Abstract
Genetically modified (GM) crops may bring new proteins with immunogenic and allergenic properties into the food and feed chains. The most commonly grown GM maize, MON810, expresses a modified version of the insecticidal Cry1Ab protein originating in the soil bacterium Bacillus thuringiensis (Bt). Immune reactions following inhalation of pollen and debris from such plants have been scarcely studied. We exposed BALB/c mice to purified Cry1Ab proteins and Cry1Ab-containing MON810 plant materials by intranasal installation. No anti-Cry1Ab antibodies were detected following exposure to the plant materials. Exposure to purified Cry1Ab resulted in specific anti-Cry1Ab IgG1 and IgE production, indicating inherent immunogenicity and allergenicity. Mice exposed to leaf extracts from both MON810 and unmodified maize demonstrated influx of lymphocytes and eosinophils in the broncho-alveolar lavage, and increased cytokine release in mediastinal lymph node cells. The results indicate that the airway exposure to Cry1Ab proteins may be a route of practical relevance.
1. Introduction
Genetically modified (GM) crops are created by direct transfer, insertion and ligation into the plant genome of a transgene, coding for a protein that may confer an advantageous trait, e.g. pest insect resistance, to the recipient plant (reviews in Traavik & Lim Li, Citation2007). As many GM crops bring new proteins into the food and feed chains, concerns have been raised about potential adverse effects in terms of toxicity (Domingo & Gine Bordonaba, Citation2011; Hammond, Kough, Herouet-Guicheney, & Jez, Citation2013), impaired nutrition value (EFSA, Citation2008) and undesirable immune responses (Adel-Patient et al., Citation2011; Bernstein et al., Citation2003; EFSA, Citation2010; Finamore et al., Citation2008; Kroghsbo et al., Citation2008; Taylor & Hefle, Citation2001). In predisposed individuals, immunogenic proteins (i.e. proteins that are able to induce specific humoral and/or cellular immune responses) may be capable of stimulating a type-I hypersensitivity reaction through specific immunoglobulin (Ig) E responses, resulting in allergy against the protein (Poulsen & Hummelshoj, Citation2007).
The assessment of potential immunogenicity and/or allergenicity of novel proteins expressed in a GM plant is a challenging task, as no single validated test or type of information exists to predict allergic or other immune responses against them (EFSA, Citation2010; Kimber & Dearman, Citation2002). For ethical reasons, experimental sensitisation to potential allergens cannot be performed in humans, while epidemiological investigations have not been published so far. Current allergenicity assessment strategies, such as the pepsin resistance test and in vitro digestibility tests, the search for sequence and structural similarities between newly expressed proteins and known allergens, as well as in vitro IgE binding tests, have severe limitations (Bernstein et al., Citation2003; Guimaraes et al., Citation2010). Most importantly, in vitro testing cannot completely mimic the complex interaction between the many factors that are involved in the induction of an immune response. The EU scientific guidance document on the assessment of allergenicity of GM plants (EFSA, Citation2010) recognises the weight of evidence approach and case by case evaluation to be the most appropriate way of assessing the allergenicity of GM food and feed. Animal models, in particular, are mentioned as helpful to evaluate the potential of a newly expressed protein to sensitise and/or elicit allergic reactions, and to evaluate the immunogenic and/or adjuvant capacity of these proteins.
The most commonly grown GM maize event, MON810, expresses an insecticidal Cry1Ab protein from a genome-inserted cry1Ab transgene originating in the soil bacterium Bacillus thuringiensis (Bt). The Cry1Ab protein is currently not considered an allergen in part based on the observation that specific IgE responses against Cry1Ab were not detected in exposed food-allergic humans (Nakajima, Teshima, Takagi, Okunuki, & Sawada, Citation2007). Moreover, it has been stated for Cry1Ab protoxin that there is no sequence similarity to known allergens (Randhawa, Singh, & Grover, Citation2011), and the protein is rapidly digested in simulated gastric and intestinal fluids (EFSA, Citation2009). However, sequence similarity, protein source and digestibility alone cannot predict allergenicity. Several studies in mammals and fish have reported effects on the immune system after feeding of MON810-containing diets, including elevated levels of allergic and inflammatory-associated cytokines in serum and altered percentage of B and T cells in the gut (Finamore et al., Citation2008) and a changed proportion of ileal B cells and macrophages (Walsh et al., Citation2011). Assessments of potential Cry1Ab immunogenicity and/or allergenicity have mostly been performed with purified protein isolated from the naturally occurring Bt spore Cry(stal) inclusions, or with a version produced in Escherichia coli that is trypsinised to mimic the activated toxin expressed in GM plants. The many important structural and contextual differences between bacterial and plant Cry1Ab may, however, make extrapolations inadequate, and may have led to irrelevant conclusions. In the Bt bacterium, Cry1Ab is expressed as a protoxin that must be activated by enzymatic cleavage in the lepidopteran gut to obtain its toxicity. The MON810 transgene, on the other hand, encodes only the N-terminal half of the protoxin. Hence it expresses an already activated version of the Cry1Ab toxin, which is probably structurally and immunogenically different from the Bt protoxin (Guerrero, Dean, & Moreno-Fierros, Citation2004; Guerrero, Russell, & Moreno-Fierros, Citation2007; Kitami et al., Citation2011). Furthermore, transgenic insertions into a maize genome may cause a complex recombination event resulting in expression of “new” unanticipated proteins (Rosati, Bogani, Santarlasci, & Buiatti, Citation2008). The different origins of the Cry1Ab protein (bacterium- or plant-expressed), as well as the specific version (protoxin, toxin, size, molecular conformation, etc.) or context (purified bacterial, part of plant cell or tissue), thus prerequisite careful consideration and interpretation.
Although the mucous membranes of the respiratory and gastro-intestinal tracts may be the most important portals of exposure (Kitami et al., Citation2011; Vazquez-Padron et al., Citation2000) little information exist on the potential immune effects after airway exposure to the Cry1Ab proteins. Inhalation of pollen and plant debris may be a realistic exposure route for humans and domestic as well as wild animals in the fields, and also of workers during processing of food and feed. To our knowledge, airway exposure to adequate plant Cry1Ab proteins has not yet been investigated. Purified Cry1A protoxins and trypsin-activated toxins have demonstrated specific humoral as well as cellular anti-Cry1A immune responses after intranasal administration in mice (Guerrero et al., Citation2004, Citation2007). The same authors emphasised that the immune responses after intranasal exposure were unique, and suggested that binding of the lectin motifs of Cry-proteins to the lectin binding receptors in nasal-associated lymphoid tissue might contribute to that (Takata, Ohtani, & Watanabe, Citation2000). The immunological potential of airway exposure to Cry1Ab-containing feed debris was directly illustrated, although unintended, when control fed and Cry1Ab-containing rice fed rats kept in the same room all developed Cry1Ab-specific antibodies (Kroghsbo et al., Citation2008). In humans, Cry1Ab-specific antibodies have been detected after occupational inhalation of Bt-based insecticidal sprayings (Bernstein et al., Citation1999; Doekes, Larsen, Sigsgaard, & Baelum, Citation2004). Studies like these indicate that specific immune responses may be induced after inhalation of Cry1Ab proteins.
Given the expansion of cultivation areas for cry-transgenic maize varieties, the current knowledge gaps concerning immunogenicity and allergenicity of Cry1Ab proteins in general, and inhalation of such proteins in particular, warrant further research. We hypothesised that airway exposure to Cry1Ab-expressing plant materials may induce immunological reactions. Since the origin/version/context of the Cry1Ab protein may give subtle structural modifications and thus affect the immune reactions, we investigated the various versions of the Cry1Ab protein to elicit immune and/or allergic responses in mice after intranasal exposure to (1) Cry1Ab protoxin, (2) trypsinised protoxin Cry1Ab (trypCry1Ab), (3) MON810 pollen and (4) MON810 leaf extract.
2. Materials and methods
2.1. Animals
Five- to six-week old female BALB/c mice (Charles River, Sulzfeld, Germany) were allowed to rest for one week. The mice were randomly allocated to groups (8–10 mice per group) and housed 3–5 animals together in cages with Nestpack bedding (Datesand Ltd, Manchester, UK). Pelleted Teklad Global 18% protein rodent diet 2018 (Harlan Laboratories, Madison, Wisconsin, USA) and tap water were given ad libitum. The mice were exposed to a 12/12 hour light/dark cycle, room temperature (RT) of 21 ± 2°C and 55 ± 10% humidity. The experiments were performed in conformity with laws and regulations for live animals, and approval was given by the North-West University Office of Ethics Committee (Ethical approval reference: NWU-00025-10-A3 Potchefstroom) and by the Norwegian Animal Research Authority under the Ministry of Agriculture (reference FOTS 4050) for Experiments 1 and 2 (described below), respectively.
2.2. Experiments
Experiment 1 was conducted at North-West University (NWU) in Potchefstroom, South Africa, in 2011, while Experiment 2 was performed at the Norwegian Institute of Public Health (NIPH), Oslo in 2012. Although the two experiments were not supposed to be replicates (different origin/versions of the Cry1Ab protein were tested), the experimental conditions were kept as similar as possible (otherwise stated) and performed by the same investigator (M.A.).
2.3. Experimental protocol
The mice were anaesthetised and exposed intranasally (i.n.) to 35 µL of test solutions on days 0, 1 and 2, and booster i.n. on days 21, 22 and 23, and the experiments were terminated on day 26 (). A 100 µl blood sample was collected from vena saphena lateralis prior to the exposures on days 0 and 21. The mice were exsanguinated by heart puncture under anaesthesia on day 26, and blood and 3 × 0.8 mL broncho-alveolar lavage fluid (BALF) were collected as previously described by Samuelsen, Nygaard, and Løvik (Citation2009). BALF samples were kept on ice until processing. In Experiment 2, mediastinal lymph nodes (MLNs) were located according to Van den Broeck, Derore, and Simoens (Citation2006), excised, and single cell suspensions were prepared as described by Hansen, Alberg, Rasmussen, Lovik, and Nygaard (Citation2011).
2.4. Cry1Ab protein
Purified Cry1Ab protoxin isolated from B. thuringiensis spores, prepared in phosphate buffered saline (PBS; pH 7.4) and lyophilised, was purchased from Abraxis via Analytical Solutions (Banbury, South Africa). Trypsinised Cry1Ab (trypCry1Ab) protein was purchased from Case Western Reserve University, (Ohio, USA, Dr Marianne Carey). The origin of the cry1Ab gene inserted in E. coli was the Bt kurstaki HD-1 strain. The inclusion bodies were solubilised at pH 10.5 in the presence of a reducing agent and the precipitated protoxins were digested by commercial bovine trypsin and subsequently purified by ion exchange high-performance liquid chromatography (HPLC). The relevant fractions were analysed by gel filtration, HPLC and SDS-PAGE, desalted and lyophilised (M. Carey, personal communication). The protein preparations were dissolved in a sterile physiological buffer [Hanks Balanced Salt Solution (HBSS) or PBS]. The total doses of protoxin Cry1Ab and trypCry1Ab were 60 and 16.5 µg per mouse, respectively.
2.5. Maize plant material
The maize event MON810 (cultivar DKC78-15B) and its non-GM conventional counterpart (cultivar CRN 3505) were grown under identical conditions in pots in a greenhouse at NWU. The presence of Cry1Ab in MON810 plants was confirmed with the QuickStix kit for Cry1Ab corn leaf and seed (Envirologix, Portland, Maine, USA). On days 0, 1 and 2 of the experiment, individual plant flowers from the two cultivars were carefully enclosed in plastic bags and shaken to dislodge pollen from flowers 2–3 hours before exposure to mice. After sieving twice to remove fragments other than pollen, 5 mg pollen was suspended in 350 µL sterile HBSS less than 1 hour before exposure. Additional pollen was frozen immediately and kept at –80°C until exposures on days 21, 22 and 23. Frozen pollen was thawed in RT for 2–3 hours before exposure to mice and liquefied as described above.
For leaf extract preparation, whole leaves were collected from greenhouse-cultivated MON810 (cultivar 6Q-308Bt) and non-GM (cultivar 6Q-121) maize plants at NWU. The leaves were enclosed in wet paper, sent by airmail to Norway. At arrival, the leaves were cut into 1–2 cm long pieces before manual grinding using a mortar and pestle. HBSS of 10 mL was added to 1 gram of ground leaves and left overnight at 4°C. After centrifugation at 877 g for 5 min, the supernatant was collected and frozen at –18°C until exposure.
The content of plant-expressed Cry1Ab in both pollen suspension and leaf extract was determined using a semi-quantitative enzyme-linked immunosorbent assay (ELISA) kit (Agdia, Elkhart, Indiana, USA) following manufacturer instructions. Standard curves were obtained using progressive dilutions of a purified trypCry1Ab solution, ranging from 0.03–0.96 ng/µL and 3–96 ng/µL for the quantification of Cry1Ab in pollen and leaves, respectively.
2.6. Anaesthetics
At NWU, an initial dose of 1.4 mL of Halothane (SafeLine Pharmaceuticals (Pty) Ltd., South Africa) was added to an inhalation chamber, and the mice were kept individually in the chambers until sedated. A supplementary dose of 0.4 mL of Halothane was then added once the sedative effect declined. At NIPH, 3.5% Isofluran gas (Isoba vet; Intervet/Schering-Plough Animal Health, Lysaker, Norway) was administered in surgical O2 in an inhalation chamber until sedation.
2.7. Intranasal exposure
Each individual mouse was put on its back, as droplets of the test solution were applied with a pipette onto the left and right nostril repeatedly until the complete volume of 35 µL was taken up.
2.8. Detection of endotoxin in test solutions
Endotoxin levels were measured in the pollen suspension (0.131 EU/mL in both MON810 and non-GM), leaf extracts (0.130 and 0.129 EU/mL for MON810 and non-GM, respectively) and the trypCry1Ab solution (0.046 EU/mL) using ToxinSensor TM Chromogenic LAL endotoxin Assay kit (GenScript, Piscataway, New Jersey, USA) according to the manufacturers' description.
2.9. Detection of Cry1Ab-specific antibodies
In-house ELISA protocols were established to detect specific anti-Cry1Ab IgG1, IgG2a and IgE in mouse sera. For the IgG1 assay, microtiter 96-well plates (Maxisorp, Nunc-Immuno Plate, Thermo Fisher Scientific, Roskilde, Denmark) with 96 wells were coated with 2ng/µL purified trypCry1Ab protein per well and incubated for 1 hour at RT and then overnight at 4°C. Plates were washed with 50 mM Tris/HCl buffer pH8 with 0.05% Tween 20 (Tris/Tween) and incubated with blocking solution [5% skimmed milk powder (Fluka Analytical, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) in phosphate buffered saline (PBS)] for 1 hour at RT. After subsequent washing, diluted sera (1:50) were added and plates were incubated for 1 hour at RT and again overnight at 4°C. The plates were washed, 200 ng biotinylated rat anti-mouse IgG1 (Experimental Immunology Unit, University of Louvain, Belgium) was added per well and plates were incubated for 1 hour at RT. After subsequent washing, poly-HRP-streptavidin (Thermo-Scientific, Pierce Biotechnology Inc., Rockford, IL, USA) diluted 1:40,000 was added and incubated for 1 hour at RT. The plates were washed and colour development was obtained by adding stabilised chromogen tetramethylbenzidine (TBM; Invitrogen). The reaction was stopped with 2N H2SO4 solution, after incubation in darkness for a maximum of 15 min.
Considering the small relative amounts of IgE compared to other classes of Igs, competitive displacement of IgE by the latter must be avoided. Therefore, we used anti-mouse IgE antibodies as capture antibody. Microtiter plates with 96 wells were coated with 200 ng rat anti-mouse IgE antibodies (clone LO-ME-3, Experimental Immunology Unit, University of Louvain, Belgium,) per well, incubated for 1 hour at RT and then overnight at 4°C. Subsequently, the plates were washed with Tris/Tween and blocked with 5% skimmed milk in PBS for 1 hour at RT. After subsequent washing, diluted sera (1:10) were added and plates were incubated for 1 hour at RT and then again over night at 4°C. The following day plates were washed, 300 ng trypCry1Ab were added to each well and incubated for 1 hour at RT. After subsequent washing, 300 ng biotinylated rabbit anti-mouse Cry1Ab antibody (Abraxis, Warminster, Pennsylvania, USA) was added per well as detection antibody and plates were incubated for 1 hour at RT. Plates were washed and detection was performed with poly-HRP-streptavidin and stabilised chromogen TBM as described above. The reaction was stopped with 2N H2SO4 solution after incubation in darkness for a maximum of 15 min.
The relative amount of specific IgG2a is apparently small, and a Cry1Ab-specific IgG2a ELISA protocol was established using the same reasoning as for detection of Cry1Ab-specific IgE. Microtiter plates with 96 wells were coated with 200 ng biotinylated rat anti-mouse IgG2a antibodies (clone R19-15, BD Biosciences Pharmingen, San Diego, California, USA) per well, and the protocol for Cry1Ab-specific IgE detection was subsequently followed as described above. To accelerate the reactivity of each step, incubations were performed at 37°C for the detection of specific anti-Cry1Ab IgG2a.
Standard curves were made from duplicates of diluted serum pools from mice immunised with trypCry1Ab and Al(OH)3, and included for all antibody assays on each plate. As the amount of specific IgG1, IgE and IgG2a in the standards is unknown, the levels are presented as arbitrary units. Absorbance was measured at 450 nm on a BioTek Elx808 Absorbance Microplate reader with the Gen5™ Microplate Data Collection and Analysis Software (BioTek® Instruments, Inc., Winooski, Vermont, USA).
2.10. Cytokine analyses
Cytokine levels in BALF and in supernates from MLN cells were determined by cytometric bead array flex set kit from BD Biosciences (San Diego, California, USA). Beads dyed with fluorescence red and covalently coupled to antibodies that capture specific cytokines, allow simultaneous quantification of several cytokines. IL-5, IL-10, IFNγ, TNFα and MCP-1 were measured in BALF from both experiments. IL-10, IFNγ, IL-4, IL-13 and IL-17 were determined in the supernates from MLN cells stimulated with 172 µg/mL trypCry1Ab and incubated at 37°C for 120 hours, in Experiment 2.
2.11. Differential counts
Cells (125,000 cells/mL) from all three BALF collections from each individual mouse were spun down on to cytoslides using a cytocentrifuge (Shandon Scientific Ltd., Astmoor, Runcorn, UK) at 70 g for 6 min, and stained with the Hemacolour rapid staining according to the manufacturer's protocol (Merc KGaA, Darmstadt, Germany). A total number of 200 cells were counted on each slide in a light microscope (Axioplan2, Zeiss, Göttingen, Germany) at 100× magnification, differentiating macrophages, eosinophils, neutrophils, lymphocytes and epithelial cells based on their morphological characteristics. Cell differential counts for all animals were performed blindly by the same investigator (M.A.).
2.12. Statistical analyses
Statistical analyses were performed using SigmaPlot 12.3 and Minitab 16 Statistical software. All normally distributed parameters with comparable variances were tested by one-way analysis of variance. When necessary, data were log10 transformed to obtain normal distribution and comparable variances. In the case of significant overall differences, pair-wise comparisons between treatment and control or between pairs of treatments were performed with the Dunnets post hoc test. Data that could not be transformed to normal distribution and comparable variances were tested with the non-parametric Kruskal-Wallis test, using Mann-Whitney post hoc tests for pair-wise comparisons between treatments versus control or between pairs of treatments. Differences between outcomes were considered significant when p-values were <0.05.
3. Results
3.1. Cry1Ab exposure dose
Based on the measured concentrations (), the calculated total dose of Cry1Ab given per mice exposed to pollen or leaf extracts was 0.02 µg and 12.86 µg, respectively. Non-GM pollen and leaves had no detectable content of Cry1Ab protein.
Table 1. Total exposure doses of Cry1Ab in MON810 pollen and leaves per mouse.
3.2. Serum Cry1Ab-specific antibodies
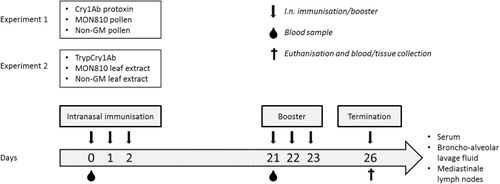
Cry1Ab-specific antibodies were analysed in sera collected prior to the intranasal exposures on days 0 and 21, and at termination on day 26. Cry1Ab-specific antibodies were not detected on day 0. Cry1Ab-specific IgG1 was elevated on day 21 in mice exposed to both Cry1Ab protoxin and trypCry1Ab compared to controls (data not shown). On day 26, mice exposed to Cry1Ab protoxin, but not maize pollen, had significantly higher serum levels of both Cry1Ab-specific IgE and IgG1, and a tendency towards a higher serum level of Cry1Ab-specific IgG2a, compared to the control group (–). Furthermore, mice exposed to trypCry1Ab protein, but not maize leaf extracts, had elevated serum level of Cry1Ab-specific IgG1 and Cry1Ab-specific IgE, on day 26 (–). Cry1Ab-specific IgG2a levels were not determined on days 0 and 21 due to limited amounts of serum, and the levels on day 26 did not significantly differ in mice from either group.
Note: Asterisks (*) denote groups that are significantly different (p < 0.05) from control group exposed to physiological buffer only.
3.3. Cytokine levels in BALF
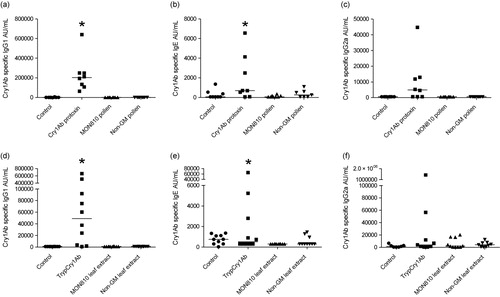
IL-5, IL-17, MCP-1, TNFα and IFNγ cytokine levels determined in BALF collected on day 26 were very low (). Although statistically significant differences between the groups were observed, levels of cytokines in mice exposed to Cry1Ab protoxin or MON810 pollen were not significantly different from those exposed to the non-GM pollen. Cytokine levels in BALF collected on day 26 after exposure of mice to trypCry1Ab and maize leaf extracts were below the detection limit irrespective of the exposure scheme (data not shown).
Note: Asterisks (*) denote groups that are significantly different (p < 0.05) from the control group (CTRL) exposed to physiological buffer only.
3.4. Cell differential counts
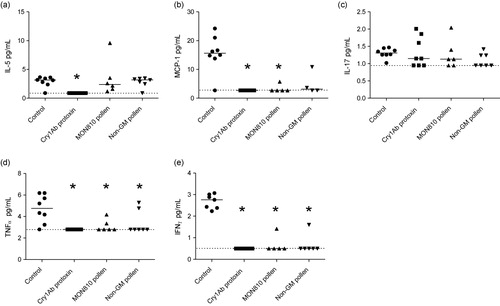
The percentage of neutrophils, macrophages, lymphocytes and eosinophils was determined in BALF from mice exposed to trypCry1Ab and maize leaf extracts. Mice exposed to MON810 leaf extract had a significantly higher percentage of lymphocytes and eosinophils, and significantly lower percentage of macrophages, than mice in the control group (). However, these numbers were not significantly different from what was observed in mice exposed to the non-GM leaf extract. The percentage of neutrophils was not different between any groups of mice irrespective of exposure scheme.
Note: Asterisks (*) denote groups that are significantly different (p < 0.05) from the control group exposed to physiological buffer only.
3.5. Ex vivo cytokine secretion from MLN cells
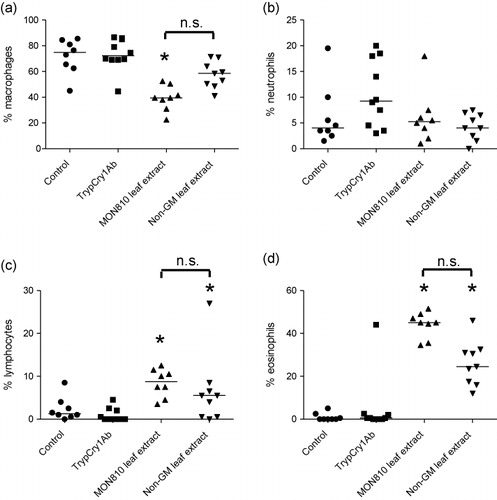
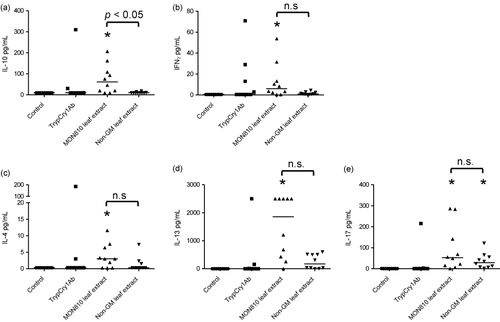
Cytokines were measured in supernates from MLN cells collected on day 26 and stimulated with trypCry1Ab. IL-10 was significantly elevated in mice exposed to MON810 leaf extract compared to mice exposed to non-GM extract, and compared to control mice (). Furthermore, mice exposed to MON810 leaf extract had significantly higher levels of IL-4, IL-13, IFNγ and IL-17 compared to mice in the PBS-control group, however the levels were not significantly different from mice exposed to non-GM leaf extract ().
Note: Asterisks (*) denote groups that are significantly different (p < 0.05) from the control group exposed to physiological buffer only.
4. Discussion
The MON810 plant material did not elicit humoral immune responses in mice after airway exposure. However, the production of specific IgG1 against the two purified protein versions indicates that Cry1Ab protein as such is capable of provoking immunogenic responses, and the production of specific IgE further indicates the capability of Cry1Ab to induce pro-allergic responses, in mammals. In the field, the airway exposure of Cry1Ab proteins (e.g. trough pollen and dust) is a highly relevant route of exposure and our results therefore warrant further studies. Our findings are in agreement with previous investigations showing immunogenic responses after Cry1Ab administration through other routes. For example, Cry1Ab-specific IgG, IgE and IgG2a antibody responses were detected in BALB/c mice immunised intraperitoneally (i.p.) with the protoxin Cry1Ab, however this was not the case after intragastric (i.g.) administration (Adel-Patient et al., Citation2011). The authors did not, like us, detect specific antibody responses after immunisation with a protein extract from MON810 maize, via neither the i.p. nor the i.g. route. On the other hand, our results are only partly in line with the findings of Guerrero et al. (Citation2004). While an i.p. immunisation with a total dose of 150 µg purified protoxin Cry1Ab (produced in recombinant E.coli; 133 kDa) resulted in a strong specific IgG antibody response in BALB/c mice, i.n. immunisations with the same dose gave no such response. Furthermore, 150 µg of toxin Cry1Ab (trypsinised protoxin; 70 kDa) induced a strong specific IgG response after both the i.p. and i.n. immunisation route.
The precise measurement of Cry1Ab concentrations in plant material has been proven to be a difficult task, with significant inter-laboratory differences (Székács et al., Citation2012) and huge variations in concentrations reported and methods used. Furthermore the expression levels of Cry1Ab as well as the whole proteome of a given GM plant may vary considerably when it is grown under different environmental conditions (Agapito-Tenfen, Guerra, Wikmark, & Nodari, Citation2013). The calculated Cry1Ab content in our plant material was based on values form an adjusted semi-quantitative assay and is thus not absolutely, but reflects a relative difference in the Cry1Ab content in different parts of the plant, suggesting that leaves contain approximately 90 times more Cry1Ab than pollen. Nevertheless, mice exposed to both plant materials received a considerably lower dose of Cry-proteins compared to those exposed to purified protein solutions, which could, at least in part, explain the lack of humoral responses against the plant Cry1Ab. On the other hand, immunisation with low doses of allergens has been shown to induce higher IgE production than high doses (Arps, Sudowe, & Kölsch, Citation1998; Hansen et al., Citation2011), i.e. not supporting a simple positive dose–response relationship. This was also the case in a study by Adel-Patient et al. (Citation2011), where 1 µg protoxin Cry1Ab induced a specific IgE response, while the dose of 100 µg did not. In addition, structural differences between the Cry1Ab proteins we used in our four treatments may be accountable for the difference in immune responses. The trypsin activation of the protein produced in E. coli is supposed to give a Cry1Ab that is similar to the activated version expressed in plants, although the degree of similarity has not been confirmed. Also post-translational differences could explain the difference in immune response between purified protein and plant material preparations, as suggested by Prescott et al. (Citation2005). It was demonstrated in a mouse model that transgenic expression of the bean alpha-amylase inhibitor-1 (αAI) in peas led to the synthesis of a protein variant with altered immunogenicity. However, these findings could not be reproduced by Lee et al. (Citation2013). Whether the dissimilar responses to plant and purified protein preparations are due to discrepancy in doses or structural differences can however not be answered by the present study.
To our knowledge, a Cry1Ab-specific IgE response has not previously been demonstrated after airway exposure to Cry1Ab proteins. Since the amount of specific IgE antibodies in general are relatively low compared to other classes of immunoglobulins, and thus vulnerable to be outcompeted by immunoglobulins in abundance (Ladics et al., Citation2010), our sandwich ELISA design trapped Cry1Ab-specific IgE on the well surface by coating microtiter plates with anti-IgE monoclonal antibodies, before incubating with trypCry1Ab protein and biotinylated anti-Cry1Ab as the detection antibody. The use of the trypsinised version of the Cry1Ab protein in the assay could theoretically result in lack of detection of the fraction of specific antibodies towards epitopes present on the protoxin or the plant version. However, previous studies suggest that most epitopes are on the N-terminal present on both protoxin and trypsinised versions of Cry1Ab (Guerrero et al., Citation2004), and our data demonstrate the presence of Cry-specific IgE responses in animals treated with both protein versions.
It should be noted that the clinical relevance of the detected specific IgE was not evaluated. The inbred BALB/c is an “allergy prone” mouse strain that easily adopt the allergy-associated Th2 response pathway. The strain has been employed in previous allergy models and usually requires an adjuvant to elicit an allergic response (Nygaard, Aase, & Løvik, Citation2005). Here, a clear specific IgE response against both protoxin and trypsinised Cry1Ab was elicited even without an adjuvant, suggesting that genetically predisposed (or atopic) subjects may develop Cry1Ab IgE antibody response after airway exposure. Furthermore it is important to recognise that the model is optimised to induce allergy as the protocol with three consecutive immunisations and thereafter three booster immunisations is more likely to provoke IgE responses.
Because we observed a specific antibody response against the trypsinised Cry1Ab protein we might also anticipate an effect on cell influx in lungs and cytokine release from MLN cells from mice exposed to the trypCry1Ab protein compared to controls. However, these parameters were not significantly altered by trypCry1Ab. Interestingly, a significant influx of lymphocytes and eosinophils in the BALF was evident in the MON810 leaf extract exposed mice compared to controls, and also the cytokine release of IL10, IFNγ, IL4, IL-13 and IL-17 was higher in MLN cells from the MON810 leaf exposed mice. When MLN cells were stimulated in vitro with the trypCry1Ab protein, cytokine release was induced only in MON810 treated mice, indicating a specific response to the Cry1Ab epitopes, i.e. suggesting immunogenicity of Cry1Ab protein of plant origin. The increase of cytokines both related to Tregs (IL-10), Th2 (IL-4 and IL-13), Th1 (IFNγ) and Th17 (IL-17) cells suggests a general immune stimulation rather than skewing of the immune response in a particular direction. In agreement, the antibody response towards the Cry1Ab induced by protoxin and trypCry1A were both associated with Th1 and Th2 associated antibody classes (IgG2a, and IgE and IgG1, respectively). However, neither the cell influx nor the cytokine levels (with IL-10 as the only exception) were significantly different from the non-GM exposed mice, suggesting that the local immune response was elicited in response to the plant material as such, and not related to the Cry1Ab protein.
It has previously been demonstrated that immunisation dose, as well as sex and age, highly influence the allergy outcome (Hansen et al., Citation2011). The immunogenic and allergenic effects seen in prime aged females (6–11weeks old) included in the present experiment and in males (8–10 weeks old) in Guerrero et al. (Citation2004) indicate that Cry1Ab proteins could affect the mature immune system. Little is known, however, concerning the impact of these proteins on vulnerable groups. Finamore et al. (Citation2008) reported that the immune systems of young and old rats were more affected by Cry1Ab-containing feed, than prime aged individuals. It would therefore be reasonable to assume that the present findings would be of even greater importance in vulnerable age groups or in hypersensitive individuals.
Although no Cry protein immunogenicity could be observed at the present dose of MON810 pollen or leaf extract, the specific antibody response against the purified Cry1Ab protoxin and toxin preparations demonstrates the principle that these proteins may affect the immune system, i.e. act as immunogens, after intranasal administration. The elicitation of specific IgE antibodies indicates allergenicity of the purified Cry1Ab proteins. However, this was not observed after exposure to (lower doses of) Cry1Ab in plant materials. Notably, the observed immune responses were elicited in the absence of an adjuvant. Given the importance of Bt-transgenic maize as food and feed across the world, a considerable number of individuals may be exposed to Cry1Ab by inhalation, in the field as well as along the food/feed chain. Thus, our results warrant further clarification and testing, both in animal models and in humans, including emphasis on vulnerable age groups and hypersensitive individuals.
Acknowledgements
We are grateful for the help and technical skills of Åse Eikeset, Bodil Hasseltvedt, Berit Stensby, Else-Carin Groeng, Astri Grestad, Hege Hjertholm, Tone Rasmussen, Trude Olsen, Kari Løken and Henrik Rasmussen at Norwegian Institute of Public Health.
Additional information
Funding
References
- Adel-Patient, K., Guimaraes, V. D., Paris, A., Drumare, M.-F., Ah-Leung, S., Lamourette, P., … Wal, J. M. (2011). Immunological and metabolomic impacts of administration of Cry1Ab protein and MON 810 maize in mouse. PLoS ONE, 6(1), e16346. doi:10.1371/journal.pone.0016346.t001
- Agapito-Tenfen, S. Z., Guerra, M. P., Wikmark, O. G., & Nodari, R. O. (2013). Comparative proteomic analysis of genetically modified maize grown under different agroecosystems conditions in Brazil. Proteome Science, 11(1), 46. doi:10.1021/ac950914h
- Arps, V., Sudowe, S., & Kölsch, E. (1998). Antigen dose-dependent differences in IgE antibody production are not due to polarization towards Th1 and Th2 cell subsets. European Journal of Immunology, 28, 681–686. doi:10.1002/(SICI)1521-4141(199802)28:02<681::AID-IMMU681>3.0.CO;2-A
- Bernstein, I. L., Bernstein, J. A., Miller, M., Tierzieva, S., Bernstein, D. I., Lummus, Z., … Seligy, V. L. (1999). Immune responses in farm workers after exposure to Bacillus thuringiensis pesticides. Environmental Health Perspectives, 107, 575–582. doi:10.1289/ehp.99107575
- Bernstein, J. A., Bernstein, I. L., Bucchini, L., Goldman, L. R., Hamilton, R. G., Lehrer, S., … Sampson, H. A. (2003). Clinical and laboratory investigation of allergy to genetically modified foods. Environmental Health Perspectives, 111, 1114–1121.
- Doekes, G., Larsen, P., Sigsgaard, T., & Baelum, J. (2004). IgE sensitization to bacterial and fungal biopesticides in a cohort of Danish greenhouse workers: The BIOGART study. American Journal of Industrial Medicine, 46, 404–407. doi:10.1002/ajim.20086
- Domingo, J. L., & Giné Bordonaba, J. (2011). A literature review on the safety assessment of genetically modified plants. Environment International, 37, 734–742. doi:10.1016/j.envint.2011.01.003
- EFSA (European Food Safety Authority). (2008). Safety and nutritional assessment of GM plants and derived food and feed: The role of animal feeding trials. Food Chemical Toxicology, 46, S2–S70.
- EFSA (European Food Safety Authority). (2009). Scientific opinion of the panel on genetically modified organisms. Applications (EFSA-GMO-RX-MON810) for renewal of authorisation for the continued marketing of (1) existing food and food ingredients produced from genetically modified insect resistant maize MON810; (2) feed consisting of and/or containing maize MON810, including the use of seed for cultivation; and of (3) food and feed additives, and feed materials produced from maize MON810, all under Regulation (EC) No 1829/2003 from Monsanto. The EFSA Journal, 1149, 1–85.
- EFSA (European Food Safety Authority). (2010). Scientific opinion on the assessment of allergenicity of GM plants and microorganisms and derived food and feed. Panel on genetically modified organisms. The EFSA Journal, 8, 1700.
- Finamore, A., Roselli, M., Britti, S., Monastra, G., Ambra, R., Turrini, A., & Mengheri, E. (2008). Intestinal and peripheral immune response to MON810 maize ingestion in weaning and old mice. Journal of Agricultural and Food Chemistry, 56, 11533–11539. doi:10.1021/jf802059w
- Guerrero, G. G., Dean, D. H., & Moreno-Fierros, L. (2004). Structural implication of the induced immune response by Bacillus thuringiensis Cry proteins: Role of the N-terminal region. Molecular Immunology, 41, 1177–1183. doi:10.1016/j.molimm.2004.06.026
- Guerrero, G. G., Russell, W. M., & Moreno-Fierros, L. (2007). Analysis of the cellular immune response induced by Bacillus thuringiensis Cry1A toxins in mice: Effect of the hydrophobic motif from diphtheria toxin. Molecular Immunology, 44, 1209–1217. doi:10.1016/j.molimm.2006.06.007
- Guimaraes, V., Drumare, M. F., Lereclus, D., Gohar, M., Lamourette, P., Nevers, M. C., … Adel-Patient, K. (2010). In vitro digestion of Cry1Ab proteins and analysis of the impact on their immunoreactivity. Journal of Agricultural and Food Chemistry, 58, 3222–3231.
- Hammond, B., Kough, J., Herouet-Guicheney, C., & Jez, J. M. (2013). Toxicological evaluation of proteins introduced into food crops. Critical Reviews in Toxicology, 43(Suppl 2), 25–42.
- Hansen, J. S., Alberg, T., Rasmussen, H., Lovik, M., & Nygaard, U. C. (2011). Determinants of experimental allergic responses: Interactions between allergen dose, sex and age. Scandinavian Journal of Immunology, 73, 554–567. doi:10.1111/j.1365-3083.2011.02529.x
- Kimber, I., & Dearman, R. J. (2002). Approaches to assessment of the allergenic potential of novel proteins in food from genetically modified crops. Toxicological Sciences, 68(1), 4–8. doi:10.1093/toxsci/68.1.4
- Kitami, M., Kadotani, T., Nakanishi, K., Atsumi, S., Higurashi, S., Ishizaka, T., … Sato, R. (2011). Bacillus thuringiensis Cry toxins bound specifically to various proteins via domain III, which had a galactose-binding domain-like fold. Biosci Biotechnol Biochem, 75, 305–312.
- Kroghsbo, S., Madsen, C., Poulsen, M., Schrøder, M., Kvist, P. H., Taylor, M., … Knudsen, I. (2008). Immunotoxicological studies of genetically modified rice expressing PHA-E lectin or Bt toxin in Wistar rats. Toxicology, 245(1–2), 24–34. doi:10.1016/j.tox.2007.12.005
- Ladics, G. S., Knippels, L. M., Penninks, A. H., Bannon, G. A., Goodman, R. E., & Herouet-Guicheney, C. (2010). Review of animal models designed to predict the potential allergenicity of novel proteins in genetically modified crops. Regulatory Toxicology and Pharmacology, 56, 212–224. doi:10.1016/j.yrtph.2009.09.018
- Lee, R. Y., Reiner, D., Dekan, G., Moore, A. E., Higgins, T. J., & Epstein, M. M. (2013). Genetically modified alpha-amylase inhibitor peas are not specifically allergenic in mice. PLoS ONE, 8, e52972.
- Nakajima, O., Teshima, R., Takagi, K., Okunuki, H., & Sawada, J. (2007). ELISA method for monitoring human serum IgE specific for Cry1Ab introduced into genetically modified corn. Regulatory Toxicology and Pharmacology, 47(1), 90–95. doi:10.1016/j.yrtph.2006.08.003
- Nygaard, U. C., Aase, A., & Løvik, M. (2005). The allergy adjuvant effect of particles – genetic factors influence antibody and cytokine responses. BMC Immunology, 6(1), 11. doi:10.1186/1471-2172-6-11
- Poulsen, L. K., & Hummelshoj, L. (2007). Triggers of IgE class switching and allergy development. Annals of Medicine, 39, 440–456. doi:10.1080/07853890701449354
- Prescott, V. E., Campbell, P. M., Moore, A., Mattes, J., Rothenberg, M. E., Foster, P. S., … Hogan, S. P. (2005). Transgenic expression of bean alpha-amylase inhibitor in peas results in altered structure and immunogenicity. Journal of Agricultural and Food Chemistry, 53, 9023–9030. doi:10.1021/jf050594v
- Randhawa, G. J., Singh, M., & Grover, M. (2011). Bioinformatic analysis for allergenicity assessment of Bacillus thuringiensis Cry proteins expressed in insect-resistant food crops. Food and Chemical Toxicology, 49, 356–362. doi:10.1016/j.fct.2010.11.008
- Rosati, A., Bogani, P., Santarlasci, A., & Buiatti, M. (2008). Characterisation of 3' transgene insertion site and derived mRNAs in MON810 YieldGard maize. Plant Molecular Biology, 67, 271–281. doi:10.1007/s11103-008-9315-7
- Samuelsen, M., Nygaard, U. C., & Løvik, M. (2009). Particle size determines activation of the innate immune system in the lung. Scandinavian Journal of Immunology, 69, 421–428. doi:10.1111/j.1365-3083.2009.02244.x
- Székács, A., Weiss, G., Quist, D., Takács, E., Darvas, B., Meier, M., … Hilbeck, A. (2012). Inter-laboratory comparison of Cry1Ab toxin quantification in MON 810 maize by enzyme-immunoassay. Food and Agricultural Immunology, 23(2), 99–121. doi:10.1080/09540105.2011.604773
- Takata, S., Ohtani, O., & Watanabe, Y. (2000). Lectin binding patterns in rat nasal-associated lymphoid tissue (NALT) and the influence of various types of lectin on particle uptake in NALT. Archives of Histology and Cytology, 63, 305–312. doi:10.1679/aohc.63.305
- Taylor, S. L., & Hefle, S. L. (2001). Will genetically modified foods be allergenic? Journal of Allergy and Clinical Immunology, 107, 765–771. doi:10.1067/mai.2001.114241
- Traavik, T., & Lim Li, C. (2007). Biosafety first: Holistic approaches to risk and uncertainty in genetic engineering and genetically modified organisms. Trondheim: Tapir Academic Press.
- Van den Broeck, W., Derore, A., & Simoens, P. (2006). Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. Journal of Immunological Methods, 312, 12–9.
- Vazquez-Padron, R. I., Gonzales-Cabrera, J., Garcia-Tovar, C., Neri-Bazan, L., Lopez-Revilla, R., Hernandez, M., … de la Riva, G. A. (2000). Cry1Ac protoxin from Bacillus thuringiensis sp. kurstaki HD73 binds to surface proteins in the mouse small intestine. Biochemical and Biophysical Research Communications, 271, 54–58.
- Walsh, M. C., Buzoianu, S. G., Gardiner, G. E., Rea, M. C., Gelencsér, E., Jánosi, A., … Lawlor, P. G. (2011). Fate of transgenic DNA from orally administered Bt MON810 maize and effects on immune response and growth in pigs. PLoS ONE, 6(11), e27177. doi:10.1371/journal.pone.0027177.t007
