Abstract
Nitric oxide (NO) is synthesised by activated inflammatory macrophages and is involved in the pathogenesis of inflammatory conditions. Dorema kopetdaghense Pimenov (Apiaceae) has been used in traditional medicine from ancient times. In order to investigate anti-inflammatory potential, new kopetdaghins A, C and E were isolated and their non-toxic concentrations, as defined using MTT assay, were evaluated for anti-inflammatory activity. J774A.1 macrophages were stimulated with lipopolysaccharide (1 µg/ml) and kopetdaghins (10–100 μg/ml) for 24 h. The amount of NO production was assessed using Griess reagent. Western blot analysis was employed for evaluating the inducible nitric oxide synthase (iNOS) expression. Kopetdaghins at tested concentrations (10–100 μg/ml) did not reduce cell viability, while they significantly inhibited NO release. The expression of induced iNOS was decreased. Present experiment for the first time revealed the remarkable anti-inflammatory activity of new isolated kopetdaghins from D. kopetdaghense which might be potential candidates for further therapeutic investigations.
Introduction
During the last decades, discovery of endogenous formation of nitric oxide (NO) has led to an explosion in research on NO-induced cellular injury (Abramson, Attur, Amin, & Clancy, Citation2001; Brown, Citation1995). NO that is synthesised from L-arginine in several mammalian cells and tissues is a well-established marker of inflammation. NO plays various physiological roles and may also contribute towards pathological processes. When NO is synthesised in large amounts by activated inflammatory macrophages, it shows cytotoxic properties that may be involved in the pathogenesis of acute and chronic inflammations (Moilanen, Whittle, & Moncada, Citation2003). Supraphysiological amount of NO is produced by inducible nitric oxide synthase (iNOS), which is induced by several stimuli such as bacterial lipopolysaccharide (LPS). During the inflammation, NO production increases remarkably and may show cytotoxic activity. Moreover, the free radical nature of NO makes it as a potent pro-oxidant molecule, which is able to provoke oxidative damage and to be potentially harmful for cellular targets (Epe, Ballmaier, Roussyn, Brivida, & Sies, Citation1996; Kharitonov et al., Citation1994). Therefore, inhibition of NO production in response to inflammatory stimuli might be a valuable therapeutic approach in inflammatory diseases (Hobbs, Higgs, & Moncada, Citation1999; Sautebin, Citation2000).
The genus Dorema (Apiaceae) is represented by seven species in Iranian flora (Mozaffarian, Citation2003, Citation2007). Among them, Dorema kopetdaghense Pimenov and D. ammoniacum D. Don are endemic to Iran and have been collected for their resins in Persia since 4000 years ago (Duthie, Citation1956; Hooper, Citation1937; Jafari, Chahouki, Tavili, Azarnivand, & Amiri, Citation2004;). Moreover, members of the Dorema species possess several biological activities (Asnaashari, Dadizadeh, Talebpour, Eskandani, & Nazemiyeh, Citation2011; Bahraminejad, Citation2012; Nabavi, Nabavi, & Ebrahimzadeh, Citation2012; Prashanth Kumar, Chauhan, Padh, & Rajani, Citation2006; Rajani, Saxena, Ravishankara, Desai, & Padh, Citation2002; Ram, Balachandar, Vijayananth, & Singh, Citation2011; Yousefzadi et al., Citation2011).
D. ammoniacum has been used in folk medicine for the treatment of dermatitis, inflammation, spasm, asthma and bronchitis (Ram et al., Citation2011). It exhibits remarkable antibacterial and antifungal properties that support its folkloric application in the treatment of a number of infectious diseases (Prashanth Kumar et al., Citation2006; Rajani et al., Citation2002). Moreover, the essential oil from its ripe fruits shows in vitro toxicity on human cancer cell lines (Yousefzadi et al., Citation2011). Extracts and derivatives of D. aitchisonii Korovin ex Pimenov show excellent antihaemolytic and antifungal but weak antioxidant activities (Bahraminejad, Citation2012; Nabavi et al., Citation2012). Sesquiterpenes of D. glabrum Fisch. & C.A. Mey. exhibited free radical scavenging activity. Also, based on common folk beliefs, it can treat some disorders especially different cancers (Asnaashari et al., Citation2011; Ibadullayeva, Movsumova, Gasymov, & Mamedli, Citation2001).
There are only a few reports concerning the chemical constituents of plants within the genus Dorema. However, we previously reported the isolation of three new compounds including two prenylated coumarins (kopetdaghins A, C) and a sesquiterpene derivative (kopetdaghin E) from D. kopetdaghense (Iranshahi, Shaki, Mashlab, Porzel, & Wessjohann, Citation2007). In the present study, three kopetdaghins were isolated from the root of D. kopetdaghense and their structures were elucidated using various nuclear magnetic resonance (NMR) and high-resolution positive ion ESIMS techniques. Then, their anti-inflammatory effects were evaluated on LPS-induced NO release by J774A.1 macrophages. Finally, we examined if these compounds diminish the expression of iNOS enzyme.
Materials and methods
Plant material
The plant material (D. kopetdaghense M. Pimen.) was collected in 2007 from Khor valley, Khorasan Razavi province, Iran. It was identified by Mohammad Reza Joharchi, Ferdowsi University of Mashhad Herbarium (FUMH). A voucher specimen (No. 1001) has been deposited at the herbarium of Faculty of Pharmacy, Mashhad University of Medical Sciences (MUMS).
Isolation of kopetdaghins
Isolation of kopetdaghins (A, C and E) from D. kopetdaghense and elucidation of their structures have been done as described in previous paper (Iranshahi et al., Citation2007).
Cell culture
The J774A.1 murine macrophages were obtained from National Cell Bank of Iran (NCBI, Tehran, Iran) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco Laboratories, Detroit, MI) in 95% air and 5% CO2 humidified atmosphere at 37°C.
Determination of cell growth
Cell growth was assessed by the mitochondrial respiration-dependent reduction method of MTT 3-(4,5 dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide to formazan (Emami, Zamani Taghizadeh Rabe, Ahi, Mahmoudi, & Tabasi, Citation2009; Ghazanfari, Zamani Taghizadeh Rabe, Tabasi, & Mahmoudi, Citation2011; Mahmoudi, Zamani Taghizadeh Rabe, Ahi, & Emami, Citation2009; Mosmann, Citation1983; Zamani Taghizadeh Rabe, Mahmoudi, Ahi, & Emami, Citation2011). J774A.1 macrophages were mechanically scraped and seeded 125 × 103 cells/well in a 96-well plate. Then, cells were incubated with increasing concentrations of kopetdaghins (10–100 μg/ml) in 5% CO2 at 37°C. After 24 h of incubation, 25 μl of MTT solution (Sigma Chemical Co, St Louis, MO) was added in each well. After that, the medium was removed and cells were lysed with DMSO to dissolve the formazan crystals produced in viable cells. The optical density of the formazan product in each well was measured with a microplate reader at 545 nm. The cell growth percent was calculated by the following formula: Cell growth (%) = [(ODcontrol – ODtreated)/ODcontrol] ×100.
Measurement of NO production
J774A.1 macrophages were seeded in 24-well plates at 8 × 105 cells/well. After 3 h, cells were treated with various concentrations of kopetdaghins (10–100 μg/ml) and stimulated for 24 h with or without 1 µg/ml of LPS (Serotype 0111:B4, Sigma Chemical Co, St Louis, MO). Nitrite accumulation, as an indicator of NO synthesis, was measured in the culture medium using Griess method (Emami, Zamani Taghizadeh Rabe, Iranshahi, Ahi, & Mahmoudi, Citation2010; Green et al., Citation1982). Briefly, equal amounts of cell culture supernatants were mixed with Griess reagent [equal volumes of 1% (w/v) sulphanilamide in 5% (v/v) phosphoric acid and 0.1% (w/v) naphtylenediamine-HCl] and incubated at room temperature for 20 min. Then the absorbance at 545 nm was measured in a microplate reader. Nitrite concentration (in µM) was calculated from a sodium nitrite standard curve.
Western blot analysis
Treated and untreated cells were lysed in freshly prepared lysis buffer [20 mM HEPES, pH 7.9, 400 mM NaCl, 0.1% Nonidet (N) P-40, 10% glycerol, 1 mM sodium vanadate, 1 mM sodium fluoride, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulphonyl fluoride (PMSF) along with protease inhibitor cocktail] for 45 min on ice. The protein concentrations of cytosolic extracts were determined by Bradford assay. Equal amounts of proteins (30–50 μg/ml) of each cell lysate were dissolved in Laemmli’s sample buffer and subjected to 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Western blotting was performed by transferring proteins from a slab to a sheet of polyvinylidene difluoride (PVDF) membrane at 240 mA for 40 min. The filter was then blocked with 5% skim milk in phosphate buffered saline (PBS) overnight at 4°C. After extensive washing with PBS, the membrane was incubated with anti-mouse iNOS polyclonal antibody (Panomics, Inc, Redwood city, CA) as primary antibody at room temperature for 2 h. Membranes were then incubated with a goat anti-rabbit-horseradish peroxide conjugated antibody (KOMA biotech, Seoul, Korea) as secondary antibody for 1 h at room temperature. β-actin protein was used as housekeeping control. Subsequently, blots were extensively washed with PBS and developed using ECL-detection reagents (Amersham, Cardiff, UK).
Statistical analyses
Data are reported as mean ± SEM values of three independent determinations. All experiments were performed at least three times. Statistical analysis was performed using analysis of variance (ANOVA) test and multiple comparisons were made using Bonferroni’s test. P-values less than 0.05 were considered statistically significant.
Results
Elucidation the structure of kopetdaghins
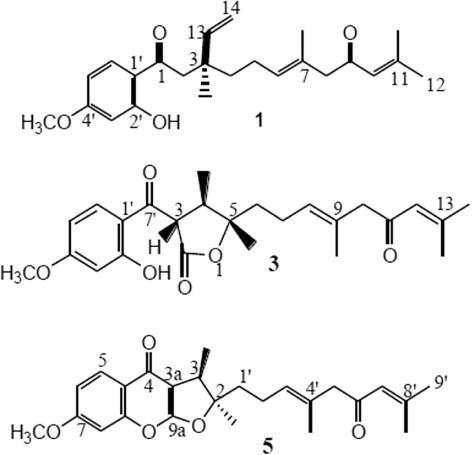
Three new compounds were isolated from D. kopetdaghense. Elucidation of their structures using various techniques revealed that kopetdaghins A and C were prenylated coumarins, but kopetdaghin E was a sesquiterpene derivative ().
Effect of kopetdaghins on cell growth
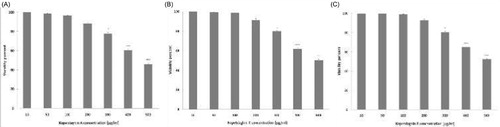
Growth of macrophages treated with kopetdaghins A, C and E (10–100 μg/ml) was measured using MTT colorimetric assay. Exposure to 10–100 μg/ml of kopetdaghins A, C and E for 24 h did not change the growth of J774A.1 macrophages (). These results suggest that isolated kopetdaghins were not toxic for J774A.1 macrophages up to the concentration 100 μg/ml. Therefore, macrophages were treated with kopetdaghins A, C and E in the concentration range of 10–100 μg/ml during follow-up experiments. The concentrations producing 50% growth inhibition (IC50) values of kopetdaghins A, C and E on J774A.1 macrophages were 474.1 ± 0.9, 496.4 ± 0.7 and 514.3 ± 0.4 μg/ml, respectively.
Inhibitory effects of kopetdaghins on LPS-induced NO production
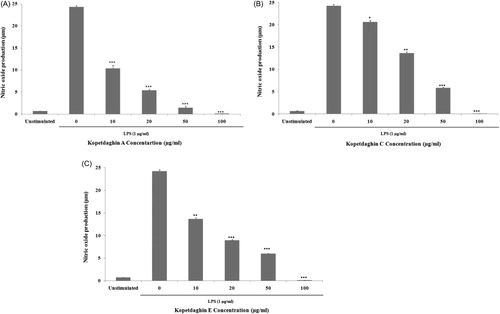
To assess the effects of kopetdaghins A, C and E on LPS-induced NO production by macrophages, cell culture supernatants were assessed for quantitation of their nitrite contents using Griess reagent. A released basal level of NO in unstimulated J774.A1 macrophages was 0.7 ± 0.01 µM, while LPS-stimulation increased NO production (24.2 ± 0.17 µM). However, pre-treatment with kopetdaghins A, C and E ( and , respectively) significantly decreased LPS-induced NO production in a concentration-dependent manner. The inhibitory percent of produced NO after pre-treatment with 10, 20, 50 and 100 μg/ml concentrations of kopetdaghins A, C and E were 57.30 ± 0.66, 77.81 ± 0.16, 94.20 ± 0.38, 100; 14.97 ± 0.32, 43.68 ± 0.28, 75.75 ± 0.12, 100 and 43.59 ± 0.24, 63.09 ± 0.10, 75.37 ± 0.11, 100%, respectively.
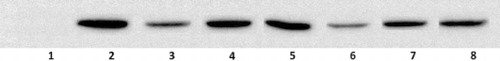
Effects of kopetdaghins on LPS-induced iNOs expression
To determine the anti-inflammatory mechanism of kopetdaghins on NO production as an inflammatory mediator, we evaluated the expression of iNOS using Western blot analysis. As demonstrated in , the experiment showed a concentration-dependent inhibitory activity of kopetdaghins A, C and E (20 and 50 μg/ml) on LPS-stimulated iNOS expression. The inhibitory profile of kopetdaghins examined on iNOS expression overlapped with their inhibitory activity on NO production.
Discussion
Activated macrophages produce a large amount of NO through the action of iNOs. This inflammatory mediator stimulates other immune cells and can cause inflammatory diseases such as rheumatoid arthritis and endotoxemia-induced multiple organ injury (Hattori, Kasai, & Gross, Citation2004; Katsuyama, Shichiri, Marumo, & Hirata, Citation1998; Stuehr, Citation1997). Some anti-inflammatory drugs prevent the development of human acute and chronic inflammatory diseases by suppressing the production of pro-inflammatory mediators including NO. Moreover, inhibition of iNOS with neutralising antibodies or gene targeting considerably alleviates the development and progression of inflammatory diseases (Hattori et al., Citation2004; Katsuyama et al., Citation1998; Stuehr, Citation1997).
Natural products have played a significant role in drug discovery and development for the treatment of several diseases that have been existed from ancient times to the present. Plants contain many phytochemical agents with various bioactivities (Mollazadeh et al., Citation2011; Neshati et al., Citation2009). Numerous experiments have been reported on the effectiveness of isolated natural compounds as anti-inflammatory agents (Maia et al., Citation2009).
The present study was undertaken to elucidate the anti-inflammatory activity of isolated kopetdaghins on the production of inflammatory mediators in vitro. Accordingly, three kopetdaghins (A, C and E) were isolated from D. kopetdaghense. Initially, we determined their toxicity in J774A.1 macrophages and utilised their non-toxic concentrations for further examinations. Subsequently, their anti-inflammatory effects were examined on LPS-induced inflammation in J774A.1 macrophages.
We showed that isolated kopetdaghins (A, C and E) inhibited the production of NO by LPS-stimulated J774A.1 macrophages. Moreover, the expression of iNOS was suppressed in kopetdaghins-treated macrophages. These findings may account for their anti-inflammatory properties.
Conclusions
In summary, we established that isolated kopetdaghins (A, C and E) from D. kopetdaghense possessed anti-inflammatory activity through the inhibition of NO production and iNOS expression in LPS-stimulated macrophages. As a result, this inhibitory effect has important implications for the development of anti-inflammatory drugs and strategies to limit pathological inflammation.
Acknowledgements
This study was supported by Mashhad University of Medical Sciences [grant number 87180].
References
- Abramson, S. B., Attur, M., Amin, A. R., & Clancy, R. (2001). Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Current Rheumatology Reports, 3(6), 535–541. doi:10.1007/s11926-001-0069-3
- Asnaashari, S., Dadizadeh, E., Talebpour, A. H., Eskandani M, & Nazemiyeh H. (2011). Free radical scavenging potential and essential oil composition of the Dorema glabrum Fisch. C.A. Mey roots from Iran. BioImpacts, 1, 241–244.
- Bahraminejad, S. (2012). In vitro and in vivo antifungal activities of Iranian plant species against Pythium aphanidermatum. Annals of Biological Research, 3, 2134–2143.
- Brown, G. C. (1995). Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Letters, 369(2–3), 136–139. doi:10.1016/0014-5793(95)00763-Y
- Duthie, J. F. (1956). The umbelliferae group. British Homoeopathic Journal, 45(2), 77–88. doi:10.1016/S0007-0785(56)80035-5
- Emami, S. A., Zamani Taghizadeh Rabe, S., Ahi, A., Mahmoudi, M., & Tabasi, N. (2009). Study the cytotoxic and pro-apoptotic activity of Artemisia annua extracts. Pharmacologyonline, 3, 1062–1069.
- Emami, S. A., Zamani Taghizadeh Rabe, S., Iranshahi, M., Ahi, A., & Mahmoudi, M. (2010). Sesquiterpene lactone fraction from Artemisia khorassanica inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression through the inactivation of NF-κB. Immunopharmacology and Immunotoxicology, 32(4), 688–695. doi:10.3109/08923971003677808
- Epe, B., Ballmaier, D., Roussyn, I., Brivida, K., & Sies, H. (1996). DNA damage by peroxynitrite characterized with DNA repair enzymes. Nucleic Acids Research, 24(21), 4105–4110. doi:10.1093/nar/24.21.4105
- Ghazanfari, T., Zamani Taghizadeh Rabe, S., Tabasi, N., & Mahmoudi, M. (2011). Study of cytotoxicity and pro-apoptotic effect of medical mushroom Pleurotus florida in cancer cell lines. Pharmacologyonline, 3, 774–783.
- Green, L. C., Wagner, D. A., Glogowski, J., Skipper, P. L., Wishnok, J. S., & Tannenbaum, S. R. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry, 126(1), 131–138. doi:10.1016/0003-2697(82)90118-X
- Hattori, Y., Kasai, K., & Gross, S. S. (2004). NO suppresses while peroxynitrite sustains NF-κB: A paradigm to rationalize cytoprotective and cytotoxic actions attributed to NO. Cardiovascular Research, 63(1), 31–40. doi:10.1016/j.cardiores.2004.03.014
- Hobbs, A. J., Higgs, A., & Moncada, S. (1999). Inhibition of nitric oxide synthase as a potential therapeutic target. Annual Review of Pharmacology and Toxicology, 39(1), 191–220. doi:10.1146/annurev.pharmtox.39.1.191
- Hooper, D. (1937). Useful plants and drugs of Iran and Iraq. Chicago, IL: Field Museum Press.
- Ibadullayeva, S., Movsumova, N., Gasymov, H., & Mamedli, T. (2001). Protection of some rare and endangered vegetable plants in the flora of the Nakhichevan AR. International Journal of Biodiversity and Conservation, 3, 224–229.
- Iranshahi, M., Shaki, F., Mashlab, A., Porzel, A., & Wessjohann, L. A. (2007). Kopetdaghins A-E, sesquiterpene derivatives from the aerial parts and the roots of Dorema kopetdaghense. Journal of Natural Products, 70(8), 1240–1243. doi:10.1021/np070043u
- Jafari, M., Chahouki, M. A. Z., Tavili, A., Azarnivand, H., & Amiri, Gh. Z. (2004). Effective environmental factors in the distribution of vegetation types in Poshtkouh rangelands of Yazd Province (Iran). Journal of Arid Environments, 56(4), 627–641. doi:10.1016/S0140-1963(03)00077-6
- Katsuyama, K., Shichiri, M., Marumo, F., & Hirata, Y. (1998). NO inhibits cytokine-induced iNOS expression and NF-kappa B activation by interfering with phosphorylation and degradation of IkappaB-alpha. Arteriosclerosis, Thrombosis, and Vascular Biology, 18(11), 1796–1802. doi:10.1161/01.ATV.18.11.1796
- Kharitonov, S. A., Yates, D., Robbins, R. A., Barnes, P. J., Logan-Sinclair, R., Shinebourne, E. A., & Barnes, P. J. (1994). Increased nitric oxide in exhaled air of asthmatic patients. Lancet, 343(8890), 133–135. doi:10.1016/S0140-6736(94)90931-8
- Mahmoudi, M., Zamani Taghizadeh Rabe, S., Ahi, A., & Emami, S. A. (2009). Evaluation of the cytotoxic activity of different Artemisia khorasanica samples on cancer cell lines. Pharmacologyonline, 2, 778–786.
- Maia, D. C. G., Benzatti, F. P., Lopes, T. I. B., Gianini, A. S., Brum, R. L., Vilegas, W., dos Santos, L. C., & Honda, N. K. (2009). Lichen metabolites modulate hydrogen peroxide and nitric oxide in mouse macrophages. Zeitschrift für Naturforsch C, 64, 664–672.
- Moilanen, E., Whittle, B., & Moncada, S. (2003). Nitric oxide as a factor in inflammation. In J. I.Gallin & R. Snyderman (Eds.), Inflammation: Basic principles and clinical correlates 1999 (pp. 787–801). Philadelphia: Lippincott, Williams & Wilkins.
- Mollazadeh, S., Matin, M. M., Bahrami, A. R., Iranshahi, M., Behnam-Rassouli, M., Rassouli, F. B., & Neshati, V. (2011). Feselol enhances the cytotoxicity and DNA damage induced by cisplatin in 5637 cells. Zeitschrift für Naturforschung C, 66, 555–561. doi:10.5560/ZNC.2011.66c0555
- Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65(1–2), 55–63. doi:10.1016/0022-1759(83)90303-4
- Mozaffarian, V. (2003). A dictionary of Iranian plant names. Tehran: Farhang Moasser, 56–58.
- Mozaffarian, V. (2007). Flora of Iran, Umbelliferae. Tehran: Research Institute of Forests and Rangelands.
- Nabavi, S. M., Nabavi, S. F., & Ebrahimzadeh, M. A. (2012). Free radical scavenging and antioxidant activities of Dorema aitchisonii. Journal of Food & Drug Analysis, 20, 34–40.
- Neshati, V., Matin, M. M., Iranshahi, M., Bahrami, A. R., Behravan, J., Mollazadeh, S., & Behnam Rassouli, F. (2009). Conferone enhances vincristine cytotoxicity in 5637 cell line. Zeitschrift für Naturforsch C, 64, 317–322.
- Prashanth Kumar, V., Chauhan, N. S., Padh, H., & Rajani, M. (2006). Search for antibacterial and antifungal agents from selected Indian medicinal plants. Journal of Ethnopharmacology, 107(2), 182–188. doi:10.1016/j.jep.2006.03.013
- Rajani, M., Saxena, N., Ravishankara, M. N., Desai, N., & Padh, H. (2002). Evaluation of the antimicrobial activity of Ammoniacum gum from Dorema ammoniacum. Pharmaceutical Biology, 40(7), 534–541. doi:10.1076/phbi.40.7.534.14686
- Ram, A., Balachandar, S., Vijayananth, P., & Singh, V. P. (2011). Medicinal plants useful for treating chronic obstructive pulmonary disease (COPD): Current status and future perspectives. Fitoterapia, 82(2), 141–151. doi:10.1016/j.fitote.2010.09.005
- Sautebin, L. (2000). Prostaglandins and nitric oxide as molecular targets for anti-inflammatory therapy. Fitoterapia, 71, S48–S57. doi:10.1016/S0367-326X(00)00181-7
- Stuehr, D. J. (1997). Structure-function aspects in the nitric oxide synthases. Annual Review of Pharmacology and Toxicology, 37(1), 339–359. doi:10.1146/annurev.pharmtox.37.1.339
- Yousefzadi, M., Heidari, M., Akbarpour, M., Mirjalili, M. H., Zeinali, A., & Parsa, M. (2011). In vitro cytotoxic activity of the essential oil of Dorema ammoniacum D. Don. Middle-East Journal of Scientific Research, 7, 511–514.
- Zamani Taghizadeh Rabe, S., Mahmoudi, M., Ahi, A., & Emami, S. A. (2011). Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharmaceutical Biology, 49(9), 962–969. doi:10.3109/13880209.2011.559251