Abstract
The transport of heterogeneous IgG to Fc receptor-expressing leukocytes and serum was investigated by the complement fixation test in mice. The results showed that heterogeneous IgG could be transported into the blood by neonatal Fc receptor (FcRn) on the gut mucosal surface. The interactions between human FcRn and IgG of different animal species were determined. It showed that rabbit IgG had the highest affinity (KD) to human FcRn, and the following species were listed as: human, sheep, horse and cattle. These results indicated the functions of heterogeneous IgG depended on the transport through FcRn, which might protect IgG from being diluted or degraded.
Introduction
Immune milk is a range of products of the bovine mammary gland that has been used to treat several human diseases (Morimoto, Kanda, Shinde, & Isobe, Citation2012). Many products derived from immune milk have been confirmed with beneficial immunological effects. In particular, IgG-rich dairy products are recommended for such human disease conditions as rheumatoid arthritis, gastroenteritis, urinary tract infections and certain types of allergies (Ligaarden, Lydersen, & Farup, Citation2012; Sekimura et al., Citation2009). In addition, many dairy products, especially baby milk powders, claim to contain abundant immunoglobulins, such as IgG, which is mainly from bovine or other animal milk.
One intriguing application of bovine colostrus and milk immunoglobulin is to provide passive immunity against diseases in other species, especially humans (Walter & Peter, Citation2011). The ability of producing antigen-specific antibodies in colostrums and milk that may be used to provide protection against a specific disease continues to be an area of interest. For example, the widespread consumption of immune milk from cows has been suggested to be a potential means of slowing outbreaks of avian influenza, SARS and other human respiratory diseases (Alisky, Citation2009). Nevertheless, the mechanism through which the bovine IgG provides passive immunity to protect humans from disease remains poorly defined up to now.
Human IgGs can bind to the high-affinity receptor, neonatal Fc receptor (FcRn), which is a unique major histocompability complex (MHC) class I-like molecule binding the Fc portion of IgG instead of peptides (Burmeister, Huber, & Bjorkman, Citation1994). It plays a major role in protecting IgGs from degradation (Junghans & Anderson, Citation1996). Therapeutic antibodies benefit from this protection and their half-life can be augmented by enhancing the affinity of their Fc domain for FcRn (Ward & Ober, Citation2009). Since FcRn was first described in the intestine of neonatal rats (Simister & Rees, Citation1985), it has been found in many species including humans, primates, mice, rabbits, ruminants and pigs, which distribute in multiple tissues (such as kidney, mammary gland, lung, placenta and vascular endothelium) (Baker et al., Citation2009; Roopenian & Akilesh, Citation2007).
In recent years, FcRn has also been reported in human and porcine intestinal epithelial cells, which can transport IgG molecules bidirectionally across this polarised cell monolayers (Claypool et al., Citation2004; Tesar, Tiangco, & Björkman, Citation2006; Ye, Tuo, Liu, Simister, & Zhu, Citation2008). The slightly acidic environment of the small intestine might help FcRn (expressed at the apical surface of enterocytes) to bind both IgG and antigen-IgG immune complexes (ICs) with high affinity (Jones & Waldmann, Citation1972; Rodewald, Citation1976). After binding, FcRn transports the bound IgGs or ICs through the epithelial barrier and releases them into the underlying extracellular space. Alternatively, FcRn can also deliver pathogen-specific IgGs to the intestinal lumen, which might help to enhance resistance to intestinal infection (Yoshida et al., Citation2006). Through these mechanisms, the pH-dependent binding of IgG to FcRn allows IgG transport through a cell layer along a concentration gradient of IgG. These findings clearly illustrate the role of FcRn-mediated transepithelial transport as an important mechanism for dendritic cells to sample luminal antigens for the control of mucosal pathogens.
Although the IgG products might come from various mammalian origins (human, bovine, rabbit, goat and horse), the mechanisms of heterogeneous IgG transporting across the gastrointestinal barrier and their destination in vivo remained unclear. On the other hand, whether the oral heterogeneous IgG can really exhibit the same protective activity for human health is still essential to be investigated further. In this study, a series of experiments were performed to investigate whether there is a pathway for transport of heterogeneous IgG to the body through FcRn and their interactions with human FcRn.
Materials and methods
Materials and reagents
Freeze dried complement (C3), hemolysin, sheep red blood cell and bovine IgG were provided by Shijiazhuang Huarui Innovate Biotechnology Co. Ltd; PcAb rabbit anti-bovine IgG by Zhengzhou Yikang Biotechnology Co. Ltd; rabbit anti-bovine IgG whole antiserum by Beijing Bioss Biotechnology Co. Ltd.; standard rabbit serum by CKRG Biolotechnology & Services Co. Ltd; and white blood cell separating medium by Tianjin Haoyang Biological Manufacture Co. Ltd.
IgGs of human, bovine, rabbit, goat and horse were all purchased from Beijing Bioss Biotechnology Co. Ltd. Recombinant human FCGRT & B2M heterodimer (human FcRn) was purchased from Sino Biological Inc. The recombinant human FcRn was composed of the recombinant human FCGRT (P55899) with B2M (P61769), which consists of 385 amino acid residues and has a molecular weight of 43.5 KD.
Apparatus
3K15 high-speed refrigerated centrifuge was provided by Sigma-Aldrich Co. LLC; −70°C Freezer by SANYO Electric Co. Ltd; Thermo Labsystem MK3 Microplate Reader by Thermo Electron Corporation.
96-pore plate (655209) was provided by CKRG Biological Engineering Technology & Services Co. Ltd; PD-10 desalting column by Amersham Biosciences; Octet RED96 system by ForteBio Inc.
Experimental animals and their grouping and sampling
All the experimental procedures were performed under the national guidelines on the proper care and use of animals in laboratory research.
Two-week-old female, healthy Kunming suckling mice were used in this study. Twenty-eight mice were randomly divided into two groups of 14 animals each (control and experimental groups) after adaptive feeding for one week. Mice were fasted overnight before experiments. Rabbit anti-cattle IgG was administered to the experimental group at a dose of 300 mg/kg orally. The control group received 0.9% physiological saline at the same dose orally. The blood samples were collected at 4-h post-administration from orbit, and serum was promptly isolated by centrifugation at 3200 rpm for 10 min at 4°C. The serum was stored at −20°C until assay.
Separation of white blood cells
Blood samples were immediately mixed with anticoagulant, then mixed with an equal volume of white blood cell separating medium and centrifuged at 800 rpm for 25 min at 4°C. After centrifugation, the sample was composed of four layers. The second and third upper layers were collected in an appropriate amount Hanks solution, then mixed and isolated by centrifugation at 1500 rpm for 20 min at 4°C. The pellet was collected and the washing step was repeated three times.
Determination of IgG transport across the mucosal barrier by the complement fixation test (CFT)
To investigate the transport of rabbit anti-cattle IgG into the body, the CFT was utilised to assay the transported IgG in serum (free) and white cells (bound to the FcRs).
All sera obtained were tested by the CFT. The CFT is a complex serological reaction that requires an antigen (e.g. the cattle IgG in this experiment), an antibody (PcAb rabbit anti-bovine IgG) and complement (the first system), sheep red blood cells and hemolytic serum (the second system). The principle of this assay is that complement binds to the antigen–antibody complex if there is an antigen–antibody reaction. If there is no antigen–antibody reaction, the complement will not bind and will remain free in solution. The free complement will then lyse the sensitised sheep red blood cells when they are added, yielding a negative test result.
The CFT was adapted to a microtiter plate and performed as described in the previous report (Krivoshein, Citation1989). The standard rabbit anti-bovine IgG antiserum was the positive control and the blank rabbit serum was negative control. This method enabled the quantitative determination of PcAb rabbit anti-bovine IgG bound to the FcRs of white cells or free in the blood (serum).
The human FcRn interaction with IgG from different animal species
A ForteBio Octet was used for the observation of different IgG interactions with human FcRn. Human FcRn lyophilized powder was dissolved in sterile water and then serially diluted by physiological saline. IgGs from different animal species were also diluted to specified final concentrations. Samples were processed as follows. (1) The desalting column was washed five times with the same phosphate buffered saline (PBS) as IgG diluent. (2) IgG was biotinylated by mixing with biotin at a 1:5 ratio and then incubated for 30 min at room temperature. (3) The mixed IgG/biotin solution was brought to 2.5 mL with PBS before being poured onto the column and eluted by 3 mL PBS. (4) The sensor requires prewetting for 20 min to remove the sucrose which covered the sensor as a blocking agent. In this study, streptavidin was used to couple biotinylated IgG to the surface of the sensor.
Binding assays were performed in 96-well microplates using the Octet Red system (Abdiche, Malashock, Pinkerton, & Pons, Citation2008). Streptavidin-coated tips were loaded with biotinylated IgG. Human FcRn was allowed to associate in PBS (pH 6.0), and its dissociation from different species' IgG was monitored. Binding of FcRn to an uncoated tip was used as a reference control. All measurement processes were under computer control. The run was carried out by placing the sensors in the wells and measuring changes in layer thickness. Data were analysed using the ForteBio Data Analysis package 6.2.
Data analysis
All the data were analysed using the statistical software package Statistical Product and Service Solutions (version 16). Independent-samples t-tests were used for the analysis.
Results and discussion
IgG transport across the mucosal barrier by the CFT
FcRn-mediated IgG transfer occurs in humans through placental and intestinal pathways (Roopenian et al., Citation2003). In rodents, the intestinal expression of FcRn rapidly declines after weaning, while FcRn expression occurs in both human neonates and adults (Israel et al., Citation1997). FcRn is expressed in human intestinal epithelial cells and can transport IgG molecules bidirectionally across these polarised cell monolayers. However, whether heterogeneous IgG in the milk products can be transported across the gastrointestinal mucosal barrier by human FcRn?
The optical density (OD) values of serum and white cells were shown in . Compared with the control group, the OD values of experiment group were lower obviously. In the study of determining the rabbit anti-bovine IgG in serum, the statistic data (t-test) demonstrated the variances appears significantly different between the experiment and control groups (P < 0.05). Therefore, it indicated that the heterogeneous antibody was able to be transported from lumen to serum by FcRn on the gut mucosal surface. In addition, it also indicated that heterogeneous antibodies could be transported from the lumen to where it turns the antibody to other Fc receptor (FcR) on the surface of leukocyte by FcRn.
Table 1. The detected OD values of serum and white cells (384 nm).
The results also indicated that heterogeneous antibody binding to FcR on the surface of leukocytes was significantly higher than levels of free antibody in the serum (0.116218: 0.101103). Binding of these antibodies to surface FcR has been shown to exert antibody-dependent cell-mediated cytotoxicity (ADCC) and protect IgG from lysosomal degradation, resulting in prolonged serum half-life to 22 days in humans and 45 days in mice (Kacskovics et al., Citation2006; Ober, Martinez, Lai, Zhou, & Ward, Citation2004).
Based on the results of this study, we hypothesised that the mechanism of FcRn transport of heterogeneous IgG and protection of IgG from degradation or immune elimination may depend on the following: (1) many cells, mostly of hematopoietic origin, express FcR on their plasma membrane. When heterogeneous IgG binds to FcRn on the apical surface of an epithelial cell, FcRn then transcytoses the bound IgG and releases it into the underlying extracellular space. This space is at physiological pH, so the IgG then releases from FcRn to bind FcR-expressing cells including myeloid cells of all types and some lymphoid cells; (2) FcRn binds the Fc portion CH2–CH3 hinge region of IgG that is distinct from the binding sites for the classical FcγRs or the C1q component of complement, and then initiates the classical pathway of complement activation (Martin, West, Gan, & Bjorkman, Citation2001). In contrast, IgG–Fc is functionally divalent for ligands binding at the CH2–CH3 interface (e.g. FcRn), causing heterogeneous IgG bound to FcγR on the surface of a leukocyte to remain unrecognised by the immune system (Jefferis, Citation2012; Taylor, Fabiane, Sutton, & Calvert, Citation2009); (3) FcRn is a non-classical MHC class-I molecule that salvages both IgG and albumin from degradation. FcRn-mediated fractional recycling rates for IgG and albumin were 142% and 44% of their fractional catabolic rates, respectively. FcRn-mediated recycling is a major contributor to the high endogenous concentrations of these two important plasma proteins. These unique characteristics can be explained by a saturable, receptor-mediated mechanism that protects both IgG and albumin from intracellular degradation after non-specific pinocytic uptake (Brandtzaeg, Citation2007; Holmgren & Czerkinsky, Citation2005). This allows for recycling and exchange between the cell surface and into the extracellular milieu for continued circulation (Mowat, Citation2003). These present results allowed us to evaluate the health benefits of immune milk, colostrums, IgG-rich dairy products and even therapeutic monoclonal antibodies (mAbs).
The interactions between human FcRn and IgG from different animal species
In the late 1980s, murine mAbs were in clinical development but had significant drawbacks. Murine mAbs are often associated with allergic reactions and the induction of anti-drug antibodies. They also exhibit a relatively short half-life in man compared to human IgG as a consequence of their relatively weak binding to human FcRn. Finally, murine mAbs are relatively poor inducers of effectors' functions such as ADCC, which are often critical for their efficacy in oncology indications (Stern & Herrmann, Citation2005). As a result, Chimeric mouse–human antibodies were developed to overcome the inherent immunogenicity and reduced the effectors' function of murine mAbs in man. These are generated by grafting the entire antigen-specific variable domain of a mouse Ab onto the constant domains of a human Ab using genetic engineering techniques (Buss, Henderson, McFarlane, Shenton, & Haan Citation2012; Morrison, Johnson, Herzenberg, & Oi, Citation1984). To evaluate the ability of heterogeneous IgG transporting across barriers and affecting the antibody's function, it is essential to know the affinity of different species' IgG for human FcRn.
FcRn has been well characterised in the transfer of passive humoral immunity from a mother to her foetus. Furthermore, FcRn protects IgGs from degradation throughout the life of an organism, controlling the long half-life of IgG and albumin content in the serum. It has become clear that FcRn is expressed in various sites in adults where potential functions are beginning to emerge. Recent studies have examined the interaction between FcRn and the Fc portion of IgG aiming to either improve the serum half-life of therapeutic mAbs or reduce the half-life of pathogenic antibodies (Roopenian & Akilesh, Citation2007).
The association and dissociation processes of IgG from different animal species with human FcRn were shown just as in – (human and rabbit). The degrees of fitting for the binding dynamics of IgG (from human, rabbit, horse, bovine, goat) with FcRn (R2) were 0.8, 0.984, 0967, 0.914 and 0.924, which indicated that the fittings were satisfying.
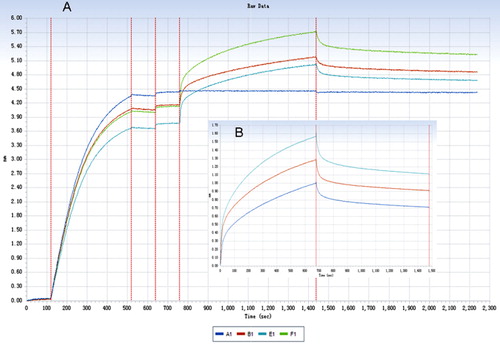
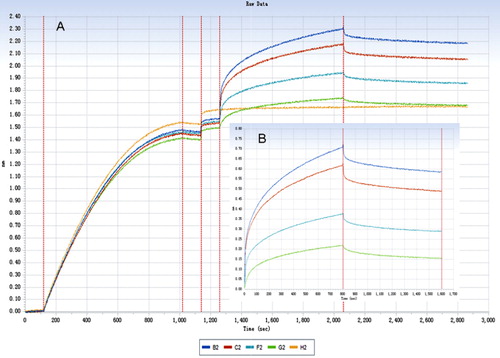
FcRn can bind internalised IgG depending on its affinity and protects IgG from lysosomal degradation, thus prolonging its half-life. All binding constant data were shown in . The results showed that FcRn can bind internalised IgG from different species with various affinities, thereby protecting IgG from lysosomal degradation and thus prolonging its half-life. Furthermore, a non-interacting two-site binding model (low affinity and high affinity) was commonly used to investigate the interaction between Fc and FcRn (Datta-Mannan et al., Citation2007). Compared with low-affinity site, the high-affinity binding site had a stronger effect on the half-life of IgG. As a result, the high-affinity KD was used to evaluate the interaction between FcRn and different species IgG ().
Table 2. The binding constants of FcRn with different species IgG.
The results showed that rabbit IgG has the highest affinity KD with human FcRn, followed in decreasing affinity by human, sheep, horse and cattle IgG. It is interesting that rabbit IgG has a higher affinity than human IgG with human FcRn, but this result requires further validation and study.
The results from this study showed that heterogeneous IgG had important roles in ADCC that are dependent on the transport of FcRn and protection IgG from degradation, facilitating the transfer of IgG to FcRs on the surface of leukocytes in vivo. These results also suggested that interactions between human FcRn and different species IgG may provide a valuable index for the evaluation of immunoglobulin additives. The conclusions from this study may form the basis for the development of immune milk, passive immunity milk products and therapeutic mAb development.
Conclusions
The present study demonstrated that heterogeneous IgG could be transported into the blood by FcRn, and the heterogeneous IgG possess various affinities with human FcRn. The functions of heterogeneous IgG might depend on the transport through FcRn, and the binding of IgG to the FcR might protect IgG from being diluted or degraded in vivo. In addition, the research provided a novel method to evaluate the immunoglobulin additives in passive immunity milk products.
Additional information
Funding
References
- Abdiche, Y., Malashock, D., Pinkerton, A., & Pons, J. (2008). Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the Octet. Analytical Biochemistry, 377, 209–217. doi:10.1016/j.ab.2008.03.035
- Alisky, J. (2009). Bovine and human-derived passive immunization could help slow a future avian influenza pandemic. Medical Hypotheses, 72(1), 74–75. doi:10.1016/j.mehy.2008.08.016
- Baker, K., Qiao, S. W., Kuo, T., Kobayashi, K., Yoshida, M., Lencer, W. I., & Blumberg, R. S. (2009). Immune and non-immune functions of the (not so) neonatal Fc receptor, FcRn. Seminars in Immunopathology, 31, 223–236. doi:10.1007/s00281-009-0160-9
- Brandtzaeg, P. (2007). Induction of secretory immunity and memory at mucosal surfaces. Vaccine, 25, 5467–5484. doi:10.1016/j.vaccine.2006.12.001
- Burmeister, W. P., Huber, A. H., & Bjorkman, P. J. (1994). Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature, 372, 379–383. doi:10.1038/372379a0
- Buss, N. A. P. S., Henderson, S. J., McFarlane, M., Shenton, J. M., & Haan, L. (2012). Monoclonal antibody therapeutics: History and future, monoclonal antibody therapeutics: History and future. Current Opinion in Pharmacology, 12, 615–622. doi:10.1016/j.coph.2012.08.001
- Claypool, S. M., Dickinson, B. L., Wagner, J. S., Johansen, F. E., Venu, N., Borawski, J. A., … Blumberg, R. S. (2004). Bidirectional transepithelial IgG transport by a strongly polarized basolateral Fcgamma-receptor. Molecular Biology of the Cell, 15, 1746–1759. doi:10.1091/mbc.E03-11-0832
- Datta-Mannan, A., Witcher, D. R., Tang, Y., Watkins, J., Jiang, W., & Wroblewski, V. J. (2007). Humanized IgG1 variants with differential binding properties to the neonatal Fc receptor: Relationship to pharmacokinetics in mice and primates. Drug Metabolism and Disposition, 35, 86–94. doi:10.1124/dmd.106.011734
- Holmgren, J., & Czerkinsky, C. (2005). Mucosal immunity and vaccines. Nature Medicine, 11, S45–S53. doi:10.1038/nm1213
- Israel, E. J., Taylor, S., Wu, Z., Mizoguchi, E., Blumberg, R. S., Bhan, A., & Simister, N. E. (1997). Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology, 92(1), 69–74. doi:10.1046/j.1365-2567.1997.00326.x
- Jefferis, R. (2012). Isotype and glycoform selection for antibody therapeutics. Archives of Biochemistry and Biophysics, 526, 159–166. doi:10.1016/j.abb.2012.03.021
- Jones, E. A., & Waldmann, T. A. (1972). The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. Journal of Clinical Investigation, 51, 2916–2927. doi:10.1172/JCI107116
- Junghans, R. P., & Anderson, C. L. (1996). The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proceedings of the National Academy of Sciences of the United States of America, 93, 5512–5516. doi:10.1073/pnas.93.11.5512
- Kacskovics, I., Kis, Z., Mayer, B. A., West, P. J., Tiangco, N. E., Tilahun, M., … Hmmarstrom, L. (2006). FcRn mediates elongated serum half-life of human IgG in cattle. International Immunology, 18, 525–536. doi:10.1093/intimm/dxh393
- Krivoshein, Y. S. (1989). Handbook on microbiology laboratory diagnosis of infectious diseases (p. 319). Moscow: MIR Publishers.
- Ligaarden, S. C., Lydersen, S., & Farup, P. G. (2012). IgG and IgG4 antibodies in subjects with irritable bowel syndrome: A case control study in the general population. BMC Gastroenterology, 12, 166–173. doi:10.1186/1471-230X-12-166
- Martin, W. L., West, A. P., Gan, J. L., & Bjorkman, P. J. (2001). Crystal structure at 2.8 Å of an FcRn/heterodimeric Fc complex: Mechanism of pH-dependent binding. Molecular Cell, 7, 867–877. doi:10.1016/S1097-2765(01)00230-1
- Morimoto, K., Kanda, N., Shinde, S., & Isobe, N. (2012). Effect of enterotoxigenic Escherichia coli vaccine on innate immune function of bovine mammary gland infused with lipopolysaccharide. Journal of Dairy Science, 95, 5067–5074. doi:10.3168/jds.2012-5498
- Morrison, S. L., Johnson, M. J., Herzenberg, L. A., & Oi, V. T. (1984). Chimeric human antibody molecules: Mouse antigen-binding domains with human constant region domains. Proceedings of the National Academy of Sciences of the United States of America, 81, 6851–6855. doi:10.1073/pnas.81.21.6851
- Mowat, A. M. (2003). Anatomical basis of tolerance and immunity to intestinal antigens. Nature Reviews Immunology, 3, 331–341. doi:10.1038/nri1057
- Ober, R. J., Martinez, C., Lai, X., Zhou, J., & Ward, E. S. (2004). Exocytosis of IgG as mediated by the receptor, FcRn: An analysis at the single-molecule level. Proceedings of the National Academy of Sciences of the United States of America, 101, 11076–11081. doi:10.1073/pnas.0402970101
- Rodewald, R. (1976). pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. Journal of Cell Biology, 71, 666–669. doi:10.1083/jcb.71.2.666
- Roopenian, D. C., & Akilesh, S. (2007). FcRn: The neonatal Fc receptor comes of age. Nature Reviews Immunology, 7, 715–725. doi:10.1038/nri2155
- Roopenian, D. C., Christianson, G. J., Sproule, T. J., Brown, A. C., Akilesh, S., Jung, N., … Anderson, C. L. (2003). The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. Journal of Immunology, 170, 3528–3533. doi:10.4049/jimmunol.170.7.3528
- Sekimura, Y., Onodera, N., Banno, M., Hata, I., Hamano, K., Shimosato, T., & Otani, H. (2009). Preparation of goat milk anti-Saccharomyces cerevisiae immunoglobulin G (IgG)-rich fraction and immunological functions of mice orally administered a mixture of the antigen and its specific IgG-rich fraction. Milk Science, 58, 119–128.
- Simister, N. E., & Rees, A. R. (1985). Isolation and characterization of an Fc receptor from neonatal rat small intestine. European Journal of Immunology, 15, 733–738. doi:10.1002/eji.1830150718
- Stern, M., & Herrmann, R. (2005). Overview of monoclonal antibodies in cancer therapy: Present and promise. Critical Reviews in Oncology, 54, 11–29. doi:10.1016/j.critrevonc.2004.10.011
- Taylor, A. I., Fabiane, S. M., Sutton, B. J., & Calvert, R. A. (2009). Isotype and glycoform selection for antibody therapeutics. Biochemistry, 3, 558–562. doi:10.1021/bi8019993
- Tesar, D. B., Tiangco, N. E., & Björkman, P. J. (2006). Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic, 7, 1127–1142. doi:10.1111/j.1600-0854.2006.00457.x
- Walter, L. H., & Peter, K. T. (2011). Perspectives on immunoglobulins in colostrum and milk. Nutrients, 3, 442–474. doi:10.3390/nu3040442
- Ward, E. S., & Ober, R. J. (2009). Multitasking by exploitation of intracellular transport functions the many faces of FcRn. Advances in Immunology, 103, 77–115.
- Ye, L., Tuo, W., Liu, X., Simister, N. E., & Zhu, X. (2008). Identification and characterization of an alternatively spliced variant of the MHC class I-related porcine neonatal Fc receptor for IgG. Developmental & Comparative Immunology, 32, 966–979. doi:10.1016/j.dci.2008.01.008
- Yoshida, M., Masuda, A., Kuo, T. T., Kobayashi, K., Claypool, S. M., Takagawa, T., … Blumberg, R. S. (2006). IgG transport across mucosal barriers by neonatal Fc receptor for IgG and mucosal immunity. Springer Seminars in Immunopathology, 28, 397–403. doi:10.1007/s00281-006-0054-z
