Abstract
A simple and rapid method for determination of carbofuran was developed using the fluorescence polarisation immunoassay (FPIA). Three tracers with different lengths of bridge (0-, 2- and 6-carbon bridge) between the hapten molecule 4-[[(2,3-dihydro-2,2-dimethyl-7-benzofuranyloxy)carbonyl]-amino]-butanoic acid (BFNB) and 5-aminofluorescein (AF), fluoresceinthiocarbamyl ethylenediamine (EDF), fluoresceinthiocarbamyl hexylenediamine (HDF), were synthesised and their binding response with anti-carbofuran-specific antibody were evaluated. The physicochemical parameters were optimised for the FPIA. The AF-labelled BFNB conjugate (BFNB-AF) was found to be the optimal tracer for FPIA of carbofuran. The detection limit of carbofuran, IC50 value and the working range were 2.3, 48.8 and 7.4−202.2 µg/L, respectively; and the reaction time was only 10 min. The average recovery from spiked water and vegetable samples was 86.9−95.4% and the mean coefficient of variation was 6.2% for inter-assay and 8.7% for intra-assay, which showed good reproducibility for FPIA. Thus, the developed FPIA method exhibited the potential for the rapid and accurate determination of carbofuran in agricultural and environmental samples.
Introduction
The irrational use of pesticides in recent years have resulted in the environment problems and the contaminated food or water, which becomes an important risk to human health (Gupta, Citation1994; Jiang et al., Citation2008; Rezg, Mornagui, El-Fazaa, & Gharbi, Citation2010; Suri et al., Citation2009; Yu et al., Citation2005).
The detection of harmful pesticides and their metabolites in the environmental water and food samples is an imminent task. Carbofuran (2,3-dihydro-2,2-dimethyl-7-benzofuranylmethyl carbamate) is a pesticide widely and effectively used to control insects. But it is a potent cholinesterase inhibitor and thus exhibits a high toxicity to human beings and wildlife, the residues of which are reported to be potential in air, soil, water and food (Campbell, David, Woodward, & Li, Citation2004; Tomlin, Citation1997). In recent years, some assay methods of chromatography have been developed to detect the residue of carbofuran, such as gas-chromatography, electro-chromatography and high-performance liquid chromatography (Caballo-López, & Luque de Castro, Citation2003; Richter, Sepulveda, Oliva, Calderon, & Seguel, Citation2003). Among these assays, the HPLC method with postcolumn derivatization and fluorescence detection is preferred (McGarvey, Citation1993; Syrago-Styliani, Evagelos, Anthony, & Panayotis, 2006; Yang, Goldsmith, & Smetena, Citation1996). However, the method requires specialised instrumentation as well as the complicated clean-up procedures of the samples before analyses, which is time-consuming.
Affinity-based immunoassays have recently emerged as an alternative to the traditional chromatographic methods that are sensitive, inexpensive, reliable and sometimes effective for field monitoring (Suri et al., Citation2009). Over the past 20 years, the development of immunochemical methods for carbofuran, such as the enzyme-linked immunosorbent assay (ELISA) and electrochemical immunosensors, is frequently reported (Bacigalupo, Meroni, & Longhi, Citation2006; Jin, Guo, Wang, Wu, & Zhu, Citation2009; Liang, Gui, Zhao, Qi, & Zhu, Citation2008; Sun, Du, Wang, Zhao, & Li, Citation2011; Yang et al., Citation2008; Zhu et al., Citation2008). However, the main drawbacks of ELISA as a heterogeneous method require a good separation procedure for the free and antibody-bound analytes and involve long reaction time and multiple washing steps, and electrochemical immunosensors own poor reproducibility. Amongst the immunoassay methods, fluorescence polarisation immunoassay (FPIA), which is based on interactions between fluorescein-labelled antigen (tracer) and specific antibody, can meet the requirements for simple, reliable, fast and cost-effective analysis, which can become a promising alternative to replace ELISA, especially in pesticide detection (Eremin et al., Citation2002; Eremin & Smith, Citation2003; Kumar, Suvardhan, Rekha, Krishnaiah, & Chiranjeevi, Citation2006; Smith & Eremin, Citation2008; Xu et al., Citation2011; Yakovleva et al., Citation2004). FPIA can be performed very quickly in both the laboratory and the field, and it is particularly suitable for the assay of antigen with low-molecular weight compounds because of its simplicity, precision and possible automation. FPIA has been used in determining pesticides, detergent metabolite, veterinary drugs, biological toxins and therapeutic drugs (Eremin et al., Citation2002; Gasilova & Eremin, Citation2010; Nielsen & Gall, Citation2001; Wang, Zhang, Shen, & Eremin, Citation2007). However, little information about the FPIA is available for determination of carbofuran residue. This study focused on the development of FPIA for determination of carbofuran residues in food and environmental water samples.
Experimental
Reagents and materials
N, N'-dicyclohexylcarbodiimide, N-hydroxysuccinimide, N, N-dimethylformamide, fluorescein isothiocyanate isomer I and 5-aminofluorescein (AF) were purchased from Sigma Co., Ltd (USA). Carbofuran (99.8%) was obtained from Shennong Chemical Co., Ltd (Jiangsu, China). Silica gel G glass sheets for thin layer chromatography (TLC) were purchased from Taizhou Shenghua Co., Ltd (Zhejiang, China). The anti-carbofuran monoclonal antibodies (MAbs), semi-synthetic 4-[[(2,3-dihydro-2,2-dimethyl-7-benzofuranyloxy)carbonyl]-amino]-butanoic acid (BFNB; Yang et al., Citation2007), fluoresceinthiocarbamyl ethylenediamine (EDF), fluoresceinthiocarbamyl hexylenediamine (HDF) were provided by the key laboratory of food quality and safety of Guangdong Province, all other chemicals and organic solvents were of reagent grade or better. Microplates (96 Black wells) were obtained from Jinchanhua Co., Ltd (Shenzhen, China). Fluorescence polarisation values were recorded using a multilabel counter (Wallac1420 VICTOR3, PerkinElmer Co., Ltd, USA).
Buffers and standard solutions: 50 mmol/L of borate buffer with 0.01% sodium azide (pH 8.5) was used as working diluent buffer for all FPIA experiments. Stock solutions (20 g/L) of carbofuran were prepared by dissolving 20 mg in 1 mL methanol and then stored at –20°C. Standard solutions of carbofuran in the range from 0.1 to 50,000 µg/L were prepared by dilution of stock solution with borate buffer (pH 8.5) and then stored at 4°C.
Preparation and characterization of tracers
BFNB-HDF
BFNB-HDF was prepared according to the earlier literature (Xu et al., Citation2011). The tracer concentrations were estimated spectrophotometrically at 492 nm, assuming the absorbance in borate buffer (pH 8.5) to be the same as for fluorescein (ε = 8.78 × 104 M−1 cm−1) (Gasilova & Eremin, Citation2010). The tracer solution was further diluted in borate buffer (pH 8.5) and used for FPIA measurements.
BFNB-EDF and BFNB-AF
The method was the same as the synthesis of BFNB-HDF described above. The supernatant was added to 4.5 mg (10 µmol/L) of EDF and 3.6 mg (10 µmol/L) of AF, respectively. After 5 min, when the red-coloured EDF and AF changed to yellow, a small portion of the reaction mixture was purified by TLC with developing agents CHCl3:CH3OH = 6:1 (v:v) and CHCl3:CH3OH = 10:1 (v:v), respectively. Several yellow bands were observed on the TLC plate. The yellow bands at the top of plate were collected, and eluted with methanol and stored at 4°C. The tracer concentration was estimated as described above.
And characterization of tracers was used in the same way according to the literature (Xu et al., Citation2011).
Competitive FPIA calibration curves
FPIA calibration curves were obtained by adding 20 µL standard solution or sample and 100 µL of the tracer solution in borate buffer (pH 8.5) to the microplate well, then 100 µL of the MAbs were added to the well. The fluorescene polarisation was measured and plotted against the concentration of analyte. A four-parameter logistic equation (according to the following formula) was used to fit the sigmoidal curve by using OriginPro 7.5 software (OriginLab Corp., Northampton, MA, USA).
Kinetic behaviour of the carbofuran FPIA
100 µL of the tracer solution (1 nmol/L) in borate buffer (pH 8.5) and 20 µL standard solution (10, 100 and 500 µg/L) were mixed, and then 100 µL of the MAbs (1.135 g/L) was added, the dilution of MAbs was about 70% of tracer bound, then plot fluorescence polarisation (FP) value against time to get the curves. In this work, the effect of the antibody–tracer incubation time on the carbofuran FPIA response was investigated.
Optimization of tracer concentration
During fixed MAbs concentration, the concentration of tracers was 0.5, 1.0 and 2.0 nmol/L, the carbofuran concentration was 0.1, 1, 10, 50, 100, 500, 1000 and 5000 µg/L, competitive curves were obtained, then the optimization of tracer concentration was got based on standard curves of δmP, IC50 and δmP/IC50.
Optimization of MAbs concentration
To 100 µL of the tracer solution (1 nmol/L) in borate buffer (pH 8.5), 100 µL of each dilution of MAbs were added and mixed in microplate. The FP value was determined and plotted against the MAbs dilution factor to assess the antibody titration level. According to the MAbs dilution curves, the optimal dilution of MAbs was about 70% of tracer bound.
Physicochemical parameter influence and optimization
The effects of several physicochemical factors on the performance of FPIA were studied. Modification of δmP and IC50 parameters of the standard curves was evaluated under different conditions. Buffer pH and solvent effects were optimised using tracers with higher sensitivity. Newly optimised assay conditions were used in subsequent experiments.
pH
Competitive curves were performed with buffers of different pH values in constant ionic concentration of 50 mmol/L. A stock solution of 200 mmol/L boracic acid (pH = 5.38) and 50 mmol/L borate sodium (pH = 9.49) was prepared. The necessary pH (6.6, 7.0, 7.4, 8.0, 8.6 and 9.0) was achieved by adjusting the ratio of two kinds of solvents.
Solvent effects
The assay tolerance to methanol was evaluated between 0 and 25% final solvent concentration (v/v). Standard curves of carbofuran were prepared in borate buffer (pH 8.5), and antibody or tracer was dissolved in borate buffer (pH 8.5) containing different amount of organic solvents. The curve parameters δmP, IC50 and δmP/IC50 were estimated for solvent effects.
Cross-reactivity studies
The cross-reactivity (CR) towards related compounds has been calculated according to the equation CR (%) = (IC50, analyte/IC50, analogue) × 100 where IC50, analogue values were obtained from calibration curves plotted for other compounds, these compounds included bendiocarb, propoxur, 3-Hydroxycarbofuran, isoprocarb, benfuracarb, carbosulfan, carbaryl and methomyl. Each measurement for date points was repeated at least three times.
Spiked sample analysis
Pond water was obtained from Po Yang river in South China Agriculture University, tap water was obtained from our laboratory. The pond water was filtered over a mixed cellulose ester microporous membrane to remove particles larger than 0.45 µm. These water samples were fortified with several concentrations of carbofuran covering the assay working range and analyzed by FPIA without any further pretreatment.
The cabbage and potato samples were got from local market. 10 g of samples were homogenised and fortified with several concentrations of carbofuran covering the assay working range and soaked overnight. Next day the sample was added by methanol 20 mL, then using ultrasonic extraction 15 min for 3 times. The merger of the extractions was concentrated almost dry under vacuum at 40°C, and then diluted with the borate buffer (pH 8.5) solution to 10 mL and used for analysis of FPIA.
Results and discussion
Synthesis and characterization of tracers
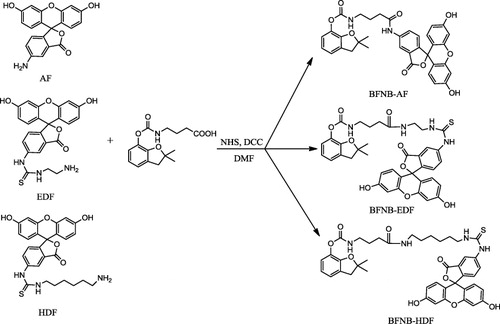
In FPIA for small molecules, the structure of the tracer may have a significant influence on the assay characteristics (Chun, Choi, Chang, Choi, & Eremin, Citation2009). The sensitivity of the immunoassay could be improved by careful choice of the structure of optimal tracer (Krikunova, Eremin, Smith, & Landon, Citation2003; Yakovleva et al., Citation2002). To study the influence of the structure of the tracer on assay sensitivity, three tracers with different lengths of bridge between the hapten and fluorescein (AF, EDF and HDF) were synthesised for carbofuran FPIA (). The tracers were purified by preparative TLC. The tracer concentrations were estimated spectrophotometrically at 492 nm, assuming the absorbance in borate buffer (pH 8.5) to be the same as for fluorescein (ε = 8.78 × 104 M−1 cm−1) (Gasilova & Eremin, Citation2010). The results are shown in .
Table 1. The concentration of three tracers.
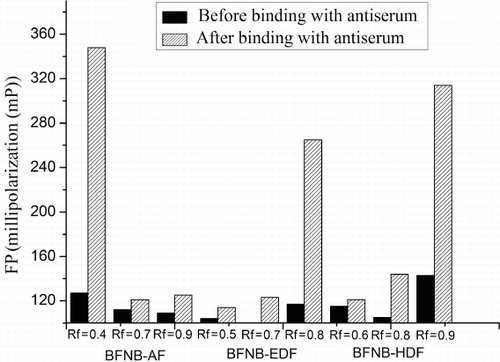
The synthesised tracers were separated by TLC. The ability to bind the antibody by all tracers was tested and only the main yellow band, Rf = 0.4 for BFNB-AF, Rf = 0.8 for BFNB-EDF and Rf = 0.9 for BFNB-HDF showed sufficient binding with the antibody (). BFNB-AF:ESI-MS (negative) m/z 621.57 [M‒H]−; BFNB-EDF:ESI-MS (negative) m/z 723.64 [M‒H]−; and BFNB-HDF:ESI-MS (negative) m/z 779.46 [M‒H]−.
Kinetic behaviour of tracers for carbofuran FPIA
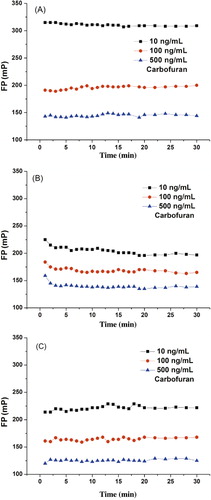
One of the major advantages of FPIA is that the antibody–tracer interaction can reach the equilibrium rapidly, which enabled the fast determination of small molecules (Chun et al., Citation2009; Krikunova, Eremin, Smith, & Landon, Citation2003; Yakovleva et al., Citation2002). In this work, the effect of the antibody–tracer incubation time on the carbofuran FPIA response was investigated. The result indicated that the equilibrium was achieved by tracer BFNB-AF and BFNB-HDF for 1 min, tracer BFNB-EDF for 10 min (), which showed that the ability of tracer BFNB-EDF to combine with antibody is slightly higher than the analyte. With the extension of time for reaction, the analyte could replace some tracers and the equilibrium was achieved finally, so this incubation time (10 min) was used in all further experiments.
Optimization of tracers and MAb concentration
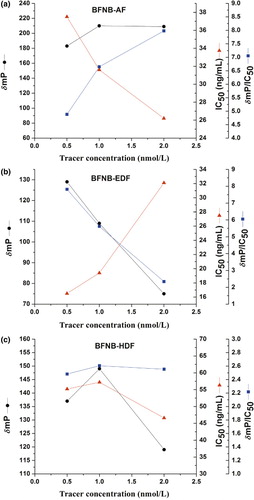
The concentrations of antibody and tracer are important for FPIA for small molecules (Eremin et al., Citation2002; Gasilova & Eremin, Citation2010; Nielsen & Gall, Citation2001; Yang et al., Citation2007). It was pointed out that limiting concentrations of antibody and tracer immunoreactants are required for a competitive immunoassay to obtain the required analytical sensitivity (Chun et al., Citation2009). The lowest possible tracer concentration, which allows the reliable detection of label and does not affect the competition, is desirable for highest sensitivity (Wang et al., Citation2007). Based on fixed MAb concentration, FPIA standards for carbofuran using different tracers with different concentrations were obtained. The effect of tracer concentration on FPIA performance (δmP, IC50 and δmP/IC50) is shown in . The concentration of tracer that showed the best δmP/IC50 in the FPIA was chosen for the optimal tracer concentration, which was 2.0, 0.5 and 1.0 nmol L–1 for BFNB-AF, BFNB-EDF and BFNB-HDF respectively.
For each tracer, MAb combination standard curves were obtained using the optimal concentrations of tracer and the MAbs of different dilution ratio. The optimal titer value of MAbs was defined from the concentration corresponding 70% binding of the tracer (Hatzidakis, Tsatsakis, Krambovitis, Spyros, & Eremin, Citation2002; Mi, Wang, Eremin, Shen, & Zhang, Citation2013; Zhang, Wang, Mi, Wenren, & Wen, Citation2014). The titers of MAbs were 1/2000, 1/4500 and 1/2000 for BFNB-AF, BFNB-EDF and BFNB-HDF, respectively.
Selection of tracers
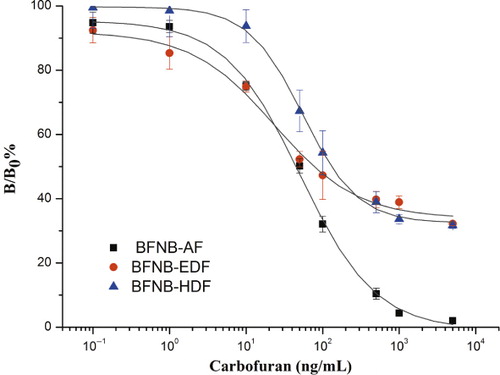
Based on the optimal tracers and MAb concentration, the standard curves for carbofuran using different tracers were obtained as shown in . The important parameters of the three assays were summarised in . It can be seen that when the tracer BFNB-EDF was used, the assay gave the best sensitivity (IC50 of 19 µg/L). However, from we can see that the use of BFNB-AF gave a highest δmP and a lowest background. The high background might result in false positive in the practical application of FPIA for carbofuran. Therefore, the BFNB-AF was chosen for the optimal tracer.
Table 2. The standard curve parameters of three tracers.
In FPIA, both the structural features of the tracer hapten itself and the structure and length of the bridge between the hapten and the fluorescein label markedly influenced the recognition of the tracer by antibody (Chun et al., Citation2009; Krikunova, Eremin, Smith, & Landon, Citation2003; Yakovleva et al., Citation2002). The short bridge tracers were reported to show lower titers than the long bridge tracer 2,4-D-HMDF, but their IC50 values showed that they had greater assay sensitivity (Krasnova, Eremin, Natangelo, Tavazzi, & Benfenati, Citation2001; Nasir & Jolly, Citation2003; Yakovleva et al., Citation2002). In this study, the results showed that the tracer with short bridge (BFNB-AF) gave the best performance for FPIA of carbofuran than the tracers with long bridge (BFNB-EDF and BFNB-HDF).
Effects of buffer pH and methanol concentration
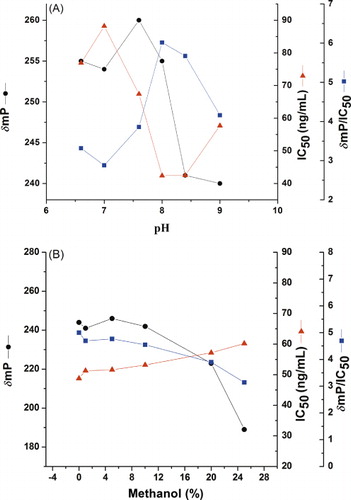
Because antigen–antibody binding is characterised by weak intermolecular bonds, a change in pH value could affect this interaction (Schneider et al., Citation1995). In order to examine the influence of pH on the assay, the buffer was changed pH value range (from 6.6 to 9.0), and the results were shown in . It indicated that the condition (pH value from 6.6 to 7.0) has a higher δmP on the assay but lower sensitivity, while alkaline conditions (pH value from 8.4 to 9.0) have higher sensitivity and lower δmP. The δmP/IC50 was highest at pH 8.0. Therefore, it was selected as the optimum pH. The marked effect of pH on FPIA parameters could be related to analysis of ionising in various pH conditions.
Most pesticides are not readily soluble in water; therefore, some polar organic solvents are often added to the assay buffer of pesticide immunoassays (Liang, Jin, Gui, & Zhu, Citation2007). Methanol was reported to cause the least negative effects on pesticide immunoassays (Lee, Ahn, Park, Kang, & Hammock, Citation2001; Liang et al., Citation2007). Therefore, methanol was used in the extraction of carbofuran in food an environmental samples. The tolerance of methanol was evaluated from 0 to 25% solvent contents in the optimised FPIA. δmP, IC50 and δmP/IC50 were recorded, and the results were shown in . Methanol did not adversely affect assay performance when its concentration lower than 10%, while the sensitivity and signal strength will be reduced when its concentration was higher to 10%. Therefore, the tolerance of methanol concentration is lower than 10%.
Calibration curve
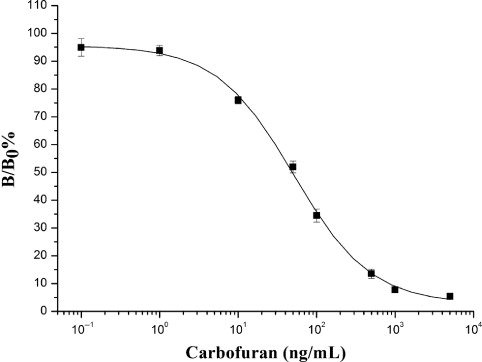
The standard curve was established by using pH 8.0, 50 mmol/L borate buffer (pH 8.5) as buffer solution for the system. The optimal FPIA for carbofuran was obtained using tracer BFNB-AF. The LOD for carbofuran was 2.3 µg/L, IC50 was 48.8 µg/L, and carbofuran could be determined in the range of 7.4–202.2 µg/L. The whole reaction only took 10 min. The calibration curve was shown in .
Cross-reactivity studies
The CR studies were performed with the data in . As it can be seen from this table, The MAb showed a slight recognition of bendiocarb, propoxur, 3-Hydroxycarbofuran, isoprocarb, benfuracarb and carbosulfan (the CR% were 3.0, 2.9, 1.5, 1.2, 0.5 and 0.3, respectively), whereas the CR to carbaryl and methomyl was negligible (the CR% was <0.01). Therefore, it indirectly proved that the bismethyl dihydrobenzofuran ring and the methyl methylcarbamate group formed the immunodominant region of the carbofuran, which is consistent with the previous report (Yang et al., Citation2007, Citation2008).
Table 3. Cross-reactivity of the antibody to carbofuran and related compounds by FPIA.
Analysis of spiked food and environmental water samples
Food and environmental water samples were spiked at several concentrations of carbofuran (10, 50 and 100 µg/L). Spiked samples were conditioned as described and analyzed without further treatment. The matrix effects of samples were studied by preparing carbofuran calibration curves with sample dilution compared to standard dilution. No matrix effects were observed from samples. Results were presented in . The mean recovery for tap water and pond water samples were 93.0 and 86.9%, while the data for cabbage and potato samples were 95.4 and 89.5%, respectively. The coefficient of variation (CV)% was between 2.4 and 11.1%. The FPIA was characterised by good reproducibility: the mean CV was 6.2% for inter-assay and 8.7% for intra-assay. The results indicated acceptable recoveries were obtained for the assay system.
Table 4. Recovery of carbofuran from spiked water and vegetables samples.
Conclusions
Three tracers were synthesised and used to develop an FPIA for the determination carbofuran in food and environmental water samples. The results indicated that the tracer with short bridge between the hapten and the fluorescein gave best FPIA performance for carbofuran than the tracers with long bridges. The FPIA based on the optimal conditions showed an IC50 value and LOD of 48.8 and 2.3 µg/L for carbofuran. The recoveries test from spiked food and environmental water samples indicated good reproducibility of the assay. These results confirmed the suitability of this immunoassay for the rapid, accurate and precise determination of carbofuran in samples. The simplicity in instrumentation and measurements allows this assay to be used in routine analyses.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/09540105.2014.914890.
Supplementary_Material.pdf
Download PDF (140 KB)Additional information
Funding
References
- Bacigalupo, M. A., Meroni, G., & Longhi, R. (2006). Determination of carbofuran in water by homogeneous immunoassay using selectively conjugate mastoparan and terbium/dipicolinic acid fluorescent complex. Talanta, 69, 1106–1111. doi:10.1016/j.talanta.2005.12.018
- Caballo-López, A., & Luque de Castro, M. D. (2003). Continuous ultrasound-assisted extraction coupled to on line filtration–solid-phase extraction–column liquid chromatography–post column derivatisation–fluorescence detection for the determination of N-methylcarbamates in soil and food. Journal of Chromatography A, 998(1–2), 51–59. doi:10.1016/S0021-9673(03)00646-0
- Campbell, S., David, M. D., Woodward, L. A., & Li, Q. X. (2004). Persistence of carbofuran in marine sand and water. Chemosphere, 54, 1155–1161. doi:10.1016/j.chemosphere.2003.09.018
- Chun, H. S., Choi, E. H., Chang, H.-J., Choi, S.-W., & Eremin, S. A. (2009). A fluorescence polarization immunoassay for the detection of zearalenone in corn. Analytica Chimica Acta, 639(1–2), 83–89. doi:10.1016/j.aca.2009.02.048
- Eremin, S. A., Ryabova, I. A., Yakovleva, J. N., Yazynina, E. V., Zherdev, A. V., & Dzantiev, B. B. (2002). Development of a rapid, specific fluorescence polarization immunoassay for the herbicide chlorsulfuron. Analytica Chimica Acta, 468, 229–236. doi:10.1016/S0003-2670(01)01361-7
- Eremin, S. A., & Smith, D. S. (2003). Fluorescence polarization immunoassays for pesticides. Combinatorial Chemistry and High Throughput Screening, 6, 257–266. doi:10.2174/138620703106298301
- Gasilova, N. V., & Eremin, S. A. (2010). Determination of chloramphenicol in milk by a fluorescence polarization immunoassay. Journal of Analytical Chemistry, 65, 255–259. doi:10.1134/S1061934810030081
- Gupta, R. C. (1994). Carbofuran toxicity. Journal of Toxicology and Environmental Health, 43, 383–418. doi:10.1080/15287399409531931
- Hatzidakis, G. I., Tsatsakis, A. M., Krambovitis, E. K., Spyros, A., & Eremin, S. A. (2002). Use of l-Lysine fluorescence derivatives as tracers to enhance the performance of polarization fluoroimmunoassays. A study using two herbicides as model antigens. Analytical Chemistry, 74, 2513–2521. doi:10.1021/ac011051x
- Jiang, X., Li, D., Xu, X., Ying, Y., Li, Y., Ye, Z., & Wang, J. (2008). Immunosensors for detection of pesticide residues. Biosensors & Bioelectronics, 23, 1577–1587. doi:10.1016/j.bios.2008.01.035
- Jin, R. Y., Guo, Y. R., Wang, C. M., Wu, J. X., & Zhu, G. N. (2009). Development of a bispecific monoclonal antibody to pesticide carbofuran and triazophos using hybrid hybridomas. Journal of Food Science, 74(1), T1–T6. doi:10.1111/j.1750-3841.2008.01002.x
- Krasnova, A. I., Eremin, S. A., Natangelo, M., Tavazzi, S., & Benfenati, E. (2001). A polarization fluorescence immunoassay for the herbicide propanil. Analytical Letters, 34, 2285–2301. doi:10.1081/AL-100107295
- Krikunova, V. S., Eremin, S. A., Smith, D. S., & Landon, J. (2003). Preliminary screening method for dioxin contamination using polarization fluoroimmunoassay for chlorinated phenoxyacid pesticides. International Journal of Environmental Analytical Chemistry, 83, 585–595. doi:10.1080/0306731021000060001
- Kumar, K. S., Suvardhan, K., Rekha, D., Krishnaiah, L., & Chiranjeevi, P. (2006). New reagents for the facile and sensitive spectrophotometric determination of carbofuran in its formulations and environmental samples. Journal of Analytical Chemistry, 61, 560–565. doi:10.1134/S1061934806060086
- Lee, J. K., Ahn, K. C., Park, O. S., Kang, S. Y., & Hammock, B. D. (2001). Development of an ELISA for the detection of the residues of the insecticide imidacloprid in agricultural and environmental samples. Journal of Agricultural and Food Chemistry, 49, 2159–2167. doi:10.1021/jf001140v
- Liang, C., Gui, W., Zhao, L., Qi, Q., & Zhu, G. (2008). Optimization of a direct competitive enzyme-linked immunoassay for carbofuran and application to water samples. Analytical Letters, 41, 1304–1317. doi:10.1080/00032710802119129
- Liang, C., Jin, R., Gui, W., & Zhu, G. (2007). Enzyme-linked immunosorbent assay based on a monoclonal antibody for the detection of the insecticide triazophos: Assay optimization and application to environmental samples. Environmental Science and Technology, 41, 6783–6788. doi:10.1021/es070828m
- McGarvey, B. D. (1993). High-performance liquid chromatographic methods for the determination of N-methylcarbamate pesticides in water, soil, plants and air. Journal of Chromatography A, 642(1–2), 89–105. doi:10.1016/0021-9673(93)80079-N
- Mi, T., Wang, Z., Eremin, S. A., Shen, J., & Zhang, S. (2013). Simultaneous determination of multiple (fluoro)quinolone antibiotics in food samples by a one-step fluorescence polarization immunoassay. Journal of Agricultural and Food Chemistry, 61, 9347–9355. doi:10.1021/jf403972r
- Nasir, M. S., & Jolly, M. E. (2003). Fluorescence Polarization (FP) assays for the determination of grain mycotoxins (Fumonisins, DON Vomitoxin and Aflatoxins). Combinatorial Chemistry and High Throughput Screening, 6, 267–273. doi:10.2174/138620703106298310
- Nielsen, K., & Gall, D. (2001). Fluorescence polarization assay for the diagnosis of brucellosis: A review. Journal of Immunoassay and Immunochemistry, 22, 183–201. doi:10.1081/IAS-100104705
- Petropoulou, S. E., Gikas, E., Tsarbopoulos, A., & Siskos, P. A. (2006). Gas chromatographic–tandem mass spectrometric method for the quantitation of carbofuran, carbaryl and their main metabolites in applicators' urine. Journal of Chromatography A, 1108(1), 99–110. doi:10.1016/j.chroma.2005.12.058
- Rezg, R., Mornagui, B., El-Fazaa, S., & Gharbi, N. (2010). Organophosphorus pesticides as food chain contaminants and type 2 diabetes: A review. Trends in Food Science and Technology, 21, 345–357. doi:10.1016/j.tifs.2010.04.006
- Richter, P., Sepúlveda, B., Oliva, R., Calderón, K., & Seguel, R. (2003). Screening and determination of pesticides in soil using continuous subcritical water extraction and gas chromatography–mass spectrometry. Journal of Chromatography A, 994, 169–177. doi:10.1016/S0021-9673(03)00496-5
- Schneider, P., Gee, S. J., Kreissig, S. B., Harris, A. S., Krämer, P., Marco, M. P., … Stander, L. (Eds.). (1995). New frontiers in agrochemical immunoassay. Arlington, TX: AOAC International, pp. 103–122.
- Smith, D. S., & Eremin, S. A. (2008). Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Analytical and Bioanalytical Chemistry, 391, 1499–1507. doi:10.1007/s00216-008-1897-z
- Sun, X., Du, S., Wang, X., Zhao, W., & Li, Q. (2011). A label-free electrochemical immunosensor for carbofuran detection based on a sol-gel entrapped antibody. Sensors, 11, 9520–9531. doi:10.3390/s111009520
- Suri, C., Boro, R., Nangia, Y., Gandhi, S., Sharma, P., Wangoo, N., … Shekhawat, G. (2009). Immunoanalytical techniques for analyzing pesticides in the environment. TrAC Trends in Analytical Chemistry, 28(1), 29–39. doi:10.1016/j.trac.2008.09.017
- Tomlin, C. D. S. (Ed.). (1997). The pesticide manual: A world compendium (11th ed.). Farnham: British Crop Protection, pp. 186–188.
- Wang, Z., Zhang, S., Nesterenko, I. S., Eremin, S. A., & Shen, J. (2007). Monoclonal antibody-based fluorescence polarization immunoassay for sulfamethoxypyridazine and sulfachloropyridazine. Journal of Agricultural and Food Chemistry, 55, 6871–6878. doi:10.1021/jf070948d
- Wang, Z. H., Zhang, S. X., Shen, J. Z., & Eremin, S. A. (2007). Development and application of labeled immunoassay in pesticide and veterinary drug residues analysis. Spectroscopy and Spectral Analysis, 27, 2299–2306.
- Xu, Z. L., Wang, Q., Lei, H. T., Eremin, S. A., Shen, Y. D., Wang, H., … Sun, Y. M. (2011). A simple, rapid and high-throughput fluorescence polarization immunoassay for simultaneous detection of organophosphorus pesticides in vegetable and environmental water samples. Analytica Chimica Acta, 708(1–2), 123–129. doi:10.1016/j.aca.2011.09.040
- Yakovleva, J., Lobanova, A., Michura, I., Formanovsky, A., Fránek, M., Zeravik, J., & Eremin, S. (2002). Development of a polarization fluoroimmunoassay for linear alkylbenzenesulfonates (LAS). Analytical Letters, 35, 2279–2294. doi:10.1081/AL-120016102
- Yakovleva, J. N., Lobanova, A. Y., Shutaleva, E. A., Kourkina, M. A., Mart'ianov, A. A., Zherdev, A. V., … Eremin, S. A. (2004). Express detection of nonylphenol in water samples by fluorescence polarization immunoassay. Analytical and Bioanalytical Chemistry, 378, 634–641. doi:10.1007/s00216-003-2307-1
- Yang, S. S., Goldsmith, A. I., & Smetena, I. (1996). Recent advances in the residue analysis of N-methylcarbamate pesticides. Journal of Chromatography A, 754(1–2), 3–16. doi:10.1016/S0021-9673(96)00203-8
- Yang, J. Y., Wang, H., Jiang, Y. M., Sun, Y. M., Pan, K., Lei, H. T., … Xu, Z. L. (2008). Development of an enzyme-linked immuno-sorbent assay (ELISA) method for carbofuran residues. Molecules, 13, 871–881. doi:10.3390/molecules13040871
- Yang, J.-Y., Wu, Q., Wang, H., Pan, K., Lei, H.-T., Hu, D.-Y., … Sun, Y.-M. (2007). Production and identification of high affinity monoclonal antibodies against pesticide carbofuran. Agricultural Sciences in China, 6, 1082–1088. doi:10.1016/S1671-2927(07)60150-3
- Yu, Y. L., Wu, X. M., Li, S. N., Fang, H., Tan, Y. J., & Yu, J. Q. (2005). Bioavailability of butachlor and myclobutanil residues in soil to earthworms. Chemosphere, 59, 961–967. doi:10.1016/j.chemosphere.2004.11.009
- Zhang, J., Wang, Z., Mi, T., Wenren, L., & Wen, K. (2014). A homogeneous fluorescence polarization immunoassay for the determination of cephalexin and cefadroxil in milk. Food Analytical Methods, 7, 879–886. doi:10.1007/s12161-013-9695-4
- Zhu, G., Jin, M., Gui, W., Guo, Y., Jin, R., Wang, C., … Wang, S. (2008). Development of a direct competitive enzyme-linked immunoassay for carbofuran in vegetables. Food Chemistry, 107, 1737–1742. doi:10.1016/j.foodchem.2007.10.035