Abstract
We report the production of a specific monoclonal antibody against Nitrazepam (NZP). First, the hapten 7-aminonitrazepam (7-ANZP) was synthesized, then the hapten was coupled with bovine serum albumin and ovalbumin by the diazotization method, and used as immunogen and coating antigen. Factors affecting binding, ionic strength, pH, and methanol concentration in phosphate-buffered saline, were optimized. The monoclonal antibody had a satisfactory IC50 of 0.2 ng/mL for NZP. The cross-reactivities with other analogs were all lower than 0.1% except for 23% with 7-NZP, which suggested that the enzyme-linked immunosorbent assay method possessed high specificity. The recoveries were in the range of 84–95%, indicating that the method could be applied for the detection of NZP in urine.
Introduction
Benzodiazepines are the most commonly prescribed psychoactive drugs used all over the world. They are often used as anxiolytics, hypnotics, tranquilizers, and anticonvulsants. Nitrazepam (NZP) is one of the most common benzodiazepines and widely used for hypnosis. NZP has a low risk of tolerance and few withdrawal symptoms (Reboul, Citation2013). Some impairments have been reported, for example, the performance of psychomotor tasks (George & Bayliff, Citation2003) and driving, cognitive impairments, and in some patients may increase the risk of respiratory depression (Manikandan, Citation2014; Martinussen & Halvorsen, Citation2013). The abuse of benzodiazepines sometimes may lead to road traffic accidents (Avalos et al., Citation2014; Johnell, Laflamme, Möller, & Monárrez-Espino, Citation2014; Orriols et al., Citation2011) and drug dependence (Chaudhury, Meshram, & Azim, Citation2013; Piccoli et al., Citation2014).
In addition to their use in medical treatment, because of their hypnotic and sedative effects, some feed enterprises add these drugs to their animal feeds. Such supplementation can reduce the cost of animal transportation. A study in the USA found benzodiazepines can promote animal food intake (Baile & McLaughlin, Citation1979). Unreasonable use can lead to the accumulation of metabolites in the body, which may carry potential health hazards for humans.
In announcement No. 176 of the Chinese agriculture ministry (Yue, Wu, Li, & Xu, Citation2009), NZP, Clonazepam, and Diazepam were listed as illegal veterinary drugs, banning them from use in feed of livestock and poultry.
Many instrumental methods have been reported for the detection of benzodiazepines in various biological matrices (serum, plasma, urine, and whole blood) including thin-layer chromatography (Thangadurai, Dhanalakshmi, & Kannan, Citation2013), gas chromatography, high-performance liquid chromatography, gas chromatography/mass spectrometry (GC/MS), high-performance liquid chromatography–mass spectrometry (HPLC-MS), and ultra performance liquid chromatography–tandem mass spectrometry (Harrison & Fu, Citation2014; Szatkowska, Koba, Kośliński, Wandas, & Bączek, Citation2014). These methods all require expensive instruments, take a long time, and require complicated sample preparation. Some immunoassay methods have also been reported (Uddin, Samanidou, & Papadoyannis, Citation2014).
In this paper, we report the development of a specific monoclonal antibody against NZP. The hapten was synthesized by the iron powder reduction method (Yue et al., Citation2009). This hapten was then conjugated to protein to obtain the immunogen and coating antigen. A monoclonal antibody against NZP was obtained, and, after optimizing several factors, a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) was successfully obtained and was found to be suitable for the analysis of urine.
Materials and methods
Chemicals
All chemicals used were of analytical grade. NZP, Clonazepan (CZP), Diazepam (DZP), chlorpromazine (CPZ), reduced iron power, ammonium chloride, sodium nitrite, hydrochloric acid, bovine serum albumin (BSA), ovalbumin (OVA), Freund's complete adjuvant, Freund's incomplete adjuvant, polyethylene-sorbitan-monolaurate (Tween-20), and 3, 3ʹ, 5, 5ʹ-tetramethylbenzidine (TMB) were obtained from Sigma-Aldrich (St Louis, MO).
Buffers and solutions
(1) A stock standard solution of NZP was dissolved in methanol at a concentration of 1 mg/mL, and stored at 4°C; (2) The coating buffer used was 0.05 M carbonate buffer (pH 9.6); (3) Blocking buffer was coating buffer containing 0.2% gelatin (m/v); (4) PBST [Phosphate buffered saline containing Tween-20 (0.5%, v/v)] (0.01 M PBS with 0.05% Tween-20 (v/v)) was used as washing buffer; (5) Substrate buffer consisted of 200 µL 1% (w/v) TMB and 20 µL 6% H2O2 made up to 1 mL; and (6) Stop solution was 2 M H2SO4.
Synthesis and characterization of hapten
The hapten was synthesized by reduction reaction from NZP and iron powder (Yue et al., Citation2009). A mixture of 1 g of Fe and 5 g of NH4Cl in 20 mL of methanol was prepared and 1 N HCl was added until a pH of about 3 was achieved. The mixture was refluxed for 30 minutes at 95°C. Then the solution was cooled to 75°C and 3 g of NZP was dissolved in methanol and added to the refluxing reaction mixture. After the final addition, the reaction mixture was stirred for 5 hours under reflux. The hot mixture was filtered through a separatory funnel. The filtrate was evaporated under reduced pressure, then the residue was redissolved in water and the pH was adjusted to 10 with the addition of 1 N NaOH. The precipitate was collected and dried at 37°C for 12 hours to obtain the hapten 7-NZP. The hapten was confirmed by Ultra Performance Liquid Chromatography tandem Mass Spectrum (UPLC-MS).
Conjugation of hapten
The hapten 7-NZP was conjugated to carrier protein using the diazotization method (Ahn et al., Citation2012; Gao, Zhao, Zhang, Wang, & Wang, Citation2013). A 300 µL aliquot of 1 N HCl was added to a solution of 5 mg of 7-NZP in 300 µL dry dimethylformamide, and stirred for 15 minutes at 4°C. The solution turned a deep red color. Then 30% (w/w) NaNO2 was added to the solution and stirred for 1 hour at the same temperature, upon which the solution became slightly yellow in color. Next, 10 mg BSA or OVA was dissolved in 2 mL 0.1 M coating buffer, and the mixture was added dropwise to the protein solution. The final mixture was stirred for 4 hours at 4°C. Finally, the solution was dialyzed against PBS for two days to remove free 7-NZP. The final solution was confirmed by UV-Vis spectroscopy. These solutions were stored at −20°C.
Preparation of monoclonal antibody
Six- to eight-week-old female BALB/c mice were prepared for subcutaneous injection with the mixture of complete antigen and Freund's adjuvant (Chen et al., Citation2013). The immunization was repeated every four weeks. For the first injection, the antigen was mixed with the same volume of Freund's complete adjuvant to give a dose of 100 µg per mouse. Freund's incomplete adjuvant was used for booster injections with a dose of 50 µg per mouse (Li et al., Citation2008; Xing, Hao, Liu, Xu, & Kuang, Citation2013). Seven to ten days after the third injection, antisera were collected from the mice and tested by ELISA. The mouse with the best inhibition and the highest titer was selected from each group. This mouse then received intraperitoneal injection of a dose of 20 µg 21 days after the last booster injections. The spleen of the selected mouse was removed for hybridoma production, and fused with myeloma cells. After cell fusion, cells were selected by ELISA and subcloned three times. The selected cell line was expanded in culture, injected intraperitoneally to obtain ascites and frozen in liquid nitrogen. After the ascites were purified monoclonal antibodies were obtained (Peng et al., Citation2014; Zhang et al., Citation2013). These antibodies were stored at −20°C.
Development of ELISA
Antibody sensitivity
The ELISA was used to evaluate the properties of the antibody including sensitivity and specificity. The coating antigen NZP-ZD-OVA was diluted with coating solution, then 100 µL of this solution was added to wells of a 96-well microtiter plate and incubated for 12 hours at 4°C. After incubation the plate was washed four times with washing buffer then blocked by adding blocking buffer for 2 hours at 37°C. After washing again as before, the antibody was diluted in PBST and 50 µL was added to each well and incubated for 30 minutes at 37°C. After washing three times, 100 µL goat anti-rabbit IgG-HRP conjugate diluted (1:3000) in PBST was added per well and incubated at 37°C for 30 minutes, then 100 µL of substrate buffer was added per well and again incubated at 37°C for 15 minutes. Stop solution (50 µL/well) was then added and the plate was measured using an ELISA plate reader instrument at 450 nm (Xu et al., Citation2011).
Standard curves were obtained from the B/B0 and the log (concentration of NZP). Sensitivity was evaluated by the IC50 of the antibody. The target of optimization was to achieve better sensitivity (Ren, Zhang, Chen, & Yang, Citation2009).
The optimal concentrations of coating antigen and antibody were assessed using the checker-board titration method (ArunKumar, AbdulBasith, & Gomathinayagam, Citation2012). The effect of ionic strength on the assay was evaluated by varying the NaCl content (0%, 0.4%, 0.8%, 1.6%, and 3.2%). Four different pH values (6, 7.4, 8.6, 9.6) were selected to test the effects of pH.
To produce NZP standards, NZP was dissolved in methanol to produce the initial concentration solution. This was then diluted in PBS with different methanol concentrations (0%, 10%, 20%, and 30%, v/v) to test the effect of methanol in ELISA.
Cross-reactivity (CR)
The specificity of the ELISA depended on the NZP standard, and on other structural analogs, such as 7-NZP, CZP, 7-CZP, DZP, and CPZ. The formula for CR is as follows:
Sample detection
Swine urine free of NZP was used to study the recovery of the tests. The urine was confirmed by LC-MS (Ming & Heathcote, Citation2011), and no residual NZP was detected. Samples of urine from swine were filtered through filter paper (Feng, Wang, Dai, Harmon, & Bernert, Citation2007), then spiked with several different concentrations of NZP and analyzed by ELISA.
Results and discussion
Preparation of hapten
From the chemical structure (), it can be seen that the NZP molecule lacks functional groups (−COOH, −NH2, −OH, −SH) to link the NZP to protein, so it was necessary to prepare an antigen for the next step. In the structure of NZP, the nitryl (−NO2) group was reduced to an amino (−NH2) group through reduction reaction. The hapten obtained can be coupled to protein directly through the amino moiety.
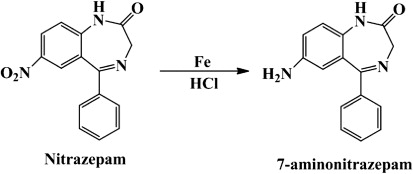
The structure of the hapten was confirmed by HPLC-MS (). On the total ion chromatogram, a strong signal peak could be seen at 2.41 minutes. On the MS chromatogram, a fragment with molecular mass of 252 Da is the predominant fragment. The hapten we obtained had a molecular mass of 252 Dalton, indicating that the synthesis of the hapten of NZP was successful.
Preparation of antigen
The hapten was conjugated to BSA or OVA to obtain the complete antigen through the diazotization method. Throughout the whole reaction, the temperature was a key factor; it should be maintained at 0–4°C. Between these temperatures, the diazonium salt remains stable, while it would be destroyed at temperatures above 4°C. The pH was another important factor affecting the reaction. In the activation phase, pH should be kept at about 3. Once the hapten is coupled to protein, the pH should be kept between 9 and 10.
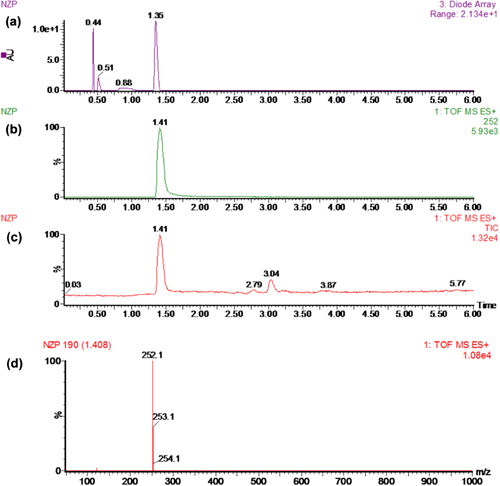
The antigens were confirmed by UV-Vis spectroscopy (), which showed that the hapten has its main UV peaks at 230, 260, and 310 nm, while those of the carrier proteins BSA and OVA were at 210 and 280 nm. It is known that the diazo bond has a characteristic UV absorption peak between 300 and 350 nm. From the UV spectrum of NZP-ZD-BSA, in addition to the main absorbance peaks at 210 and 280 nm, the antigens have another typical peak at 350 nm which is characteristic of the diazo bond. The presence of this peak indicated that the conjugation was successful.
Creation of the coating antigen NZP-ZD-OVA was also successful. The coupling ratio of hapten to protein for NZP-ZD-BSA and NZP-ZD-OVA was 25:1 and 10:1.
Preparation of monoclonal antibody
Monoclonal antibodies were purified from the ascites fluid and tested in microtiter plates. The cell lines 6F10, 5A8, and 5C11 all had approximately the same titer of between 1.5 and 1.8 while 6F10 had the lowest IC50 of 0.2 ng/mL (for both 5A8 and 5C11 the IC50 was 0.6 ng/mL). Consequently, the cell line 6F10 was chosen for further research.
Establishment of ELISA
NZP-ZD-OVA was chosen as the coating antigen and was used for optimization of the ELISA.
The lowest IC50 was obtained from the cell line 6F10. The parameters of ODmax (maximum optical density), IC50, and ODmax/IC50 were considered for optimization of the ELISA.
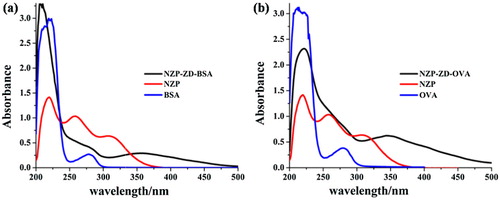
To optimize ionic strength in the assay, different NaCl contents (0%, 0.4%, 0.8%, 1.6%, and 3.2%, g/L) were used in the assay buffer, which was 0.01 M phosphate buffer. As shown in , the ODmax decreased with increasing NaCl content. The IC50 decreased from 0.62ng/mL to 0.38ng/mL as NaCl content increased from 0% to 0.4%, but between the contents of 0.8% and 3.2% the IC50 remained the same. After comprehensive consideration of IC50 and ODmax/IC50, 0.8% NaCl was chosen for subsequent tests.
To evaluate the effect of solvent, four different methanol concentrations (0%, 10%, 20%, and 30%, v/v) were tested. The methanol was dissolved in PBS. As shown in , the ODmax decreased with increasing methanol concentration, while the IC50 increased with increasing methanol concentration. Taking into account ODmax and ODmax/IC50, PBS without methanol was chosen.
To evaluate the effect of pH, four different pH values (6.0, 7.4, 8.6, 9.6) were tested and the results were shown in . PH 9.6 resulted in the lowest IC50 of 0.1 ng/mL, but the titer was below 1.0. This indicated that acidic and alkaline may cause the changes of antibody and the chemical structure, and the further effect on the interaction between antibody and antigen (Crabbe, Van Peteghem, Haasnoot, Kohen, & Salden, Citation1999). To achieve the higher ODmax/IC50 and lower IC50, pH 7.4 was selected for subsequent experiments.
Through studies of these factors, the key optimal conditions were determined: the ionic strength chosen was 0.8%, the pH was 7.4, and PBS without methanol was chosen. Under these optimized conditions, an inhibition curve was obtained, as shown in , and the IC50 was 0.2 ng/mL, the limit of detection was 0.058 ng/mL.
Cross-reactivity
To characterize the specificity of the antibody and the ELISA, several standards were tested for CR. The results are shown in . The derivative of nitrazepam (7-NZP) exhibited CR of 23%. The CR rates of other analogs were all below 0.1%, indicating no CR. Therefore, this ELISA for NZP has high specificity.
Table 1. CR of related compounds.
As shown in , low CR was obtained for the diverse chemical structures. Positions of 1, 3, 5, and 7 were most important for immune recognition. When hydrogen located at positions 1 and 3, the antibody displayed higher affinity. DZP has low CR with NZP due to methyl at position 3. The groups of −NH2 and −Cl at position 7 had no effect on the affinity. The carbon ring at position 5 has weak effect, but it has significant effect as −Cl in the ring.
Analysis of recovery
A urine sample was spiked with three different NZP concentrations (0.3, 0.5, and 1.0 ng/mL), and tested using the ELISA based on the inhibition curve. The results are shown in . The recoveries of NZP in urine ranged from 83.5% to 94.5%. The intraday coefficient of variation (CV) was 5.88%, and interday CV was 9.78%. It indicated that the developed ELISA could be successfully applied for detecting NZP in urine.
Table 2. Recoveries of NZP in swine urine samples.
Conclusion
In this study, a sensitive and specific monoclonal antibody against NZP was successfully developed. The complete antigens NZP-ZD-BSA and NZP-ZD-OVA were selected as immunogen and coating antigen. After the optimization process, the IC50 of the antibody 6F10 was 0.2 ng/mL. The low CR demonstrated that the antibody is highly specific. The good recovery means that the ELISA can be applied to practical detection of NZP in samples of urine. Consequently, this ELISA developed to detect NZP could provide a rapid method for testing in animal food production.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahn, K. C., Kasagami, T., Tsai, H.-J., Schebb, N. H., Ogunyoku, T., Gee, S. J., … Hammock, B. D. (2012). An immunoassay to evaluate human/environmental exposure to the antimicrobial triclocarban. Environmental Science & Technology, 46, 374–381. doi:10.1021/es202494d
- ArunKumar, S., AbdulBasith, S., & Gomathinayagam, S. (2012). A comparative analysis on serum antibody levels of sheep immunized with crude and thiol-purified excretory/secretory antigen of Haemonchus contortus. Veterinary World, 5, 279–284. doi:10.5455/vetworld.2012.279-284
- Avalos, M., Orriols, L., Pouyes, H., Grandvalet, Y., Thiessard, F., & Lagarde, E. (2014). Variable selection on large case-crossover data: Application to a registry-based study of prescription drugs and road traffic crashes. Pharmacoepidemiology and Drug Safety, 23, 140–151. doi:10.1002/pds.3539
- Baile, C. A., & McLaughlin, C. L. (1979). A review of the behavioral and physiological responses to elfazepam, a chemical feed intake stimulant. Journal of Animal Science, 49, 1371.
- Chaudhury, S., Meshram, S., & Azim, M. (2013). Alprazolam dependence: a case report. Pravara Medical Review, 5, 23-25.
- Chen, X., Xu, L., Ma, W., Liu, L., Kuang, H., Wang, L., & Xu, C. (2013). General immunoassay for pyrethroids based on a monoclonal antibody. Food and Agricultural Immunology, 25, 341–349. doi:10.1080/09540105.2013.794328
- Crabbe, P., Van Peteghem, C., Haasnoot, W., Kohen, F., & Salden, M. (1999). Production and characterization of polyclonal antibodies to sulfamethazine and their potential use in immunoaffinity chromatography for urine sample pre-treatment. Analyst, 124, 1569–1575. doi:10.1039/a904732h
- Feng, J., Wang, L., Dai, I., Harmon, T., & Bernert, J. T. (2007). Simultaneous determination of multiple drugs of abuse and relevant metabolites in urine by LC-MS-MS. Journal of analytical toxicology, 31, 359–368. doi:10.1093/jat/31.7.359
- Gao, F., Zhao, G. X., Zhang, H. C., Wang, P., & Wang, J. P. (2013). Production of monoclonal antibody against doxycycline for immunoassay of seven tetracyclines in bovine muscle and milk. Journal of Environmental Science and Health, Part B, 48, 92–100.
- George, C. F. P., & Bayliff, C. D. (2003). Management of insomnia in patients with chronic obstructive pulmonary disease. Drugs, 63, 379–387. doi:10.2165/00003495-200363040-00004
- Harrison, R., & Fu, S. (2014). A review of methodology for testing hair for cocaine. Journal of Forensic Investigation, 2, 8.
- Johnell, K., Laflamme, L., Möller, J., & Monárrez-Espino, J. (2014). The role of marital status in the association between benzodiazepines, psychotropics and injurious road traffic crashes: A register-based nationwide study of senior drivers in Sweden. PloS ONE, 9(1), e86742. doi:10.1371/journal.pone.0086742.t003
- Li, Y., Ji, B., Chen, W., Liu, L., Xu, C., Peng, C., & Wang, L. (2008). Production of new class-specific polyclonal antibody for determination of fluoroquinolones antibiotics by indirect competitive ELISA. Food and Agricultural Immunology, 19, 251–264. doi:10.1080/09540100802471538
- Manikandan, S. (2014). Dexmedetomidine: Its fascination, fad, and facts in neuroanaesthesia practice! Journal of Neuroanaesthesiology and Critical Care, 1, 163–165. doi:10.4103/2348-0548.139091
- Martinussen, P. E., & Halvorsen, T. (2013). The use of benzodiazepines in COPD: A population-based study of Norway. European Respiratory Journal, 42, 1803.
- Ming, D. S., & Heathcote, J. (2011). A rapid and accurate UPLC/MS/MS method for the determination of benzodiazepines in human urine. Journal of Chromatography B, 879, 421–428. doi:10.1016/j.jchromb.2010.12.029
- Orriols, L., Philip, P., Moore, N., Castot, A., Gadegbeku, B., Delorme, B., … Lagarde, E. (2011). Benzodiazepine-like hypnotics and the associated risk of road traffic accidents. Clinical Pharmacology & Therapeutics, 89, 595–601. doi:10.1038/clpt.2011.3
- Peng, J., Meng, X., Deng, X., Zhu, J., Kuang, H., & Xu, C. (2014). Development of a monoclonal antibody-based sandwich ELISA for the detection of ovalbumin in foods. Food and Agricultural Immunology, 25(1), 1–8. doi:10.1080/09540105.2012.716398
- Piccoli, S., Vitali, M., Masri, A., Hijazin, M., Russo, A., Di Prima, S., & Tolio, S. (2014). EPA-1066–Pathway of removal of clonazepam in an Italian prison: Methods and outcome indexes. European Psychiatry, 29, 1.
- Reboul, M. (2013). Observational cross-sectional study of risk-benefit balance of treatment by benzodiazepines and/or antidepressants to patients interrogated by self-questionnaire in pharmacy. Pharmaceutical Sciences. Retrieved from http://hdl.handle.net/10068/889008
- Ren, X., Zhang, F., Chen, F., & Yang, T. (2009). Development of a sensitive monoclonal antibody-based ELISA for the detection of clenbuterol in animal tissues. Food and Agricultural Immunology, 20, 333–344. doi:10.1080/09540100903365852
- Szatkowska, P., Koba, M., Kośliński, P., Wandas, J., & Bączek, T. (2014). Analytical methods for determination of benzodiazepines. A short review. Central European Journal of Chemistry, 12, 994–1007. doi:10.2478/s11532-014-0551-1
- Thangadurai, S., Dhanalakshmi, A., & Kannan, M. V. S. (2013) Separation and detection of certain benzodiazepines by thin-layer chromatography. Malaysian Journal of Forensic Sciences (MJOFS): Mission Statement, 4, 47–53.
- Uddin, M., Samanidou, V., & Papadoyannis, I. (2014). An overview on total analytical methods for the detection of 1, 4-benzodiazepines. Pharmaceutica Analytica Acta, 5, 2.
- Xing, C., Hao, C., Liu, L., Xu, C., & Kuang, H. (2013). A highly sensitive enzyme-linked immunosorbent assay for copper(II) determination in drinking water. Food and Agricultural Immunology, 25, 432–442. doi:10.1080/09540105.2013.821600
- Xu, N., Qu, C., Ma, W., Xu, L., Liu, L., Kuang, H., & Xu, C. (2011). Development and application of one-step ELISA for the detection of neomycin in milk. Food and Agricultural Immunology, 22, 259–269. doi:10.1080/09540105.2011.569882
- Yue, N., Wu, L., Li, L., & Xu, C. (2009). Multi-residue detection of benzodiazepines by ELISA based on class selective antibodies. Food and Agricultural Immunology, 20, 281–293. doi:10.1080/09540100903199475
- Zhang, X., Feng, M., Liu, L., Xing, C., Kuang, H., Peng, C., … Xu, C. (2013). Detection of aflatoxins in tea samples based on a class-specific monoclonal antibody. International Journal of Food Science & Technology, 48, 1269–1274. doi:10.1111/ijfs.12086