sundaram, Plasencia, & Bantarri, Citation1991). Antisera against protein precipitates from either culture filtrates or mycelial extracts (Gerik, Lommel, & Huisman, Citation1987; Gleason, Gharbrial, & Ferries, Citation1987) or specific fungal fractions such as enzymes are generally highly specific (Johnson, Pirone, Siegel, & Varney, Citation1982). In the present study, western blotting result revealed that generation of monospecific ATA recognised one unique immunoreactive band of molecular weight 28 kDa in the teliosporic proteins of T. indica and can be used as diagnostic antibodies for detection of quarantined fungus T. indica. Thus solubilising agent I and monospecific antibody raised against teliosporic protein of 28 kDa were found as suitable immunological reagents which can be employed for development of diagnostic method for detection of teliospores in infested and infected wheat lots. The same solubilising agent and antibody are not only be used in laboratory for development of lab-scale immunodiagnostic procedure but could also be used for the development of on-site immunoassay format which required efficient extraction of target antigen(s) for specific detection of KB.
Acknowledgements
The authors are grateful to Dean, College of Basic Sciences and Humanities, and Director, Experiment station, for providing all necessary help and facilities for carrying out this research.
Funding
The financial support provided by Department of Biotechnology, Government of India in the form of research project vide sanction order [No. 102/DBT/IFD/SAN/1826/2011-12] is gratefully acknowledged.
Additional information
Funding
References
- Banowetz, G. M., Trione, E. J., & Krygier, B. B. (1984). Immunological comparisons of teliospores of two wheat bunt fungi to Tilletia species, using monoclonal antibodies and antisera. Mycologia, 76, 51–62. 10.2307/3792835
- Barry, M. C., & Castlebury, L. A. (1999). Tilletia walkeri on annual ryegrass in wheat fields in the Southeastern United States. Plant Disease, 83(7), 685–689.
- Botelho, D., Wall, M. J., Vieira, D. B., Fitzsimmons, S., Liu, F., & Doucette, A. (2010). Top-down and bottom-up proteomics of SDS-containing solutions following mass-based separation. Journal of Proteome Research, 9, 2863–2870. 10.1021/pr900949p
- Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. 10.1016/0003-2697(76)90527-3
- Broggio, M., & Bertocci, F. (1995). ELISA detection of potato early blight agents. Phytopathologia-Mediterranea, 34, 173–179.
- Castlebury, L. A., & Carris, L. M. (1999). Tilletia walkeri, a new species on Lolium multiflorum and L. perenne. Mycologia, 91(1), 121–131. 10.2307/3761200
- Crous, P. W., Van Jaarsveld, A. B., Castlebury, L. A., Carris, L. M., Frederick, R. D., & Pretorius, Z. A. (2001). Karnal bunt of wheat newly reported from the African continent. Plant Disease, 85, 561. 10.1094/PDIS.2001.85.5.561B
- Engvall, E., & Perlmann, P. (1971). Enzyme linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry, 8, 871–874. 10.1016/0019-2791(71)90454-X
- Frederick, R. D., Snyder, K. E., Tooley, P. W., Berthier-Schaad, Y., Peterson, G. L., Bonde, M. R., … Knorr, D. A. (2000). Identification and differentiation of Tilletia indica and T. walkeri using the polymerase chain reaction. Phytopathology, 90, 951–960. 10.1094/PHYTO.2000.90.9.951
- Gerik, J. S., Lommel, S. A., & Huisman, O. C. (1987). A specific serolological staining procedure for Verticillium dehliae in cotton root tissue. Phytopathology, 77, 261–266. 10.1094/Phyto-77-261
- Gleason, M. K., Gharbrial, S. A., & Ferries, R. S. (1987). Serological detection of Phomosis longicolla in soybean seeds. Phytopathology, 77, 371–375. 10.1094/Phyto-77-371
- Gupta, V., Kumar, A., Joshi, G. K., Agarwal, V. K., & Garg, G. K. (2003). Detergent washing technique for the efficient extraction of teliospores of Tilletia indica from contaminated wheat seeds. Seed Science and Technology, 31, 95–101.
- Gupta, M. K., Narwal, S., Umapathy, V., Mall, R., & Kumar, A. (2009). Characterisation of potential antigen(s) of teliospore walls to develop a specific immunoassay for Karnal bunt detection Tilletia indica. Food and Agricultural Immunology, 20(2), 79–94. 10.1080/09540100902810791
- Holland, A. F., Denny, S. L., & Bonde, M. R. (1990). Serological differentiation of Tilletia indica and Tilletia barclayana. In Proceedings of the 7th Biennial workshop on smut fungi held at Frederick, Maryland, USA.
- Holmes, G. J., Jackson, L. F., & Perring, T. M. (1997). Imperial valley conditions limit Karnal bunt in wheat. California Agriculture, 51, 29–33. 10.3733/ca.v051n03p29
- Inman, A. J., Hughes, K. J. D., & Bowyer, R. J. (2003). EU/EPPO recommended protocol for the diagnosis of a quarantine organism: Tilletia indica, Sand Hutton, York: Central Science Laboratory. Retrieved from http://www.fera.defra.gov.uk/plants/plantHealth/pestsDiseases/documents/protocols/tipro.pdf
- Johnson, M. C., Pirone, T. P., Siegel, M. R., & Varney, D. R. (1982). Detection of Epichloe typhina in tall fescue by means of enzyme-linked immunosorbent assay. Phytopathology, 72, 647–650. 10.1094/Phyto-72-647
- Joshi, L. M., Singh, D. V., Srivastava, K. D., & Wilcoxon, R. D. (1983). Karnal bunt: A minor disease that is now a threat to wheat. Botanical Review, 49, 309–330. 10.1007/BF02861085
- Kawchuk, L. M., Kim, W. K., & Nielsen, J. (1988). A comparison of polypeptides from the wheat bunt fungi Tilletia laevis, T. tritici and T. controversa. The Canadian Journal of Botany, 66, 2367–2376. 10.1139/b88-321
- Kumar, A., Singh, U. S., Kumar, J., & Garg, G. K. (2008). Application of molecular and immuno-diagnostic tools for detection, surveillance and quarantine regulation of Karnal bunt (Tilletia indica) of wheat. Food and Agricultural Immunology, 19, 293–311. 10.1080/09540100802478194
- Pascoe, I. G., Priest, M. J., Shivas, R. G., & Cunnington, J. H. (2005). Ustilospores of Tilletia ehrhartae, a smut of Ehrharta calycina, are common contaminants of Australian wheat grain, and a potential source of confusion with Tilletia indica, the cause of Karnal bunt of wheat. Plant Pathology, 54, 161–168. 10.1111/j.1365-3059.2005.01145.x
- Poupard, P., Grare, S., & Cavalier, N. (2010). Using ELISA method for assessment of in vitro development of strains of Pseudocercosporella herpotrichoides, the cereal eyespot fungus. Proceedings of the Third International Conference on Plant Disease, Bordeaux, pp. 655–662.
- Schaad, N. W., Shaw, J. J., Vidaver, A., Leach, J., & Erlick, B. J. (1999). Crop biosecurity. APSnet Features. 10.1094/APSnetFeature-1999-1099
- Singh, A. (1994). Epidemiology and management of Karnal bunt disease of wheat (167 p). Research Bull No. 127. Pantnagar: Directorate of Experiment Station, G. B. Plant University of Agriculture and Technology.
- Singh, M., Lakhera, P. C., Singh, B., Taj, G., & Kumar, A. (2013). Identification and localisation of glycosyl moieties of surface glycoproteins of teliospore wall of Karnal bunt (Tilletia indica) of wheat. Food and Agricultural Immunology, 24(2), 203–216. 10.1080/09540105.2012.677011
- Sundaram, S., Plasencia, J. S., & Bantarri, E. E. (1991). Enzyme linked immunosorbent assay for detection of Verticillium spp. using antisera produced to V. dahliae from potato. Phytopathology, 81, 1485–1489.
- The Hindu Survey of Indian Agriculture. (2010). Retrieved from http://www.hindu.com/books/sia/2010/agri10.htm
- Warham, E. J. (1986). Karnal bunt disease of wheat. A literature review. Tropical Pest Management, 32, 229–242. 10.1080/09670878609371068
- Weber, G., & Schauz, K. (1985). Characterization of spore protein patterns in Tilletia controversa and Tilletia caries with gel electrophoresis method. Z. Pflanzenkrankh. Pflanzenschutz, 92, 600–605.
- Wright, D., Murray, G., & Tan, M. K. (2003). National diagnostic protocol for the identification of Tilletia indica, the cause of Karnal bunt. South Perth: Department of Agriculture, Government of Western Australia.
- Ykema, R. E., Floyd, J. P., Palm, M. E., & Peterson, G. L. (1996). First report of Karnal bunt of wheat in the United States. Plant Disease, 80, 1207. 10.1094/PD-80-1207B