Abstract
Saffron is a well-known spice produced from dried stigmas of Crocus sativus L. flowers. Apart from its wide use in food preparations, it also has a broad range of medical properties. We examined the potential anti-inflammatory effects of saffron ethanolic extract (SEE) using an animal model of arthritis. Adjuvant-induced arthritis was induced in Wistar rats by injection of Complete Freund's Adjuvant. The rats were then injected intraperitoneally every other day with 25–600 mg SEE/kg or dexamethasone (DEX, 2 mg/kg). Changes in body weight, paw oedema and arthritis indices were recorded over the subsequent 12 days of treatment. Results revealed that SEE particularly at the higher concentrations significantly reduced paw and tibiotarsal joint diameters and comparing with DEX caused no significant change in body weight. These observations suggest that SEE displays a considerable anti-inflammatory potency and could potentially be used as an anti-arthritic agent in control of inflammation in rheumatoid arthritis.
Introduction
Saffron, the golden spice, is the most expensive spice in the world and is produced from dried stigmas of Crocus sativus L. flowers of the Iridaceae family (Deo, Citation2003; Negbi, Citation1999; Shultes, Citation1978; Xuabin, Citation1992). It is mostly used because of its desirable and unique colouring and flavouring properties. Saffron is known to contain a wide variety of constituents, including many types of pigments, flavonoids and gums (Rios, Recio, Giner, & Manez, Citation1996; Tarantilis & Polissiou, Citation1997; Winterhalter & Straubinger, Citation2000). These constituents have made saffron a useful medicinal plant all the way back to ancient times (Winterhalter & Straubinger, Citation2000). More recently, saffron ethanol extract (SEE) has been shown to impart distinct therapeutic activities, including anti-ulcer effects in Wistar rats (Inoue et al., Citation2005; Kianbakht & Mozaffari, Citation2009), anti-cancer effect (Abdullaev & Espinosa-Aguirre, Citation2004), treatment of depression (Akhondzadeh et al., Citation2005) and treatment of premenstrual syndrome (Agha-Hosseini et al., Citation2008) in human subjects. In addition, saffron preparations have been reported to have anti-tussive effects (Hosseinzadeh & Ghenaati, Citation2006), alleviate neuropathic pains (Amin & Hosseinzadeh, Citation2012) and protect hosts against genotoxicity that could be induced by diazinon (Hariri, Moallem, Mahmoudi, & Hosseinzadeh, Citation2011; Hariri, Moallem, Mahmoudi, Memar, & Hosseinzadeh, Citation2010).
The ethanol extract of C. sativus L. stigma (saffron) was evaluated in the treatment of acute inflammation in a xylene-induced ear oedema in mice and chronic inflammation in formaldehyde-induced arthritis in rats (Hosseinzadeh & Younesi, Citation2002). It was shown that the extract contained alkaloids and saponins that had anti-nociceptive activity and that it also exerted anti-inflammatory activity against acute and chronic inflammations. The effectiveness of the SEE was also shown in experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice and this outcome was found to be due to inhibition of oxidative stress and leukocyte infiltration into the central nervous system of the mice (Ghazavi, Mosayebi, Salehi, & Abtahi, Citation2009). Ethanol is the most commonly used solvent, since the extract that is derived predominantly contains crocin and crocetin esters (Sugiura, Shoyama, Saito, & Abe, Citation1994). These compounds are known to possess various biological properties including anti-ulcer (Xu et al., Citation2009), anti-cancer (Escribano, Alonso, Coca-Prados, & Fernandez, Citation1996; Garcí-Olmo et al., Citation1999; Jagadeeswaran, Thirunavukkarasu, Gunasekaran, Ramamurty, & Sakthisekaran, Citation2000), anti-atherosclerosis (He et al., Citation2005, Citation2007) and anti-inflammatory (Nam et al., Citation2010) activities.
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that affects about 1% of people in Western countries and leads to joint destruction, deformity, disability and even premature death (Prete et al., Citation2011). The incidence of RA is increasing incidence and recent estimates indicated levels of from 20 to 50 cases/100,000 people (Tobón, Youinou, & Saraux, Citation2010). Animal models are widely used for obtaining a better understanding of the aetiology and pathologic mechanisms underlying RA. In addition, these models are extensively used for the evaluation of potential new therapeutic agents from herbal extracts and compounds (Choi & Koo, Citation2005; Ekambaram, Perumal, & Subramanian, Citation2011; Tripathi & Maiti, Citation2003; Yang et al., Citation2008). These alternative treatments are needed as many RA sufferers cannot endure some of the unpleasant side effects of commonly employed anti-RA drugs (Billingham & Davies, Citation1979).
Among the various models developed in animal model hosts, adjuvant-induced arthritis (AIA) induced in inbred rats’ strains such as Wistar rats by subcutaneous injection of Complete Freund's Adjuvant (CFA) is a systemic process that is characterised by infiltration of lymphocytes into the synovium, destruction of cartilage and bone erosion. AIA in rats has been deemed clinically and histologically identical to human RA (Di Paola & Cuzzocrea, Citation2008; Joe & Wilder, Citation1999). On this basis, the current study sought to examine the anti-arthritic property of SEE in AIA Wistar rats to determine if this product had any potential for use as an effective and safe candidate anti-RA agent.
Materials and methods
Animals
Male Wistar rats (aged 6–8 weeks, 150–180 g) were purchased from Pasteur Institute (Tehran, Iran) and housed in standard laboratory conditions (25 ± 2°C and 40–70% relative humidity) with a 12-hr day/night lighting cycle throughout the experimental period. The animals were kept in large spacious polypropylene cages and provided ad libitum access to the standard rodent chow and filtered water. All rats were allowed to acclimate for 1 week prior to the initiation of any experiment. The Ethnic Council of Mashhad University of Medical Sciences (Mashhad, Iran) approved all protocols used with these rats in the studies herein.
Herbal preparation and extraction
Saffron was purchased from Novin Saffron Co. (Mashhad, Iran). The ethanol extract of the stigma was prepared as described previously (Hosseinzadeh & Younesi, Citation2002). Briefly, the powdered stigma was extracted using maceration in 70% (v/v) ethanol (Merck, Darmstadt, Germany) for 3 days at 45°C. Subsequently, the mixture was filtered and concentrated under reduced pressure at 45°C. The yield (w/w) of ethanol extract of the saffron stigma (SEE) was 44.2%. This material was then dissolved in phosphate-buffered saline (PBS, pH 7.4) to yield a stock concentration of 1200 mg/ml for use in subsequent preparation of injections of the study's arthritic rats.
Induction of AIA in rats and SEE treatment protocol
For the induction of experimental arthritis, the plantar surface of the right hind paw of each rat was injected with 0.1 ml CFA (Sigma, St. Louis, MO). This CFA contained 10 mg heat-killed Mycobacterium tuberculosis/ml paraffin oil. The left hind paw (injected with saline only) was used as the ‘self’-control for each rat.
Fourteen days after the CFA injection (Day 0), all rats were randomly assigned into eight groups (n = 5/group). AIA rats in Groups II, III, IV, V, VI and VII were injected intraperitoneally (IP) with 25, 50, 100, 200, 400 or 600 mg SEE/kg, respectively. Rats in Groups I and VIII were injected IP with an equal amount of normal saline or dexamethasone (DEX; at 2 mg/kg body weight, as reference drug), respectively (Lage & Cantrell, Citation2009; Sahebari et al., Citation2011). Each injection was repeated every other day (Days 2, 4, 6, 8 and 10) until Day 12 when the experiment was ended. Over the injection period, the body weight of each rat was recorded every other day to assess for any possible drug toxicity and to adjust dosing preparations as needed. Differences in body weights (compared to Day 0 values) were used to calculate the net changes in body weight in each group.
Assessment of paw oedema progression and determination of arthritis index (AI)
The clinical severity of the induced arthritis was determined by measuring footpad and tibiotarsal joint diameters (as indices of oedema) of the right (CFA injected) and left (control) paws, using a digimatic caliper (Mitutoyo Co., Japan). Rats were examined on Days 3, 6, 9 and 12 for extent of induced arthritis using a well-established scoring system developed to evaluate AIA severity (Prete et al., Citation2011; Tobón et al., Citation2010; Tripathi & Maiti, Citation2003). Briefly, the extent of arthritis was judged using the grading system: 0 = normal paw, 1 = erythema in toes, 2 = erythema and swelling of paws, 3 = swelling of ankles and 4 = complete swelling of the whole leg and inability to bend. The AIs for each group were then calculated from the average of the scores of all rats in each given treatment group.
Statistical analysis of data
A one-way analysis of variance with a Dunnett post hoc test was used to calculate the statistical significance of any differences in outcomes between the experimental groups. The difference between groups was considered statistically significant at p-values < 0.05. All data were presented as mean ± SD (standard deviation).
Results
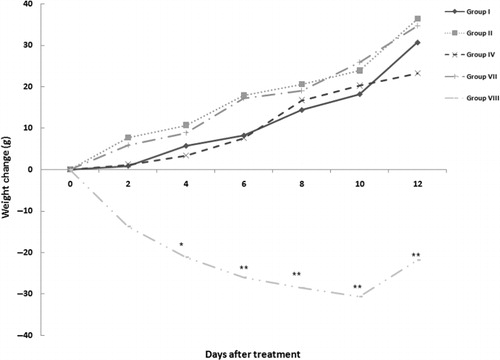
Effect of SEE treatment on body weight change
The body weight changes of rats in each different group – in comparison with Day 0 values (body weights 14 days after CFA injection) are shown in . For ease of viewing, only the data from rats in Groups I, II, IV, VII and VIII are presented. The mean body weight of CFA-treated rats increased throughout the experimental period, with weight gain among all SEE-treated rats being equal to (if trending greater) compared to those in Group I control rats. Interestingly, AIA rats treated with DEX had an immediate and persistent significant weight loss compared to the controls.
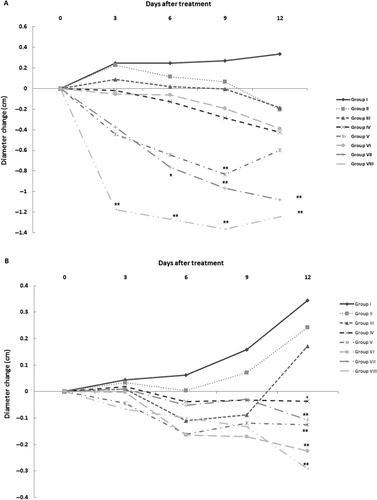
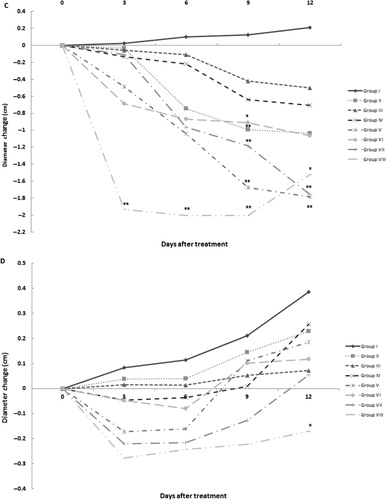
Effect of SEE treatment on the progression of paw oedema
After injection of CFA, the first manifestations of arthritis – such as erythema of ankle joints and involvement of metatarsal and interphalangeal joints – were evident in the rat paws. In addition, after CFA injection, there was a gradual increase in differences in the diameters of right and left footpad paws and in their respective tibiotarsal joints. In these studies, footpad and tibiotarsal joint oedema was assessed on Days 3, 6, 9 and 12 of the treatment regimen (). Diameter values of footpad paws and the tibiotarsal joints in Group I (arthritis only) rats increased gradually over the 12 days of treatment. In contrast, administration of different concentrations of SEE or DEX caused reductions in these changes in left paw diameters. Among them, significant decreases in left paw diameters were seen after 12 days of treatment with 100 mg SEE/kg (p < 0.05) and also after treatment with 200, 400 and 600 mg SEE/kg (Groups IV–VII) and DEX (Group VIII, p < 0.01). In addition, DEX treatment caused significant decreases in left tibiotarsal joint diameter after 12 days of treatment (p < 0.05). Treatment with DEX diminished the right paw diameters as an indicator of oedema during the early days of the regimen (p < 0.01). Treatment with 200 mg SEE/kg (p < 0.01) after 9 days and treatment with 600 mg SEE/kg (p < 0.01) during the measurements decreased right paw diameters too. Moreover, measurement of right tibiotarsal joint diameter revealed a significant decrease after treatment with DEX and 200–600 mg SEE/kg (p < 0.01).
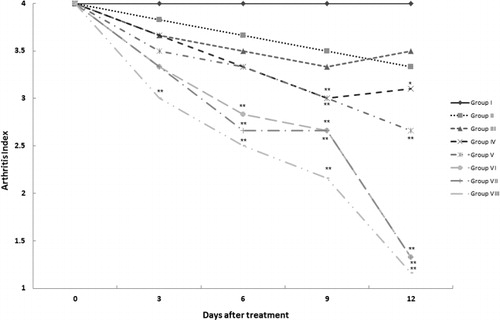
Effect of SEE treatment on AI
Symptoms of arthritis were assessed to generate the AIs in the right paws of rats in each treatment group (). The AI of each treated group was then compared with the values in Group I control rats at each assessment timepoint. After 3 days of treatments, the AI in DEX-treated rats (Group VIII) was significantly (p < 0.01) decreased. By Day 6, AI in Groups VI and VII rats that had received, respectively, 400 and 600 mg SEE/kg, were now also significantly lower than the control values (p < 0.01). After 9 days of SEE, the AI in rats in Groups IV–VIII also achieved a statistically significant lowering (p < 0.01). Only rats in Groups II and III failed to ever achieve any significant reduction in AI compared to the Group I controls.
Discussion
Saffron is produced from dried stigmas of C. sativus L. flowers (Deo, Citation2003; Negbi, Citation1999; Shultes, Citation1978; Xuabin, Citation1992). Although saffron has been used as medicinal plant in folklore all the way back to ancient times (Winterhalter & Straubinger, Citation2000), more recent studies have clearly documented a broad range biological activities of its extract, including anti-ulcer (Inoue et al., Citation2005; Kianbakht & Mozaffari, Citation2009), anti-cancer (Abdullaev & Espinosa-Aguirre, Citation2004) and anti-depressive effects (Akhondzadeh et al., Citation2005), and a utility in the treatment of premenstrual syndrome (Agha-Hosseini et al., Citation2008). Our own group has reported other effects of saffron, such as anti-tussive effect (Hosseinzadeh & Ghenaati, Citation2006), alleviation of neuropathic pains (Amin & Hosseinzadeh, Citation2012) and protection against potential genotoxicity that could be induced by diazinon (Hariri et al., Citation2010; Hariri et al., Citation2011).
Increasingly, information has become available about the anti-inflammatory property of preparations of saffron, in particular its ethanol extract (SEE). In one study, inhibitory effects of SEE were evaluated against both acute and chronic inflammation (Hosseinzadeh & Younesi, Citation2002). SEE at concentrations of 200–2000 mg/kg resulted in a significant anti-inflammatory activity [reductions in inflammation of 18.2–27.8% in comparison with 49.5% in mice that treated with DEX (at 15 mg/kg)] treatment in mice that underwent xylene-induced ear edoema. Moreover, SEE diminished chronic inflammation in rats that had undergone a formaldehyde-induced arthritis in their hind paws. Although diclofenac (10 mg/kg) as well as SEE (1400 mg/kg) showed anti-inflammatory effects on Day 1 of the treatment, neither seemed to have any demonstrable anti-inflammatory activity on Days 4 and 5. However, both did again seem to resolve the hind paw oedema after 6 days of treatment. Another study showed that SEE (500 mg/kg) was effective against EAE in C57BL/6 mice, and that this effect was due in part to significant reductions in oxidative stress and leukocyte infiltration to the central nervous system of the affected mice (Ghazavi et al., Citation2009).
It is known that the parent saffron contains an abundance of constituents, including many pigments, flavonoids, gums, tannins, anthocyanins, alkaloids and saponins (Hosseinzadeh & Younesi, Citation2002; Rios et al., Citation1996; Tarantilis & Polissiou, Citation1997; Winterhalter & Straubinger, Citation2000). It has also been shown that SEE contains many potent alkaloids and saponins (Hosseinzadeh & Younesi, Citation2002; ). Other studies have speculated that the anti-inflammatory activity of SEE might be attributed to its content of crocin (Hemshekhar et al., Citation2012; Nam et al., Citation2010), crocetin (Nam et al., Citation2010; Yang et al., Citation2012) and safranal (Boskabady, Tabatabaee, & Byrami, Citation2012), agents whose individual anti-inflammatory effects were documented in the cited studies.
Table 1. Phytochemical screening of C. sativus stigma ethanolic extract.
Conclusion
The results of the present study demonstrate that SEE was effective in alleviating the symptoms of AIA in rats. Besides to its effectiveness, the extract did not induce any overt side effects in the treated rats. From this, it could be suggested that saffron may potentially be useful as a safe effective agent against arthritis. Clearly, this needs to be evaluated in clinical trials in RA patients following more extensive evaluations of any potential toxicities of the SEE in animal models.
Funding
The authors would like to thank the authorities in research council of Mashhad University of Medical Sciences (MUMS) for their financial support [grant number 88246].
Additional information
Funding
References
- Abdullaev, F. I., & Espinosa-Aguirre, J. J. (2004). Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detection and Prevention, 28, 426–432. doi:10.1016/j.cdp.2004.09.002
- Agha-Hosseini, M., Kashani, L., Aleyaseen, A., Ghoreishi, A., Rahmanpour, H., & Zarrinara, A. R. (2008). Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: A double-blind, randomized and placebo-controlled trial. BJOG, 115, 515–519. doi: 10.1111/j.1471-0528.2007.01652.x
- Akhondzadeh, S., Tahmacebi-Pour, N., Noorbala, A., Amini, H., Fallah-Pour, H., & Jamshidi, A. (2005). Crocus sativus L. in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo controlled trial. Phytotherapy Research, 19, 148–151. doi: 10.1002/ptr.1647
- Amin, B., & Hosseinzadeh, H. (2012). Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L. and its constituents, safranal and crocin, in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia, 83, 888–895. doi: 10.1016/j.fitote.2012.03.022
- Billingham, M. E., & Davies, E. G. (1979). Experimental models of arthritis in animals as screening tests for drugs to treat arthritis in man. In J. R. Vane & S. H. Ferreira (Eds.), Anti-inflammatory drugs: Handbook of experimental pharmacology (Vol. 50, pp. 108–144). Berlin: Springer.
- Boskabady, M. H., Tabatabaee, A., & Byrami, G. (2012). The effect of the extract of Crocus sativus and its constituent safranal, on lung pathology and lung inflammation of ovalbumin sensitized guinea pigs. Phytomedicine, 19, 904–911. doi: 10.1016/j.phymed.2012.05.006
- Choi, E., & Koo, S. (2005). Anti-nociceptive and anti-inflammatory effects of the ethanolic extract of potato (Solanum tuberlosum). Food and Agricultural Immunology, 16(1), 29–39. doi: 10.1080/09540100500064320
- Deo, B. (2003). Growing saffron – The world's most expensive spice. Crop Food Research, 20, 1–4.
- Di Paola, R., & Cuzzocrea, S. (2008). Predictivity and sensitivity of animal models of arthritis. Autoimmunity Reviews, 8(1), 73–75. doi: 10.1016/j.autrev.2008.07.029
- Ekambaram, S. P., Perumal, S. S., & Subramanian, V. (2011). Strychnos potatorum Linn seed extract enhances lysosomal membrane stability and collagen formation in Freund's complete adjuvant-induced arthritic rats. Journal of Herbs, Spices and Medicinal Plants, 17, 392–402. doi: 10.1080/10496475.2011.632115
- Escribano, J., Alonso, G.-L., Coca-Prados, M., & Fernandez, J. A. (1996). Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Letters, 100(1–2), 23–30. doi: 10.1016/0304-3835(95)04067-6
- Garcí-Olmo, D. C., Riese, H. H., Escribano, J., Ontanon, J., Fernandez, J. A., & Atienzar, M. (1999). Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): An experimental study in the rat. Nutrition and Cancer, 35, 120–126. doi: 10.1207/S15327914NC352_4
- Ghazavi, A., Mosayebi, G., Salehi, H., & Abtahi, H. (2009). Effect of ethanol extract of saffron (Crocus sativus L.) on the inhibition of experimental autoimmune encephalomyelitis in C57BL/6 mice. Pakistan Journal of Biological Sciences, 12, 690–695. doi: 10.3923/pjbs.2009.690.695
- Hariri, A. T., Moallem, S. A., Mahmoudi, M., & Hosseinzadeh, H. (2011). The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine, 18, 499–504. doi: 10.1016/j.phymed.2010.10.001
- Hariri, A. T., Moallem, S. A., Mahmoudi, M., Memar, B., & Hosseinzadeh, H. (2010). Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: Protective effects of crocin and safranal. Food and Chemical Toxicology, 48, 2803–2808. doi: 10.1016/j.fct.2010.07.010
- He, S.-Y., Qian, Z.-Y., Tang, F.-T., Wen, N., Xu, G.-L., & Sheng, L. (2005). Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sciences, 77, 907–921. doi: 10.1016/j.lfs.2005.02.006
- He, S.-Y., Qian, Z.-Y., Wen, N., Tang, F.-T., Xu, G.-L., & Zhou, C.-H. (2007). Influence of crocetin on experimental atherosclerosis in hyperlipidemic-diet quails. European Journal of Pharmacology, 554, 191–195. doi: 10.1016/j.ejphar.2006.09.071
- Hemshekhar, M., Santhosh, M., Sunitha, K., Thushara, R. M., Kemparaju, K., Rangappa, K. S., & Girish, K. S. (2012). A dietary colorant crocin mitigates arthritis and associated secondary complications by modulating cartilage deteriorating enzymes, inflammatory mediators and anti-oxidant status. Biochimie, 94, 2723–2733. doi: 10.1016/j.biochi.2012.08.013
- Hosseinzadeh, H., & Ghenaati, J. (2006). Evaluation of the antitussive effect of stigma and petals of saffron (Crocus sativus) and its components, safranal and crocin in guinea pigs. Fitoterapia, 77, 446–448. doi: 10.1016/j.fitote.2006.04.012
- Hosseinzadeh, H., & Younesi, H. (2002). Anti-nociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacology, 2(1), 7–14. doi: 10.1186/1471-2210-2-7
- Inoue, E., Shimizu, Y., Shoji, M., Tsuchida, H., Sano, Y., & Ito, C. (2005). Pharmacological properties of N-095, a drug containing red ginseng, polygala root, saffron, antelope horn, and aloe wood. The American Journal of Chinese Medicine, 33(1), 49–60. doi: 10.1142/S0192415X05002655
- Jagadeeswaran, R., Thirunavukkarasu, C., Gunasekaran, P., Ramamurty, N., & Sakthisekaran, D. (2000). In vitro studies on the selective cytotoxic effect of crocetin and quercetin. Fitoterapia, 71, 395–399. doi: 10.1016/S0367-326X(00)00138-6
- Joe, B., & Wilder, R. L. (1999). Animal models of rheumatoid arthritis. Molecular Medicine Today, 5, 367–369. doi: 10.1016/S1357-4310(99)01528-2
- Kianbakht, S., & Mozaffari, K. (2009). Effects of saffron and its active constituents, crocin and safranal, on prevention of indomethacin induced gastric ulcers in diabetic and non-diabetic rats. Journal of Medicinal Plants, 8, 30–38.
- Lage, M., & Cantrell, C. L. (2009). Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin, and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Scientia Horticulturae, 121, 366–373. doi: 10.1016/j.scienta.2009.02.017
- Nam, K. N., Park, Y.-M., Jung, H.-J., Lee, J. Y., Min, B. D., Park, S.-U., … Lee, E. H. (2010). Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. European Journal of Pharmacology, 648, 110–116. doi: 10.1016/j.ejphar.2010.09.003
- Negbi, M. (1999). Saffron cultivation: Past, present and future prospects. In M. Negbi (Ed.), Saffron Crocus sativus L. (pp. 1–19). Amsterdam: Harwood Academic.
- Prete, M., Racanelli, V., Digiglio, L., Vacca, A., Dammacco, F., & Perosa, F. (2011). Extra-articular manifestations of rheumatoid arthritis: An update. Autoimmunity Reviews, 11, 123–131. doi: 10.1016/j.autrev.2011.09.001
- Rios, J. L., Recio, M. C., Giner, R. M., & Manez, S. (1996). An update review of saffron and its active constituents. Phytotherapy Research, 10, 189–193. doi: 10.1002/(SICI)1099-1573(199605)10:3%3C189::AID-PTR754%3E3.0.CO;2-C
- Sahebari, M., Mahmoudi, Z., Zamani Taghizadeh Rabe, S., Haghmorad, D., Mahmoudi, M. B., Hosseinzadeh, H., … Mahmoudi, M. (2011). Inhibitory effect of aqueous extract of saffron (Crocus sativus L.) on adjuvant-induced arthritis in Wistar rat. Pharmacology Online, 3, 802–808.
- Shultes, R. E. (1978). The kingdom of plants. In W. Thomson (Ed.), Medicines from the earth (p. 208). New York, NY: McGraw-Hill.
- Sugiura, M., Shoyama, Y., Saito, H., & Abe, K. (1994). Crocin (crocetin di-gentiobiose ester) prevents the inhibitory effect of ethanol on long-term potentiation in the dentate gyrus in vivo. Journal of Pharmacology Experimental Therapeutics, 271, 703–707.
- Tarantilis, P. A., & Polissiou, M. (1997). Isolation and identification of the aroma constituents of saffron (Crocus sativa). Journal of Agricultural and Food Chemistry, 45, 459–462. doi: 10.1021/jf960105e
- Tobón, G. J., Youinou, P., & Saraux, A. (2010). The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. Journal of Autoimmunity, 35(1), 10–14. doi: 10.1016/j.jaut.2009.12.009
- Tripathi, S., & Maiti, T. K. (2003). Efficiency of heat denatured lectins from Abrus precatorius as immunoadjuvants. Food and Agricultural Immunology, 15(3–4), 279–287. doi: 10.1080/09540100400019887
- Winterhalter, P., & Straubinger, M. (2000). Saffron-renewed interest in an ancient spice. Food Reviews International, 16(1), 39–59. doi: 10.1081/FRI-100100281
- Xu, G. L., Li, G., Ma, H. P., Zhong, H., Liu, F., & Ao, G. Z. (2009). Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. Journal of Agricultural and Food Chemistry, 57, 8325–8330. doi: 10.1021/jf901752f
- Xuabin, N. (1992). Research progresses on the saffron crocus (Crocus sativus). Zhongcaoyao, 23, 100–107.
- Yang, G., Qiao, Y., Li, B., Yang, J., Liu, D., Yao, H., … Yang, X. (2008). Adjuvant effect of di-(2-ethylhexyl) phthalate on asthma-like pathological changes in ovalbumin-immunized rats. Food and Agricultural Immunology, 19, 351–362. doi: 10.1080/09540100802545869
- Yang, R., Yang, L., Shen, X., Cheng, W., Zhao, B., Hamid Ali, K., … Ji, H. (2012). Suppression of NF-κB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. European Journal of Pharmacology, 674, 391–396. doi: 10.1016/j.ejphar.2011.08.029