Abstract
Sulfamethazine was used as target analyte to produce IgY from chicken aiming to compare the performance of these two antibodies in term of yield, titer, sensitivity, selectivity and matrix effect under parallel conditions. The results showed that the total yield of IgY produced by one chicken during 43 weeks' experimental period was about 15 g. This output is much more than IgG (150 mg from one rabbit). Besides, IgY titers increased during 10 weeks and remained stable over 30 weeks. The peak titer of IgY was 1:218. As the antibody titer rose, the sensitivity was increased. For IgG, the maximum titer was observed to be 1:223. In addition, both IgY and IgG were highly sensitive. The limits of detection were 0.54 ng/mL for IgY and 0.24 ng/mL for IgG. These results indicated that the IgY potentially provides a practical and ethical alternative to IgG in veterinary drug residue immunoanalysis.
Keywords:
1. Introduction
Immunoassays are widely used bioanalytical method for the detection of veterinary drug residues due to their low cost, specificity and high sensitivity (Dias & Tambourgi, Citation2010; Reig & Toldra, Citation2011). These methods are usually based on polyclonal or monoclonal antibodies, which are produced in mammal. However, there are certain limitations in using mammal-produced antibodies, such as complex preparation, high cost, frequent bleeding of animals and low titer against mammalian antigens (Klimentzou et al., Citation2006; Larsson & Sjoquist, Citation1990; Tu, Chen, & Chang, Citation2001). Recently, chicken yolk antibody (IgY) has attracted considerable attention for the prevention and treatment of infectious gastrointestinal diseases (Hirai et al., Citation2010; Malekshahi, Gargari, Rasooli, & Ebrahimizadeh, Citation2011; Rahman, Van Nguyen, Icatlo, Umeda, & Kodama, Citation2013; Sarker, Pant, Juneja, & Hammarstrom, Citation2007). IgY is isolated from the egg yolk of immunised hens instead of blood or ascitic fluid. It is a non-invasive method other than the generation of polyclonal or monoclonal antibodies. Moreover, this technology is able to continuously produce large quantities of IgY (Meulenaer & Huyghebaert, Citation2001; Schade et al., Citation2005; Svendsen, Crowley, Stodulski, & Hau, Citation1996). For example, one immunised chicken can produce as much as 3 grams of IgY per month, which is much more than the serum antibodies obtained from one rabbit (Tini, Jewell, Camenisch, Chilov, & Gassmann, Citation2002). Nowadays, IgY is applied in the detection of chemical compounds (Bottari, Oliveri, & Ugo, Citation2014; Meulenaer, Baert, Lanckriet, Van Hoed, & Huyghebaert, Citation2002; Sotiropoulou, Pampalakis, Prosnikli, Evangelatos, & Livaniou, Citation2012; Van Coillie, Block, & Reybroeck, Citation2004; Wang et al., Citation2013). However, few immunoassays based on IgY were reported for the detection of veterinary drugs. Probably because there was little report to investigate the advantages and disadvantages of IgY and IgG against veterinary drugs.
Sulfamethazine (SMZ) is a widely used veterinary drug to treat and prevent various diseases, such as gastrointestinal and respiratory tract infections (Agunos, Léger, & Carson, Citation2012; Clark, Mackey, & Scheel, Citation1966; O'Connor, Wellman, Rice, & Funk, Citation2010; Sampson, Bing, Grueter, Ose, & Havens, Citation1973). As a consequence, the overuse of SMZ has caused many food safety issues (Ding et al., Citation2006; Ko, Song, & Park, Citation2000; Shaikh & Chu, Citation2000). In China and the European Commission, the maximum residue level for SMZ in animal product is set at 100 µg/kg (Chinese Agriculture Ministry, Citation2002; European Community Council Regulation, Citation1990). The aim of this study was to produce IgY against SMZ for the first time and evaluate IgY and IgG by systematically comparing their advantages and disadvantages.
2. Materials and methods
2.1. Apparatus
White opaque 96-well polystyrene microtiter plates were purchased from Costar Inc. (Milpitas, CA, USA). Spectra Max M5 micro-plate reader (Molecular Devices, Sunnyvale, CA, USA) was used for this experiment. Centrifugation was performed with a refrigerated centrifuge supplied by Shanghai Anting Scientific Instrument Factory (Shanghai, China). Protein concentration was carried out using the Thermo NanoDrop 2000 (Waltham, MA, USA). Water was purified using a Milli-Q system (Bedford, MA, USA). Other reagents were analytical grade and obtained from Beijing Regent Corp. (Beijing, China).
2.2. Reagents and buffers
SMZ, sulfamerazine, sulfadimethoxine, sulfamethoxazole, sulfabenzamide, sulfapyridine, sulfaquinoxaline, sulfamethoxypyridazine, sulfamonomethoxine, sulfachlorpyrazine, sulfadoxine, sulfadiazine, sulfamethoxydiazine, sulfaclozine, sulfathiazole, sulfisoxazole, sulfabenzamine, sulfanilamide, sulfaguanidine, sulfamethizole, sulfaphenazolum, sulfaethoxypyridazine, bovine serum albumin (BSA), ovalbumin (OVA), Freund's complete adjuvant (FCA), Freund's incomplete adjuvant (FIA), 3,3′,5,5′-tetramethylbenzidine, urea hydrogen peroxide and casein were purchased from Sigma-Aldrich (St. Louis, MO, USA). The peroxidase-conjugated rabbit anti-chicken IgY (HRP-IgY) and peroxidase-conjugated sheep anti-rabbit IgG (HRP-IgG) were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Proclin-300 was purchased from Zymed Laboratories, Inc. (South San Francisco, CA, USA). Other reagents used were analytical grade from Beijing Reagent Corp. (Beijing, China).
The coating buffer was 0.05 M carbonate solution (pH 9.6), containing 0.015 M Na2CO3 and 0.035 M NaHCO3. The blocking buffer consisted of 0.01 M phosphate buffered saline (PBS) (pH 7.4), 5% bovine serum, 0.5% casein (w/v) and 0.01% proclin 300. Assay buffer was 0.01 M PBS containing 0.15 M NaCl and 2.7 mM KCl. PBS with 0.05% Tween 20 (v/v) was used as washing solution. PBS with 5% foetal bovine serum was used for the dilution of peroxidase-conjugated rabbit anti-chicken IgY and sheep anti-rabbit IgG. The substrate was 0.1% 3,3′,5,5′-tetramethylbenzidine and 0.05 M citrate buffer, pH 4.5. The stop solution was 2 M H2SO4. The stock solutions of SMZ and structure-related sulfonamides (including sulfamerazine, sulfadimethoxine, sulfamethoxazole, sulfabenzamide, sulfapyridine, sulfaquinoxaline, sulfamethoxypyridazine, sulfamonomethoxine, sulfachlorpyrazine, sulfadoxine, sulfadiazine, sulfamethoxydiazine, sulfaclozine, sulfathiazole, sulfisoxazole, sulfabenzamine, sulfanilamide, sulfaguanidine, sulfamethizole, sulfaphenazolum, sulfaethoxypyridazine) in methanol (2 mg/mL) were stored at −20°C in amber glass bottles.
2.3. Synthesis of immunogen and coating antigen
Glutaraldehyde was used for the coupling of SMZ with OVA or BSA according to the previous articles (Haasnoot et al., Citation2000; He, Shen, Suo, Jiang, & Hou, Citation2005). Briefly, 63.6 mg of SMZ and 10 mg of BSA were dissolved in 2.5 mL of PBS (0.01 M, pH 7.4). About 150 µL of 1% glutaraldehyde was added and incubated for 3 h at room temperature. Then this mixture was dialysed for three days in PBS. The obtained immunogen, SMZ-G-BSA, was stored at −20°C for further use. The coating antigen (SMZ-G-OVA) was synthesised by using 30 mg of SMZ and 5 mg of OVA in the same strategy as SMZ-G-BSA.
2.4. Immunisation
The use of experimental animal in this study was approved by the Animal Care Committee of Beijing. The animal house in China Agricultural University has been certificated in 2004 and evaluated twice each year by the same committee. Three leghorn chickens and four New Zealand white rabbits were used to raise specific antibodies. The chickens were housed in 0.5 m × 0.5 m floor pens with nest boxes in the standard animal room (20°C ± 4°C). They were fed laying hen diet, and water ad libitum. The rabbits were kept in single cages in rabbit standard animal room (20°C ± 4°C) and fed rabbit diet, water ad libitum. Care of the animals was in accordance with animal welfare act.
For the first immunisation, three chickens were immunised intramuscularly in the breast muscle with 200 µg immunogen (SMZ-G-BSA) in PBS, which was emulsified in FCA (1:1, v/v). Each of the four rabbits was subcutaneously injected on the backs, using the same antigen and dose as chicken immunisation treatment. Booster immunisations for both chickens and rabbits were carried out at weeks 2, 4, 6, 8, 11, 13 and 16 with 250 µg immunogen in PBS with FIA (1:1, v/v). Finally, the immunisation procedure was stopped.
2.5. Antibody isolation and quantity determination
The eggs were collected from each immunised chicken every day for further use. IgY was isolated from chicken yolks according to the method of Akita and Nakai (Citation1992) with slight modification. Egg yolk was separated from egg white and dried with filter paper, and then it was diluted 6-fold with water. The pH was adjusted between 5.0 and 5.2 by 1 M HCl. The mixture was incubated overnight at 4°C and then centrifuged (10,000 rpm, 30 min, 4°C). The supernatant was filtered through filter paper. Subsequently, saturated ammonium sulfate was added with stirring in order to achieve a 40% saturated solution. After overnight incubation, this solution was centrifuged (5000 rpm, 20 min, room temperature). The residue was dissolved in 5 mL PBS and stored in small aliquots at −20°C for further use. The concentration of IgY was evaluated by the NanoDrop assay according to the instructions.
In order to monitor the presence of IgG in rabbit, blood samples (1 mL) were collected from the marginal ear vein one week after each booster immunisation. Finally, each rabbit was exsanguinated by heart punctures one week after the final booster immunisation and the blood samples were collected. Then all the rabbits were euthanised by lethal injection. Blood samples were allowed to incubate at 4°C overnight, followed by centrifuging to remove particulate material. The serum was kept at −20°C before use.
2.6. Antibody titer determination
To study the response of the immunised chickens and rabbits, noncompetitive ELISA was adopted to test the titers of antibodies. Microplates were previously coated with coating antigen and blocked. Then 100 µL of IgY or IgG (at appropriate dilution) was added into each well. After incubating for 30 min at 37°C, the microplates were washed. After that, rabbit anti-chicken IgY-HRP or sheep anti-rabbit IgG-HRP (1/5000, 100 µL/well) was added, followed by another incubation. The plates were washed and substrate solution was added (100 µL/well). Finally, the enzyme reaction was stopped by sulfuric acid. The absorbance was measured by microplate absorbance reader (450 nm as test wavelength and 630 nm as reference wavelength). Preimmune IgY preparation or rabbit serum was used as negative control, and antibody titer was defined as the maximum dilution that gave an absorbance of 2.2 times that of the preimmune chickens or rabbits.
2.7. Antibody sensitivity determination
Indirect competitive ELISA was employed to test the sensitivity of IgY and IgG. The procedure was similar to the noncompetitive ELISA described above. Checkerboard titration was carried out to select the optimal concentration of coating antigen and antibody dilution. Fifty microlitres of SMZ and 50 µL of antibody were added to each well. Antibodies with relatively low IC50 values would be used in the further experiments. In this study, the IC50 value, dynamic range and limit of detection (LOD) were served as the analyte concentrations obtained at 50%, 20%–80% and 90%, respectively. Standard curves were obtained by plotting inhibition rate against the value of analyte concentration and fitted to the four parameters function. The equation was:
2.8. Antibody selectivity
The selectivity of the antibodies was tested using 22 structure-related sulfonamides. The test compounds were SMZ, sulfamerazine, sulfadimethoxine, sulfamethoxazole, sulfabenzamide, sulfapyridine, sulfaquinoxaline, sulfamethoxypyridazine, sulfamonomethoxine, sulfachlorpyrazine, sulfadoxine, sulfadiazine, sulfamethoxydiazine, sulfaclozine, sulfathiazole, sulfisoxazole, sulfabenzamine, sulfanilamide, sulfaguanidine, sulfamethizole, sulfaphenazolum and sulfaethoxypyridazine. The ELISA was performed as described above. The selectivity of antibody was revealed by the cross-reactivity (%) for each interfering compound, and it was calculated as the following equation:
2.9. Matrix effect
Dairy products are frequently contaminated with SMZ. A preliminary study was carried out to test the matrix effect of milk. Milk samples bought in a local shop were centrifuged at 4°C (10,000 rpm, 10 min), and the upper fat layer was removed. Artificially contaminated milk samples were used to obtain standard curves. These samples were diluted with PBS at different spiked levels. Finally they were compared to the curves prepared in buffer. The dilutions of spiked milk were 1:2, 1:5 and 1:10, respectively.
2.10. Recovery test
To confirm the precision and trueness of the developed ELISA, recovery test was performed. Blank milk samples were fortified with SMZ at the levels of 1/2, 1 and 2 × MRLs. Samples were thoroughly mixed and centrifuged for 10 min at 10,000 rpm to remove the upper fat layer. Then they were diluted with PBS. Fifty microlitres of these diluted milk samples were used in the indirect competitive ELISA assay.
3. Results and discussion
3.1. Antibody isolation and quantity determination
In this study, IgY was separated from egg yolk using acidified water dilution method due to its cheap cost, high purity and facility of the process (Akita & Nakai, Citation1992, Citation1993). The quantity of specific antibody was determined by NanoDrop according to the instructions. During the 43-weeks experimental period, each hen laid about 200 eggs (except one hen that barely laid eggs after immunisation). About three-fourths of those eggs were obtained during the highest activity period. And nearly 0.1 g of IgY can be recovered per yolk after treatment. These results implied that the total quantity of IgY with high titer and good sensitivity produced by one chicken was about 15 g (0.1 × 150). In the case of rabbit, considering that multiple bleeding would seriously hurt the rabbits, antiserum was collected by heart puncture. And about 150 mg of IgG were obtained from 30 mL of antiserum per rabbit. These data indicated that the IgY output per hen was significantly higher than IgG output per rabbit.
3.2. Antibody titer and sensitivity determination
Indirect competitive ELISA was introduced to detect the changes of IgY and IgG titers. Since the concentration of coating antigen may have significant effect on the antibody titer, the same concentration of coating antigen was employed in the comparison of IgY and IgG. Because one of the three chickens stopped laying eggs 30 days after the first immunisation, the IgY produced by this chicken was excluded from consideration. The remaining two chickens immunised with SMZ-G-BSA responded consistently against the antigen. As can be seen from , a sharp rise of titer was observed after the third booster immunisation. After the fourth and fifth booster immunisations, it reached a maximum titer of 1:218. Since large numbers of useful eggs were already collected, the detection of antibody titers and IC50 values were stopped at 43 weeks after the first immunisation. With the rise of IgY titer, the IC50 values of the ELISA were decreasing to a relatively low level (). The lowest IC50 value was less than 15 ng/mL, and this low IC50 value remained stable for more than 30 weeks. For rabbit IgG, the maximum IgG titer against SMZ was observed to be 1:223 after the third booster immunisation (). It is 32 times higher than IgY. But the changing trends in antibody titer and IC50 values of the ELISA were similar to IgY ().
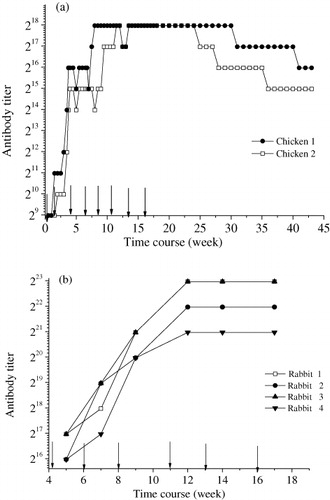
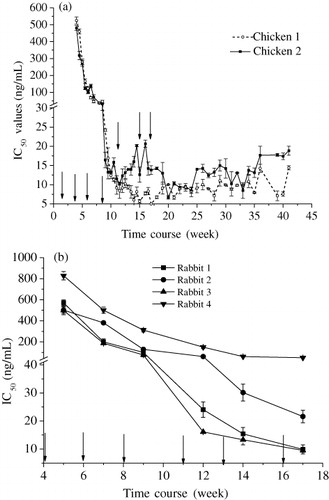
Therefore, taking both quantity and titer of the two antibodies into consideration, the final productivity of IgY obtained from one chicken was about three times greater than IgG isolated from a rabbit.
3.3. Selectivity determination
In order to determine the specificity of the IgY and IgG, cross-reactivity towards various structure-related sulfonamide analogues was evaluated. Two antibodies (Chicken 1, day 165 and Rabbit 3) with relatively low IC50 values were used in this assay. summarised the IC50 values and cross-reactivity of the two antibodies against other sulfonamides. The cross-reactivity testing showed that both IgY and IgG had a high specificity for SMZ. There was no significant cross-reactivity with the other sulfonamides except for sulfamerazine. The IC50 values towards SMZ and sulfamerazine using IgY were 6.76 ng/mL and 53.53 ng/mL, indicating a 12.63% cross-reactivity. In the case of IgG, the IC50 values towards SMZ and sulfamerazine were 4.76 ng/mL and 78.81 ng/mL, meaning 6.04% cross-reactivity. The cross-reactivity study showed that both IgY and IgG had high specificity towards SMZ. But IgG was slightly better than IgY in distinguishing SMZ from sulfamerazine.
Table 1. Cross-reactivity (CR) results of IgY (Chicken 1 day 165) and IgG (Rabbit 3) towards several related sulfonamides.
3.4. Matrix effect
Matrix effect of samples may lead to poor accuracy of immunoassays. An important step for the evaluation of immunoassay is the assessment of matrix effect. To gain basic information on matrix effect of IgY and IgG, standard curves generated in PBS were compared with curves obtained in diluted milk samples. showed that the IC50 values of sulfamerazine using IgY were 6.56, 4.31 and 6.06 ng/mL when dilution factors were 1:2, 1:5 and 1:10, respectively, while the IC50 value of standard curve was 6.76 ng/mL. Though the IC50 values showed minor differences at different dilutions of milk and PBS, the optical density values decreased significantly when the dilution factors were 1:2 and 1:5. Therefore, a 1:10 dilution would be good enough to reduce milk matrix effect on ELISA of IgG. The working range of this method was 1.32–27.81 ng/mL, and the LOD was 0.54 ng/mL. Similar results were obtained using IgG in the milk matrix effect study (). In this case, the IC50 of ELISA using IgG at 1:10 dilution was 4.18 ng/mL, similar to 4.76 ng/mL in PBS. The working range and LOD were 0.68–25.54 ng/mL and 0.24 ng/mL, respectively. The parameters of calibration curves using IgY and IgG in PBS and 10 times diluted milk were shown in . Generally, it can be presumed that IgY and IgG we obtained in the study had similar tolerance ability for milk matrix.
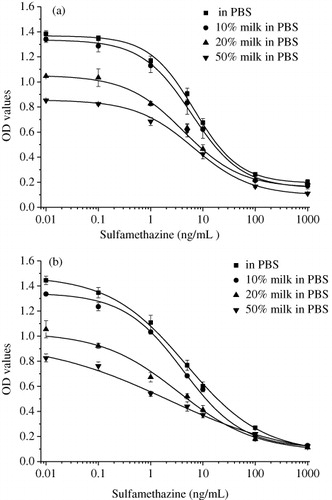
Table 2. Detailed parameters of the calibration curves using IgY (Chicken 1 day 165) and IgG (Rabbit 3) in PBS and milk samples.
3.5. Recovery test
Considering that the calibration curves prepared in 1:10 diluted milk were consistent with those generated in PBS, blank milk samples were fortified with SMZ and then diluted 10 times in PBS. The results of the recoveries of IgY and IgG were summarised in . The average recoveries (n = 5) for IgY and IgG were in the range of 96.2–131.8% and 101.2–114.4%, respectively. Assay reproducibility was satisfied. For IgY, the coefficient variation (CV) was ranging from 4.8% to 12.0%, while for IgG, it is ranging from 10.0% to 12.1%. These data indicated that both IgY and IgG could be used to determine SMZ in milk samples.
Table 3. Recoveries of sulfamethazine in milk samples using IgY (Chicken 1 day 165) and IgG (Rabbit 3).
4. Conclusion
IgY from chickens against SMZ was successfully produced. The comparison between IgY and rabbit antiserum IgG was also discussed in this study. The properties of IgY are similar to IgG in terms of sensitivity, selectivity and matrix effect. Both antibodies were highly sensitive, providing a LOD 0.54 ng/mL for IgY and 0.24 ng/mL for IgG. The IgY we reported in this study is complementary to the existing mammal antibodies, and it potentially provides a practical and ethical alternative in veterinary drug residue immunoanalysis.
Additional information
Funding
References
- Agunos, A., Léger, D., & Carson, C. (2012). Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. The Canadian Veterinary Journal, 53, 1289–1300.
- Akita, E. M., & Nakai, S. (1992). Immunoglobulins from egg yolk: Isolation and purification. Journal of Food Science, 57, 629–634. doi:10.1111/j.1365-2621.1992.tb08058.x
- Akita, E. M., & Nakai, S. (1993). Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. Journal of Immunological Methods, 160, 207–214. doi:10.1016/0022-1759(93)90179-B
- Bottari, F., Oliveri, P., & Ugo, P. (2014). Electrochemical immunosensor based on ensemble of nanoelectrodes for immunoglobulin IgY detection: Application to identify hen's egg yolk in tempera paintings. Biosensors and Bioelectronics, 52, 403–410. doi:10.1016/j.bios.2013.09.025
- Chinese Agriculture Ministry. (2002). Document No. 235/2002 of Chinese Agriculture Ministry. Beijing: China Agriculture Press.
- Clark, J. G., Mackey, D. R., & Scheel, E. H. (1966). Evaluation of sustained-release sulfamethazine in infectious diseases of cattle. Veterinary Medicine, Small Animal Clinician, 61, 1103–1105.
- Dias, S. W., & Tambourgi, D. V. (2010). IgY: A promising antibody for use in immunodiagnostic and in immunotherapy. Veterinary Immunology and Immunopathology, 135, 173–180. doi:10.1016/j.vetimm.2009.12.011
- Ding, S., Chen, J., Jiang, H., He, J., Shi, W., Zhao, W., & Shen, J. (2006). Application of quantum dot-antibody conjugates for detection of sulfamethazine residue in chicken muscle tissue. Journal of Agricultural Food and Chemistry, 54, 6139–6142. doi:10.1021/jf0606961
- European Community Council Regulation 2377/90. (1990). Official Journal of the European Communities NO L 224/1-8. Luxembourg: Publications Office of the European Union.
- Haasnoot, W., Cazemier, G., Pre, J. D., Kemmers-Voncken, A., Bienenmann-Ploum, M., & Verheijen, R. (2000). Sulphonamide antibodies: From specific polyclonals to generic monoclonals. Food and Agricultural Immunology, 12(1), 15–30. doi:10.1080/09540100099599
- He, J., Shen, J., Suo, X., Jiang, H., & Hou, X. (2005). Development of a monoclonal antibody-based ELISA for detection of sulfamethazine and N4−acetyl sulfamethazine in chicken breast muscle tissue. Journal of Food Science, 70, C113–C117. doi:10.1111/j.1365-2621.2005.tb09012.x
- Hirai, K., Arimitsu, H., Umeda, K., Yokota, K., Shen, L., Ayada, K., … Oguma, K. (2010). Passive oral immunization by egg yolk immunoglobulin (IgY) to Vibrio cholerae effectively prevents cholera. Acta Medica Okayama, 64, 163–170.
- Klimentzou, P., Paravatou-Petsotas, M., Zikos, C., Beck, A., Skopeliti, M., Czarnecki, J., … Evangelatos, G. P. (2006). Development and immunochemical evaluation of antibodies Y for the poorly immunogenic polypeptide prothymosin alpha. Peptides, 27, 183–193. doi:10.1016/j.peptides.2005.07.002
- Ko, E., Song, H., & Park, J. H. (2000). Direct competitive enzyme-linked immunosorbent assay for sulfamethazine. The Journal of Veterinary Medical Science, 62, 1121–1123. doi:10.1292/jvms.62.1121
- Larsson, A., & Sjoquist, J. (1990). Chicken IgY: Utilizing the evolutionary difference. Comparative Immunology, Microbiology and Infectious Diseases, 13, 199–201. doi:10.1016/0147-9571(90)90088-B
- Malekshahi, Z. V., Gargari, S. L., Rasooli, I., & Ebrahimizadeh, W. (2011). Treatment of Helicobacter pylori infection in mice with oral administration of egg yolk-driven anti-UreC immunoglobulin. Microbial Pathogenesis, 51, 366–372. doi:10.1016/j.micpath.2011.06.002
- Meulenaer, B. D., & Huyghebaert, A. (2001). Isolation and purification of chicken egg yolk immunoglobulins: A review. Food and Agricultural Immunology, 13, 275–288. doi:10.1080/09540100120094537
- Meulenaer, B. D., Baert, K., Lanckriet, H., Van Hoed, V., & Huyghebaert, A. (2002). Development of an enzyme-linked immunosorbent assay for bisphenol A using chicken Immunoglobulins. Journal of Agricultural and Food Chemistry, 50, 5273–5282. doi:10.1021/jf0202739
- O'Connor, A. M., Wellman, N. G., Rice, N. G., & Funk, L. (2010). Characteristics of clinical trials assessing antimicrobial treatment of bovine respiratory disease, 1979–2005. Journal of the American Veterinary Medical Association, 237, 701–705. doi:10.2460/javma.237.6.701
- Rahman, S., Van Nguyen, S., Icatlo, F. C., Jr., Umeda, K., & Kodama, Y. (2013). Oral passive IgY-based immunotherapeutics: A novel solution for prevention and treatment of alimentary tract diseases. Human Vaccines & Immunotherapeutics, 9, 1039–1048. doi:10.4161/hv.23383
- Reig, M., & Toldra, F. (2011). Patents for ELISA tests to detect antibiotic residues in foods of animal origin. Recent Patents on Food, Nutrition & Agriculture, 3, 110–114. doi:10.2174/2212798411103020110
- Sampson, G. R., Bing, R. F., Grueter, H. P., Ose, E. E., & Havens, M. (1973). Effect of tylosin and sulfamethazine on naturally-occurring bacterial pneumonia in swine. Veterinary Medicine, Small Animal Clinician, 68, 543–544.
- Sarker, S. A., Pant, N., Juneja, L. R., & Hammarstrom, L. (2007). Successful treatment of rotavirus-induced diarrhoea in suckling mice with egg yolk immunoglobulin. Journal of Health, Population, and Nutrition, 25, 465–468.
- Schade, R., Calzado, E. G., Sarmiento, R., Chacana, P. A., Porankiewica, A. J., & Terzolo, H. R. (2005). Chicken egg yolk antibodies (IgY-technology): A review of progress in production and use in research and human and veterinary medicine. Alternatives to Laboratory Animals, 33, 129–154.
- Shaikh, B., & Chu, P. S. (2000). Distribution of total 14C residue in egg yolk, albumen, and tissues following oral [14C] sulfamethazine administration to hens. Journal of Agricultural and Food Chemistry, 48, 6404–6408. doi:10.1021/jf000519e
- Sotiropoulou, G., Pampalakis, G., Prosnikli, E., Evangelatos, G. P., & Livaniou, E. (2012). Development and immunochemical evaluation of a novel chicken IgY antibody specific for KLK6. Chemistry Central Journal, 6, 148. doi:10.1111/j.1365-2621.1992.tb08058.x
- Svendsen, B. L., Crowley, A., Stodulski, G., & Hau, J. (1996). Antibody production in rabbits and chickens immunized with human IgG. A comparison of titre and avidity development in rabbit serum, chicken serum and egg yolk using three different adjuvants. Journal of Immunological Methods, 191, 113–120. doi:10.1016/0022-1759(96)00010-5
- Tini, M., Jewell, U. R., Camenisch, G., Chilov, D., & Gassmann, M. (2002). Generation and application of chicken egg-yolk antibodies. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 131, 569–574. doi:10.1016/S1095-6433(01)00508-6
- Tu, Y. Y., Chen, C. C., & Chang, H. M. (2001). Isolation of immunoglobulin in yolk (IgY) and rabbit serum immunoglobulin G (IgG) specific against bovine lactoferrin by immunoaffinity chromatography. Food Research International, 34, 783–789. doi:10.1016/S0963-9969(00)00172-1
- Van Coillie, E., Block, D. J., & Reybroeck, W. (2004). Development of an indirect competitive ELISA for flumequine residues in raw milk using chicken egg yolk antibodies. Journal of Agricultural and Food chemistry, 52, 4975–4978. doi:10.1021/jf049593d
- Wang, Y., Kececi, K., Mirkin, M. V., Mani, V., Sardesai, N., & Rusling, J. F. (2013). Resistive-pulse measurements with nanopipettes: Detection of Au nanoparticles and nanoparticle-bound anti-peanut IgY. Chemical Science, 4, 655–663. doi:10.1039/c2sc21502k
