Abstract
Humans and animals suffer a health threat to soybean allergy. The impaired mechanism of the intestinal barrier by food/feed allergen glycinin has not been clear. The effects of epithelial metabolic activity, permeability, integrity, and the tight junction (TJ) expression induced by glycinin were conducted using porcine intestinal epithelial cells (IPEC-J2) model in this study. The results showed the decreased metabolic activity (R2 = 0.964, p < 0.001) and the increased alkaline phosphatase activity (R2 = 0.528, p = 0.002) linearly correlated with glycinin levels (0–4 mg/mL). The trans-epithelial electrical resistance values declined stimulated by 4 mg/mL glycinin treatment in a time-dependent manner (0, 6, 12, or 24 h, p = 0.018), and also descended after 12 or 24 h glycinin treatment in dose-dependence (0, 0.5, 1, 2, or 4 mg/mL, p < 0.001). The glycinin-induced TJ expression of claudin-3, occludin, and ZO-1 were reduced (p < 0.05).
1. Introduction
The incidence of food allergy, estimated to be 3–4% in adults and up to 6% in children (Sicherer & Sampson, Citation2006), have generated a major concern of food safety. Babiker et al. demonstrated that at least 21 allergenic proteins in soybean have been identified and bound to IgE (Babiker et al., Citation1998). Soybeans are widely used in food/feed products because of their high nutritional value (Friedman & Brandon, Citation2001). The increasing consumption of various soybean products may lead to a rise in the incidence of soybean allergies. Soybean Glycinin, a major allergenic protein, is a hexamer with a molecular weight about 360 KD consisting of both acidic and basic polypeptide chains linked by a single disulfide bond (Kitamura & Shibasaki, Citation1975). It has negative effects on humans (Foucard & Yman, Citation1999) and animals (Guo, Piao, Cao, Ou, & Li, Citation2008; Sun, Li, Dong, Qiao, Ma, Citation2008; Zhao et al., Citation2008).
Dietary proteins are digested and absorbed primarily within the gastrointestinal tract. Only a small amount of food allergens escape the enzymatic breakdown or suffer the insufficient degradation, then they might be available for intestinal absorption. The gastrointestinal tract, as an interface between the body and the external environment, also acts as a barrier to prevent the entrance of harmful entities including bacteria, endotoxin, toxicity macromolecule, and luminal antigens. Although, the mechanisms of food allergy currently unclear, they are generally thought to sensitize an individual via the gastrointestinal tract (Bannon, Citation2004; Lehrer, Horner, Reese, & Taylor, Citation1996; Mills, Madsen, Shewry, & Wichers, Citation2003). So it is vital to consider the gut permeability for soybean-induced allergy. However, the effects of Glycinin on intestinal barrier permeability are not yet fully elucidated to our knowledge.
The gastrointestinal epithelium is an important contact surface between the organism and its external environment (Fujita, Baba, Shimamoto, Sakuma, & Fujimoto, Citation2007) and prevents food allergen from being absorbed into blood. The destruction of the mucosal barrier function augments the penetration of allergic proteins into the circulation and consequently induces the occurrence of the allergic response (Tlaskalová-Hogenová et al., Citation2002). Therefore, it is of great interest for elucidating the effects of food/feed allergen on the mucosal barrier function.
The tight junction (TJ), a major mode of cell–cell adhesion, plays an important role in regulating permeability. It is mainly composed of transmembrane proteins (occludin, claudin, and junctional adhesion molecules) and peripheral membrane proteins (zonula occludens [ZO]-1, ZO-2, ZO-3, MUPP-1) (Yoshida, Ban, & Kinoshita, Citation2009), whose quantity decide the efficacy of cell–cell adhesion.
This study was developed to provide further information on the intestinal permeability and the TJ protein expression in porcine intestinal epithelial cells (IPEC-J2) treated with Glycinin, which are essential criteria to explore the mechanism of Glycinin-induced allergy.
2. Materials and methods
2.1. Preparation of glycinin
Glycinin was isolated from defatted soy flour according to the method of Setsuko and Fumio (Citation1987). The purified production contains over 95% glycinin determined using Pierce BCA Protein Assay Kit (Thermo, Rockford, America) and SDS-PAGE analysis.
2.2. Cells culture
The IPEC-J2 cells were kindly donated by Dr Guoyao Wu of China Agricultural University. Cells culture conditions were conducted as our previous method (Zhao et al., Citation2014).
2.3. Cellular metabolic activity detected by MTT assay
IPECs were treated with 0, 0.5, 1.0, 2.0, 4.0 mg/ml glycinin media for 24 h, respectively. MTT assay was determined to evaluate the metabolic activity according to our previous procedure (Zhao et al., Citation2014).
2.4. Measurement of trans-epithelial electrical resistance
For trans-epithelial electrical resistance (TEER) measurements, IPECs were seeded at 5 × 104/cm2 in a 24-well polycarbonate Transwell filter insert microplate (Costar, Corning Inc., New York, NY, USA) in membrance 0.3 μm pore size. When monolayer of cells was completely differentiated, cells were treated with 0, 0.5, 1.0, 2.0, 4.0 mg/mL of soybean glycinin. The TEER was measured using the Millicell-ERS resistance system (Millipore, Billerica, MA, USA) after incubating 0, 6, 12, 24 h, respectively. Each well was performed in triplicate. TEER mean values were calculated according to the manufacturer's instructions and expressed as Ω·cm2.
2.5. Alkaline phosphatase activity
Alkaline phosphatase (AP) activity could reflect the cellular membrane integrity. Cells, grown in a 96-well microplate, were treated with 0, 0.5, 1.0, 2.0, 4.0 mg/mL glycinin media for 24 h, respectively. The AP activity of the supernatant was measured using the AP Test kit (Jiancheng, Nanjing, China).
2.6. Expression analysis of TJ proteins
The expression analysis of TJ proteins (occludin, claudin-3, and ZO-1) were assessed by Immunofluorescence and Western blot. IPECs, grown on cover glass within 6-well plates for complete confluence, were treated with 2 mg/mL glycinin media for 24 h for Immunofluorescence. IPEC seeded in cell culture flasks were treated with 0.0 mg/ml and 2 mg/mL of glycinin media for 24 h for Western blot. The methods of detection and analysis were carried out in accordance with our previous report (Zhao et al., Citation2014).
2.7. Statistical analysis
All data were analyzed using the general linear model procedure of Statistical Package for Social Sciences version 11.5 (SPSS Inc., Chicago, IL, USA). Duncan's multiple range tests were employed to test the differences between means. Statements of statistical significance were based upon p ≤ 0.05.
3. Results
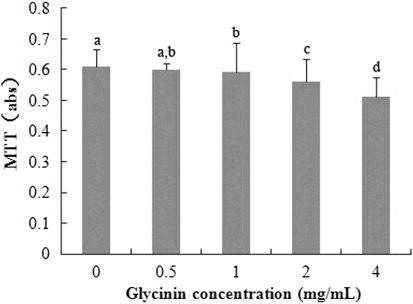
3.1. Cellular metabolic activity
As shown in , the negative correlation exists between MTT and glycinin levels (R2 = 0.964, p < 0.001) from 0–4 mg/mL treatment at 24 h. The maximal reduction of MTT (versus control) was 83.93%.
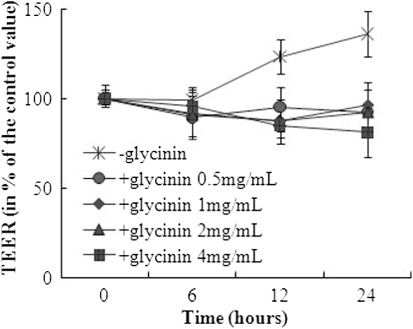
3.2. Trans-epithelial electrical resistance
Four milligram per milliliter of glycinin decreased the TEER after 0, 6, 12 or 24 h incubation in a time-dependent manner (p = 0.018; ). TEER value declined in dose-dependence after 12 or 24 h incubation, respectively (p < 0.001). The highest concentration of glycinin (4 mg/mL) reduced the TEER to 83.4% of the control after 24 h.
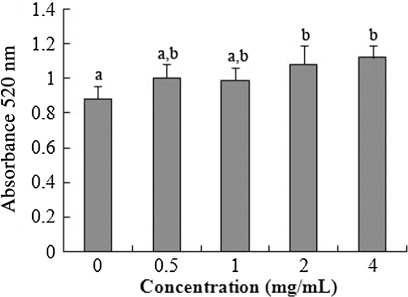
3.3. Cellular integrity assessed by AP activity
shows that AP Activity expanded for 2–4 mg/mL glycinin at 24 h compared with the control. A significant linear increase of AP activity was detected after the incubation of 0–4 mg/mL glycinin (R2 = 0.528, p = 0.002) at 24 h.
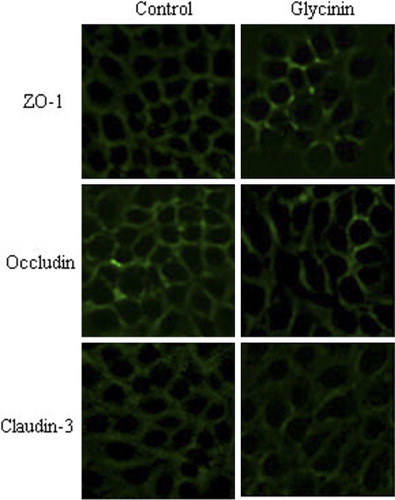
3.4. TJ protein expression
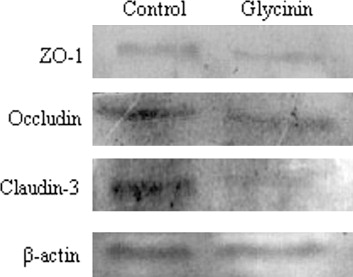
After culturing with glycinin, the immunofluorescence signal of the TJ proteins (occludin, claudin-3, and ZO-1) clearly weakened, and the cellular junction location was obscure (). Seen from , the protein expression of occludin, claudin-3, and ZO-1 were reduced versus control by 77.46%, 76.26%, and 53.17%, respectively (p < 0.05).
4. Discussion
The IPEC-J2 cell line, originally derived from native jejunal epithelia isolated from neonate piglets, was subcultured and used as cell model in the present study. Due to its similarity with the human gut, the swine intestinal cell model represents a good platform for the simulation of various human intestinal applications (Lunney, Citation2007; Nissen, Chingwaru, Sgorbati, Biavati, & Cencic, Citation2009; Pipenbaher et al., Citation2009). Many studies have been conducted using IPEC-J2 cell model to investigate the effects of epithelial barrier function induced by various additives (including soybean allergen β-conglycinin; Diesing et al., Citation2011; Lodemann, Einspanier, Scharfen, Martens, & Bondzio, Citation2013; Zhao et al., Citation2014). This study illustrated the glycinin-induced destruction of the intestinal permeability and TJ, which provides a basis to reveal the mechanism of soybean allergy.
The chances of soybean allergies are greater along with a rise in the consumption of various soybean products (Friedman & Brandon, Citation2001). Soybean protein reduced villus height, and increased crypt depth, and slow the migration rate and turnover time of epithelial cells of the small intestine in pigs as animal models owing to soybean-induced allergy (Dunsford, Knabe, & Hanesly, Citation1989; Li et al., Citation1990; Qiao et al., Citation2003; Qin et al., Citation2002). It has been identified that glycinin could lead to the bad enterocyte apoptosis, proliferation, and migration (Zhao, Qin, Sun, Zhang, & Wang, Citation2010). The above findings show that soybean allergens have severely destroyed the intestine. The intestine, a tubular structure, represents a communication organ between the nutritional environment and the mammalian metabolism. Meanwhile, its mucosa acts as an importantly selective barrier for pathogens and toxins, and participates in the innate immune response (Mariani, Palermo, Fiorentini, Lanubile, & Giuffra, Citation2009; Pitman & Blumberg, Citation2000).
Food allergy such as induced by β-conglycinin and ovalbumin could injure the intestinal epithelium barrier (André, André, Colin, & Cavagna, Citation1987; Perrier & Corthésy, Citation2011; Song et al., Citation2009; Ventura et al., Citation2006). The MTT assay, a standard method for the detection of metabolic activity, indirectly demonstrates the damage of epithelial barrier. The present study displays that there was the negative correlation between the MTT value and glycinin levels, suggesting the reduction of cellular activity with the increasing glycinin. This is accordance with our recent report using β-conglycinin incubation, another major soybean allergen (Zhao et al., Citation2014). Xu, Zhou, Wang, and Ai (Citation2010) using the mouse intestinal epithelial cells as model concluded the same result. In the present study, the intestinal permeability was amplified by glycinin seen from the TEER variation. And the AP activity showed a remarkable increase, indicating the destroyed cellular integrity induced by glycinin, which corresponds to the changed epithelial permeability. The relevant study of β-conglycinin is similar with our findings (Zhao et al., Citation2014). The only difference was the impact degree between the two soybean allergens, and the change was gentler for glycinin than β-conglycinin. The mechanism of imparity is not clear yet. The current study shows that the increasing soybean allergens augment the epithelial permeability after 12 h and 24 h incubation. And the TEER in the treatment of 4 mg/mL glycinin declined notably with the extension of incubation time. The above results indicated the change was serious with the treatment by higher level and longer time.
Various stimuli and diseases, leading to degradation, delocalization, or diminished expression of TJ proteins, can increase the paracellular permeability (Förster, Citation2008). Food allergens initially amplify allergen ingestion and translocation through the transcellular route, and subsequently enlarge the paracellular permeability associated with a disruption of TJ (Berin et al., Citation1998; Groschwitz & Hogan, Citation2009). Relevant studies showed that the reduction of occluding (Yu et al., Citation2005) and ZO-1 (Zhao et al., Citation2011) can enhance the epithelial permeability. A finding in this study is that the glycinin decreased claudin-3, occludin, and ZO-1 in IPEC-J2. The result is consistent with our recent report about β-conglycinin, another important soybean allergen. It also demonstrates that the soybean destroys the epithelial barrier owing to the reduced TJ expression.
5. Conclusion
The soybean glycinin impaired the mechanical barrier of intestinal epithelium deduced by increased permeability and reduced TJ expression.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- André, C., André, F., Colin, N., & Cavagna, S. (1987). Measurement of intestinal permeability to mannitol and lactulose as a means of diagnosing food allergy and evaluating therapeutic effectiveness of sodium cromoglycate. Annals of Allergy, 59, 127–130.
- Babiker, E. f. E., Hiroyuki, A., Matsudomi, N., Iwata, H., Ogawa, T., Bando, N., & Kato, A. (1998). Effect of polysaccharide conjugation or transglutaminase treatment on the allergenicity and functional properties of soy protein. Journal of Agricultural and Food Chemistry, 46, 866–871. doi:10.1021/jf9705072
- Bannon, G. A. (2004). What makes a food protein an allergen? Current Allergy and Asthma Reports, 4(1), 43–46. doi:10.1007/s11882-004-0042-0
- Berin, M. C., Kiliaan, A. J., Yang, P. C., Groot, J. A., Kitamura, Y., & Perdue, M. H. (1998). The influence of mast cells on pathways of transepithelial antigen transport in rat intestine. Journal of Immunology, 161, 2561–2566.
- Diesing, A. K., Nossol, C., Panther, P., Walk, N., Post, A., Kluess, J., … Kahlert, S. (2011). Mycotoxin deoxynivalenol (DON) mediates biphasic cellular response in intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2. Toxicology Letters, 200(1–2), 8–18. doi:10.1016/j.toxlet.2010.10.006
- Dunsford, B. R., Knabe, D. A., & Hanesly, W. E. (1989). Effect of dietary soybean meal on the microscopic anatomy of the small intestine in the early weaned pigs. Journal of Animal Science, 67, 1855–1863.
- Förster, C. (2008). Tight junctions and the modulation of barrier function in disease. Histochemistry and Cell Biology, 130(1), 55–70. doi:10.1007/s00418-008-0424-9
- Foucard, T., & Yman, I. M., (1999). A study on severe food reactions in Sweden—is soy protein an underestimated cause of food anaphylaxis? Allergy, 54, 261–265. doi:10.1034/j.1398-9995.1999.00924.x
- Friedman, M., & Brandon, D. L. 2001. Nutritional and health benefits of soy proteins. Journal of Agricultural and Food Chemistry, 49, 1069–1086. doi:10.1021/jf0009246
- Fujita, M., Baba, R., Shimamoto, M., Sakuma, Y., & Fujimoto, S. (2007). Molecular morphology of the digestive tract; macromolecules and food allergens are transferred intact across the intestinal absorptive cells during the neonatal-suckling period. Medical Molecular Morphology, 40(1), 1–7. doi:10.1007/s00795-006-0346-3
- Groschwitz, K. R., & Hogan, S. P. (2009). Intestinal barrier function: Molecular regulation and disease pathogenesis. Journal of Allergy and Clinical Immunology, 124(1), 3–20. doi:10.1016/j.jaci.2009.05.038
- Guo, P., Piao, X., Cao, Y., Ou, D., & Li, D., (2008). Recombinant soybean protein β-conglycinin α'-subunit expression and induced hypersensitivity reaction in rats. International Archives of Allergy and Immunology, 145(2), 102–110. doi:10.1159/000108135
- Kitamura, K., & Shibasaki, K. (1975). Isolation and some physico-chemical properties of the acidic subunits of soybean 11S globulin. Agricultural and Biological Chemistry, 39, 945–951. doi:10.1271/bbb1961.39.945
- Lehrer, S. B., Horner, W. E., Reese, G., & Taylor, S. (1996). Why are some proteins allergenic? Implications for biotechnology. Critical Reviews in Food Science and Nutrition, 36, 553–564. doi:10.1080/10408399609527739
- Li, D., Nelssen, J. L., Reddy, P. G., Belcha, F., Hancock, J. D., Allee, G. L., & Goodband, R. D. (1990). Transient hypersensitivity to soybean meal in the early-weaned pig. Journal of Animal Science, 68, 1790–1799.
- Lodemann, U., Einspanier, R., Scharfen, F., Martens, H., & Bondzio, A. (2013). Effects of zinc on epithelial barrier properties and viability in a human and a porcine intestinal cell culture model. Toxicology In Vitro, 27, 834–843. doi:10.1016/j.tiv.2012.12.019
- Lunney, J. K. (2007). Advances in swine biomedical model genomics. International Journal of Biological Sciences, 3, 179–184. doi:10.7150/ijbs.3.179
- Mariani, V., Palermo, S., Fiorentini, S., Lanubile, A., & Giuffra, E. (2009). Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-2I. Veterinary Immunology and Immunopathology, 131(3–4), 278–284. doi:10.1016/j.vetimm.2009.04.006
- Mills, E. N. C., Madsen, C., Shewry, P. R., & Wichers, H. J. (2003). Food allergens of plant origin – their molecular and evolutionary relationships. Trends in Food Science & Technology, 14(4), 145–156. doi:10.1016/S0924-2244(03)00026-8
- Nissen, L., Chingwaru, W., Sgorbati, B., Biavati, B., & Cencic, A. (2009). Gut health promoting activity of new putative probiotic/protective Lactobacillus spp. strains: A functional study in the small intestinal cell model. International Journal of Food Microbiology, 135, 288–294. doi:10.1016/j.ijfoodmicro.2009.08.027
- Perrier, C., & Corthésy, B. (2011). Gut permeability and food allergies. Clinical and Experimental Allergy, 41(1), 20–28. doi:10.1111/j.1365-2222.2010.03639.x
- Pipenbaher, N., Moeller, P. L., Weingartl, H., Dolinšek, J., Jakobsen M., Weingartl, H., & Cencič, A. (2009). Nitric oxide (NO) production in mammalian non-tumorigenic epithelial cells of the small intestine and macrophages induced by individual strains of lactobacilli and bifidobacteria. International Dairy Journal, 19, 166–171. doi:10.1016/j.idairyj.2008.09.003
- Pitman, R. S., & Blumberg, R. S. (2000). First line of defense: The role of the intestinal epithelium as an active component of the mucosal immune system. Journal of Gastroenterology, 35, 805–814. doi:10.1007/s005350070017
- Qiao, S., Li, D., Jiang, J., Zhou, H., Li, J., & Thacker, P. (2003). Effects of moist extruded full-fat soybeans on gut morphology and mucosal cell turnover time of weanling pigs. Asian-Australasian Journal of Animal Science, 16(1), 63–69.
- Qin, G. X., Xu, L. M., Jiang, H. L., van der Poel, A. F. B., Bosch, M. W., & Verstegen, M. W. A. (2002). The effect of Chinese and Argentine soybeans on nutrient digestibility and organ morphology in Landrace and Chinese Min pigs. Asian-Australasian Journal of Animal Science, 15, 555–564.
- Setsuko, I., & Fumio, Y. (1987). Determination of glycinin and β-conglycinin in soybean protein by immunological methods. Journal of Agricultural and Food Chemistry, 35, 200–205.
- Sicherer, S., & Sampson, H. (2006). Food allergy. Journal of Allergy and Clinical Immunology, 117, S470–S475. doi:10.1016/j.jaci.2005.05.048
- Song, D. J., Cho, J. Y., Miller, M., Strangman, W., Zhang, M., Varki, A., & Broide, D. H. (2009). Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clinical Immunology, 131, 157–169. doi:10.1016/j.clim.2008.11.009
- Sun, P., Li, D., Dong, B., Qiao, S., & Ma, X., (2008). Effects of soybean glycinin on performance and immune function in early weaned pigs. Archives of Animal Nutrition, 62, 313–321. doi:10.1080/17450390802066419
- Tlaskalová-Hogenová, H., Tucková, L., Lodinová-Zádniková, R., Stepánková, R., Cukrowska, B., Funda, D. P., … Sánchez, D. (2002). Mucosal immunity: Its role in defense and allergy. International Archives of Allergy and Immunology, 128(2), 77–89.
- Ventura, M. T., Polimeno, L., Amoruso, A. C., Gatti, F., Annoscia, E., Marinaro, M., … Francavilla, A. (2006). Intestinal permeability in patients with adverse reactions to food. Digestive and Liver Disease, 38, 732–736. doi:10.1016/j.dld.2006.06.012
- Xu, J., Zhou, A., Wang, Z., & Ai, D. (2010). Effects of glycinin and β-conglycinin on integrity and immune responses of mouse intestinal epithelial cells. Journal of Animal and Plant Science, 20, 170–174.
- Yoshida, Y., Ban, Y., & Kinoshita, S. (2009). Tight junction transmembrane protein claudin subtype expression and distribution in human corneal and conjunctival epithelium. Investigative Ophthalmology & Visual Science, 50, 2103–2108. doi:10.1167/iovs.08-3046
- Yu, A. S., McCarthy, K. M., Francis, S. A., McCormack, J. M., Lai, J., Rogers, R. A., … Schneeberger, E. E. (2005). Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. American Journal of Physiology. Cell Physiology, 288, C1231–C1241.
- Zhao, J., Wang, J., Dong, L., Shi, H., Wang, Z., Ding, H., … Lu, X. (2011). A protease inhibitor against acute stress-induced visceral hypersensitivity and paracellular permeability in rats. European Journal of Pharmacology, 654, 289–294. doi:10.1016/j.ejphar.2010.12.032
- Zhao, Y., Qin, G. X., Sun, Z. W., Zhang, B., & Wang, T. (2010). Effects of glycinin and ß-conglycinin on enterocyte apoptosis, proliferation and migration of piglets. Food and Agricultural Immunology, 21, 209–218. doi:10.1080/09540101003596644
- Zhao, Y., Qin, G., Sun, Z., Zhang, X., Bao, N., Wang, T., … Sun, L. (2008). Disappearance of immunoreactive glycinin and ß-conglycinin in the digestive tract of piglets. Archives of Animal Nutrition, 62, 322–330. doi:10.1080/17450390802190318
- Zhao, Y., Qin, G., Han, R., Wang, J., Zhang, X., & Liu, D. (2014). β-Conglycinin reduces the tight junction occludin and ZO-1 expression in IPEC-J2. International Journal of Molecular Sciences, 15, 1915–1926. doi:10.3390/ijms15021915