Abstract
A non-pretreatment monitoring method based on immunochromatographic test (ICT) strips was developed to test for chloramphenicol (CAP) in raw milk. This assay was designed to measure competitive binding affinities for CAP molecules in raw milk and CAP antigen immobilized on strips with colloidal gold nanoparticles (GNPs, average diameter of 30 nm) labeled with anti-CAP monoclonal antibody (CAP mAb). Several working conditions, including the CAP solvent, different types and concentrations of CAP mAb, coating antigen, and GNP, were optimized for performance. After optimization, the detection process was carried out in 8 min with a visual limit of detection (LOD) of 0.3 ng/mL for qualitative detection and a LOD of 0.064 ng/mL for semi-quantitative detection. No cross-reactivity was detected toward CAP analogs. Based on these results, this simple and ultrasensitive assay could be thoroughly applied in the on-site testing of CAP in raw milk.
Introduction
Previously (Xu, Xu, Ma, Kuang, & Xu, Citation2014), we demonstrated the development and characterization of an ultrasensitive and specific monoclonal antibody against chloramphenicol (CAP). CAP, an antibiotic, has been prohibited in milk, meat, and muscle, and for aquatic use because of its toxic side effects. CAP residues in food are monitored with a minimum required performance limit (MRPL) of 0.3 ng/g. The screening assay described herein should meet the MRPL and be a simple, convenient, and time-saving method.
Apart from instrument-based analysis methods (Berendsen, Zuidema, de Jong, Stolker, & Nielen, Citation2011; Han, Wang, Yu, Yan, & Xie, Citation2011; Han et al., Citation2011; Li, Yang, Zhang, & Li, Citation2014; Taka, Baras, & Bet, Citation2012; Tian, Gao, Zhao, Peng, & Chen, Citation2013) and immunoassays (Tao et al., Citation2014; Zhang, Zhang, Shi, Eremin, & Shen, Citation2006), CAP residues have been detected by alternative immunological (Karaseva & Ermolaeva, Citation2012; Sai et al., Citation2010; Xu et al., Citation2012; Yuan, Oliver, Aguilar, & Wu, Citation2008; Yuan et al., Citation2012) and immunochromatographic (Bai et al., Citation2013; Berlina, Taranova, Zherdev, Vengerov, & Dzantiev, Citation2013; Byzova, Zvereva, Zherdev, Eremin, & Dzantiev, Citation2010; Xia et al., Citation2013) methods. The immunochromatographic test (ICT) analysis methods have not represented a feasible solution for on-site monitoring of food stuffs, as the sensitivity was not sufficiently sensitive or pretreatment was required for the samples ().
Table 1. Comparison of different ICT assays for CAP rapid detection.
To overcome these disadvantages, CAP residues in raw milk samples were detected on ICT strips without pretreatment using the anti-CAP monoclonal antibody (CAP mAb) that we developed. Using optimized working conditions, the sensitivity of the ICT strip was recorded and calculated based on qualitative and semi-quantitative methods.
Materials and methods
Regents and materials
CAP, chloramphenicol succinate (CAP-HS), florfenicol (FFC), thiamphenicol (TAP), and thiamphenicol glycinate hydrochloride (TAP-GH) were purchased from Aladdin Reagent Company (Shanghai, China). CAP antigen and CAP mAb were prepared in our lab (Xu et al., Citation2014). Chloroauric acid (HAuCl4·4 H2O) was obtained from Sigma–Aldrich (St. Louis, MO, USA).
Materials for strip configuration, such as the sample pad (glass fiber membrane), conjugated pad (Ahlstrom 8964), absorbance pad (H5076 filter paper), nitrocellulose membrane (NC membrane), and the backing (polyethylene adhesive card), were purchased from JieYi Biotech (Shanghai, China).
All other reagents were of analytical grades. All implements were washed with ultrapure water that was purified to 18.2 MΩ·cm using a Millipore water filtration unit.
Preparation of colloidal gold nanoparticles
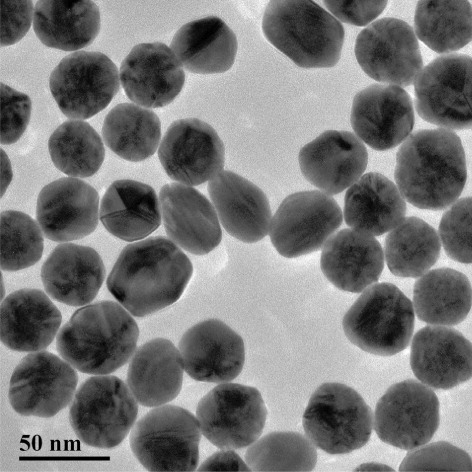
Colloidal gold nanoparticles (GNPs) with average diameters of 30 nm were synthesized based on an established protocol (Bui, Li, Han, Pham, & Seong, Citation2012; Fang, Chen, Li, & Xia, Citation2011; Kuang et al., Citation2011; Lin, Ni, Li, & Kokot, Citation2012; Lou, Ye, Li, & Wu, Citation2012; Yan et al., Citation2012) with some modifications. Briefly, 50 mL 0.01% (m/v) HAuCl4·4H2O solution was stirred while boiling constantly. Then, 1 mL 1% (m/v) freshly prepared sodium citrate solution was added quickly under continuous stirring. The mixture was boiled for another 5 min after the color changed to claret, and then it was cooled to ambient temperature and stored in a refrigerator. Before preparation, glassware was thoroughly soaked in aqua regia (HCl/HNO3 = 3:1, v/v), rinsed with Millipore-Q water and then oven-dried. Transmission electron microscopy (TEM) was used to characterize GNP distribution and size uniformity.
Conjugation of CAP mAb and GNPs
CAP mAb was conjugated to GNPs based on an established method (Chen, Liu, Kuang, Song, & Xu, Citation2013; Sun et al., Citation2012). After modification, the antibody was diluted to 0.15 mg/mL in borate buffer and was added drop-wise into 1 mL GNPs solution. This mixture was adjusted to pH 8.2 with 0.1 M K2CO3 solution and incubated for 4 h at room temperature under constant stirring. Then, the reaction was blocked with 50 μL 10% (m/v) bovine serum albumin (BSA) to eliminate non-specific binding. After 2 h incubation, the mixed solution was centrifuged at 12,000 r/min for 10 min to reduce the influence of excessive reagents; this process was repeated three times. The conjugates were then resuspended in 100 μL phosphate buffer solution (PBS), including 2% (w/v) BSA, 2% (w/v) sucrose, and 0.02% (w/v) sodium azide (Xing et al., Citation2013).
Fabrication of ICT strips
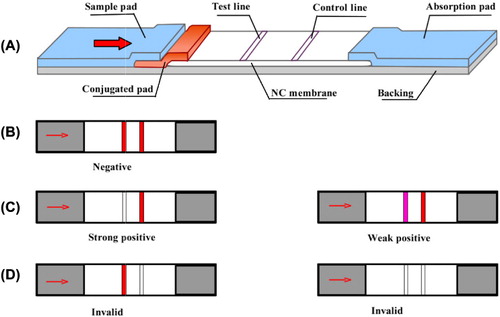
The ICT strips were fabricated as illustrated in (Wang et al., Citation2011). The conjugated pad along with the sample pad, NC membrane and absorption pad were pasted onto the backing card. The conjugated pad loaded with GNP-labeled CAP mAb was incubated at 37°C for 2 h. CAP coating antigen and goat anti-mouse IgG antibody (GAM Ab) flowed along the NC membrane to form test and control zones using a dispensing platform (BioDot Inc., Irvine, CA, USA). Finally, the assembled card was vacuum dried overnight, cut into 3-mm wide strips and stored with a desiccating agent to avoid rehydration.
Measurement of CAP in raw milk
Raw milk was obtained from a local dairy. Milk samples were mixed with different amounts of CAP or CAP analog standards and were analyzed directly by ICT assay at room temperature. The strips were placed vertically into the raw milk sample for 3 min, retrieved and displayed horizontally (Liu, Xing, Yan, Kuang, & Xu, Citation2014; Xing et al., Citation2014; Zhang et al., Citation2013). The visual results were recorded in 3-min intervals based on the color intensity of the test zone. Additionally, the intensity on the tip was also analyzed semi-quantitatively using a BioDot TSR3000 Membrane Strip reader (Gene Company Limited, Shanghai, China). All assays were repeated three times to calculate the calibration curve.
Results and discussion
A monoclonal antibody against chloramphenicol (CAP mAb) was developed and characterized; it had an IC50 value of 0.01 ng/mL and showed no cross-reactivity to CAP analogs, as we reported previously (Xu et al., Citation2014). Using this antibody, we developed a fast and sensitive on-site monitoring assay to test for CAP in raw milk without pretreatment.
Characterization and optimization of the strips
Several ICT conditions, such as concentrations of coating antigen, GAM Ab, and GNP-labeled conjugation, were closely related to the performance of strips and needed to be optimized. To improve the sensitivity of the ICT assay, concentrations of GNPs and CAP mAb were optimized on the conjugated pad.
Previous studies showed that the GNPs that could prevent aggregation and enhance detection sensitivity were between 20 nm and 40 nm in size (Fang et al., Citation2011; Huo, Peng, Xu, & Liu, Citation2006; Lou et al., Citation2012; Tian, Liu, Peng, Chen, & Xu, Citation2009). GNPs that had an average diameter of 30 nm were prepared with a uniform size distribution and dispersion (). CAP mAb at a proper concentration could yield clear coloring of the test and control lines. The stability of GNP-labeled conjugation was pH-dependent; an excessively value could cause irreversible aggregation, while a pH value that was too low could result in insufficient coupling.
The best sensitivity and stability for the ICT method were obtained under the following conditions: 0.8 mg/mL coating antigen, 0.3 mg/mL GAM Ab, 0.15 mg/mL CAP mAb, and 6 nmol/L combined GNPs.
Assessment of the ICT results
An ICT strip was used that had been used commercially for on-site rapid detection. Detection using this strip was based on the principles of competitive reaction of an immobilized antigen on the test zone and the CAP standard against GNP-labeled CAP mAb conjugation.
To start the test, 80 μL sample solution was added, which rapidly flowed laterally and later arrived on the dried conjugated pad (GNP-labeled CAP mAb conjugation). The results were recorded 10 min later. GAM antibody was conjugated on the control zone to confirm the results for validation, regardless of the presence or absence of CAP residues in the sample. If there was no CAP residue in the sample, the GNP-labeled conjugate would bind to the immobilized antigen on the test zone. Thus, the test line would negatively turn red along with the control line (). If the concentration of CAP standard increased, more GNP-labeled conjugates were captured and the red color intensity of the test zone decreased or disappeared ( and ). Three replicates were performed for each sample.
For qualitative detection, the sensitivity and visual limit of detection (LOD) were defined as the concentration of CAP standard at which the intensity on the test zone decreased conspicuously or disappeared completely. For semi-quantitative detection, the sensitivity and LOD were calculated as the concentration of half inhibition (IC50) and 10% inhibition (IC10) based on the calibration curve as measured by the scanning reader.
Sensitivity of the ICT strip in raw milk
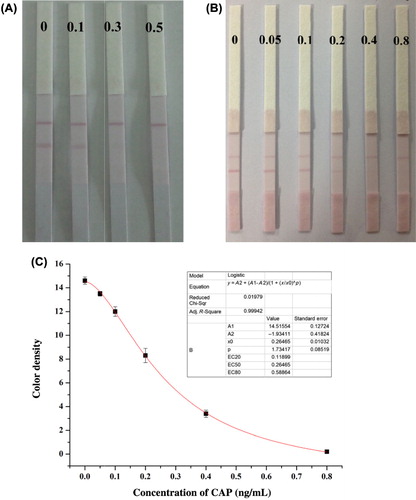
Sensitivity was considered as one of the paramount parameters to estimate the detection ability of developed method in real samples. Raw milk without pretreatment was prepared by adding a gradient dilution of CAP standard (0, 0.1, 0.3, and 0.5 ng/mL) in 0.01 M PBS. Under optimized conditions, results were observed visually (). The intensity in the test zone of the ICT strip declined remarkably at a concentration of 0.1 ng/mL, while the visual LOD was 0.3 ng/mL. A more precise gradient dilution of CAP standard (0, 0.05, 0.1, 0.2, 0.4, and 0.8 ng/mL) and a calibration curve in raw milk were calculated to record the semi-quantitative sensitivity. The IC50 value and LOD were 0.26 and 0.064 ng/mL, respectively ( and ). This result indicated that positive CAP residues in raw milk could be readily discriminated from negative ones at the boundary of the CAP MRPL (0.3 ng/g).
Compared with the indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) method, these results of ICT assay were in great consistence and agreement, showing good reproducibility and accuracy (). Based on our CAP mAb, the ICT assay that we developed was the most sensitive assay available for the on-site monitoring of CAP residues.
Table 2. Spiking results of ic-ELISA and ICT for CAP in milk.
Specificity confirmation by ICT assay
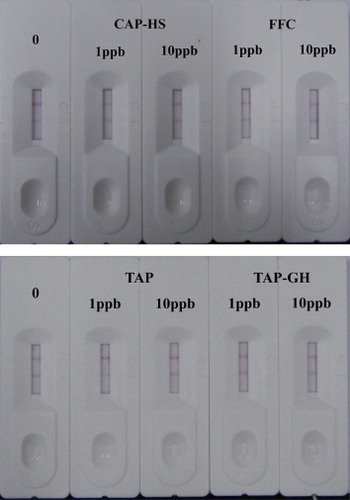
The specific reaction of antigen and antibody varies extensively depending on the particular antigenic epitope. Four CAP analogs (CAP-HS, FFC, TAP, and TAP-GH) were diluted to concentrations of 1 and 10 ng/mL and tested for cross-reactivity to confirm the specificity of CAP mAb using ICT strips. An inconspicuous reduction of color density was even detected at a concentration of 10 ng/mL (), indicating that extraordinarily high specificity against CAP was established, similar to the results of the ic-ELISA.
Conclusions
CAP is an antibiotic for which there is zero-tolerance in food stuffs. Here, we developed a simple and ultrasensitive immunochromatographic assay for CAP detection in raw milk that did not require pretreatment. Ultrasensitive and specific results against CAP analogs were obtained visually or using a reader for 8 min with the test strips. Because of the simplicity, specificity, and sensitivity of this method, it could represent a convenient and competitive assay for the on-site monitoring of CAP in the dairy market.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bai, Z., Luo, Y., Xu, W., Gao, H., Han, P., Liu, T., … Huang, K. (2013). Development of a new fluorescence immunochromatography strip for detection of chloramphenicol residues in chicken muscles. Journal of the Science of Food and Agriculture, 93, 3743–3747. doi:10.1002/jsfa.6232
- Berendsen, B. J. A., Zuidema, T., de Jong, J., Stolker, L. A. M., & Nielen, M. W. F. (2011). Discrimination of eight chloramphenicol isomers by liquid chromatography tandem mass spectrometry in order to investigate the natural occurrence of chloramphenicol. Analytica Chimica Acta, 700(1–2), 78–85. doi:10.1016/j.aca.2010.11.009
- Berlina, A. N., Taranova, N. A., Zherdev, A. V., Vengerov, Y. Y., & Dzantiev, B. B. (2013). Quantum dot-based lateral flow immunoassay for detection of chloramphenicol in milk. Analytical and Bioanalytical Chemistry, 405, 4997–5000. doi:10.1007/s00216-013-6876-3
- Bui, M. P. N., Li, C. A., Han, K. N., Pham, X.-H., & Seong, G. H. (2012). Simultaneous detection of ultratrace lead and copper with gold nanoparticles patterned on carbon nanotube thin film. Analyst, 137, 1888–1894. doi:10.1039/c2an16020j
- Byzova, N. A., Zvereva, E. A., Zherdev, A. V., Eremin, S. A., & Dzantiev, B. B. (2010). Rapid pretreatment-free immunochromatographic assay of chloramphenicol in milk. Talanta, 81, 843–848. doi:10.1016/j.talanta.2010.01.025
- Chen, X., Liu, L., Kuang, H., Song, S., & Xu, C. (2013). A strip-based immunoassay for rapid determination of fenpropathrin. Analytical Methods, 5, 6234–6239. doi:10.1039/c3ay41030g
- Fang, C., Chen, Z., Li, L., & Xia, J. (2011). Barcode lateral flow immunochromatographic strip for prostate acid phosphatase determination. Journal of Pharmaceutical and Biomedical Analysis, 56, 1035–1040. doi:10.1016/j.jpba.2011.08.008
- Han, J. A., Wang, Y., Yu, C. L., Li, C. X., Yan, Y. S., Liu, Y., & Wang, L. A. (2011). Separation, concentration and determination of chloramphenicol in environment and food using an ionic liquid/salt aqueous two-phase flotation system coupled with high-performance liquid chromatography. Analytica Chimica Acta, 685(2), 138–145. doi:10.1016/j.aca.2010.11.033
- Han, J. A., Wang, Y., Yu, C. L., Yan, Y. S., & Xie, X. Q. (2011). Extraction and determination of chloramphenicol in feed water, milk, and honey samples using an ionic liquid/sodium citrate aqueous two-phase system coupled with high-performance liquid chromatography. Analytical and Bioanalytical Chemistry, 399, 1295–1304. doi:10.1007/s00216-010-4376-2
- Huo, T., Peng, C., Xu, C., & Liu, L. (2006). Development of colloidal gold-based immunochromatographic assay for the rapid detection of medroxyprogesterone acetate residues. Food and Agricultural Immunology, 17, 183–190. doi:10.1080/09540100601089895
- Karaseva, N. A., & Ermolaeva, T. N. (2012). A piezoelectric immunosensor for chloramphenicol detection in food. Talanta, 93, 44–48. doi:10.1016/j.talanta.2011.12.047
- Kuang, H., Chen, W., Yan, W., Xu, L., Zhu, Y., Liu, L., … Xu, C. (2011). Crown ether assembly of gold nanoparticles: Melamine sensor. Biosensors & Bioelectronics, 26, 2032–2037. doi:10.1016/j.bios.2010.08.081
- Li, X. Q., Yang, Z., Zhang, Q. H., & Li, H. M. (2014). Evaluation of matrix effect in isotope dilution mass spectrometry based on quantitative analysis of chloramphenicol residues in milk powder. Analytica Chimica Acta, 807, 75–83. doi:10.1016/j.aca.2013.11.017
- Lin, X. Y., Ni, Y. N., Li, S. Z., & Kokot, S. (2012). A novel method for simultaneous analysis of three ß2-agonists in foods with the use of a gold-nanoparticle modified glassy carbon electrode and chemometrics. Analyst, 137, 2086–2094. doi:10.1039/c2an16062e
- Liu, L., Xing, C., Yan, H., Kuang, H., & Xu, C. (2014). Development of an ELISA and immunochromatographic strip for highly sensitive detection of microcystin-LR. Sensors (Basel), 14, 14672–14685. doi:10.3390/s140814672
- Lou, S., Ye, J. Y., Li, K. Q., & Wu, A. G. (2012). A gold nanoparticle-based immunochromatographic assay: The influence of nanoparticulate size. Analyst, 137, 1174–1181. doi:10.1039/c2an15844b
- Sai, N., Chen, Y. P., Liu, N., Yu, G. G., Su, P., Feng, Y., … Ning, B. A. (2010). A sensitive immunoassay based on direct hapten coated format and biotin–streptavidin system for the detection of chloramphenicol. Talanta, 82, 1113–1121. doi:10.1016/j.talanta.2010.06.018
- Sun, F. X., Liu, L. Q., Ma, W. W., Xu, C. L., Wang, L. B., & Kuang, H. (2012). Rapid on-site determination of melamine in raw milk by an immunochromatographic strip. International Journal of Food Science and Technology, 47, 1505–1510. doi:10.1111/j.1365-2621.2012.02998.x
- Taka, T., Baras, M. C., & Bet, Z. F. C. (2012). Validation of a rapid and sensitive routine method for determination of chloramphenicol in honey by LC–MS/MS. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment, 29, 596–601. doi:10.1080/19440049.2011.625047
- Tao, X., Jiang, H., Zhu, J., Wang, X., Wang, Z., Niu, L., … Shen, J. (2014). An ultrasensitive chemiluminescent ELISA for determination of chloramphenicol in milk, milk powder, honey, eggs and chicken muscle. Food and Agricultural Immunology, 25(1), 137–148. doi:10.1080/09540105.2012.753513
- Tian, W., Gao, L., Zhao, Y., Peng, W., & Chen, Z. (2013). Simultaneous determination of metronidazole, chloramphenicol and 10 sulfonamide residues in honey by LC–MS/MS. Analytical Methods, 5, 1283–1288. doi:10.1039/c2ay25998b
- Tian, Z., Liu, L. Q., Peng, C., Chen, Z., & Xu, C. (2009). A new development of measurement of 19-Nortestosterone by combining immunochromatographic strip assay and ImageJ software. Food and Agricultural Immunology, 20(1), 1–10. doi:10.1080/09540100802621017
- Wang, L. B., Ma, W. W., Chen, W., Liu, L. Q., Ma, W., Zhu, Y. Y., … Xu, C. L. (2011). An aptamer-based chromatographic strip assay for sensitive toxin semi-quantitative detection. Biosensors & Bioelectronics, 26, 3059–3062. doi:10.1016/j.bios.2010.11.040
- Xia, X. H., Xu, Y., Ke, R. Q., Zhang, H., Zou, M. Q., Yang, W., & Li, Q. G. (2013). A highly sensitive europium nanoparticle-based lateral flow immunoassay for detection of chloramphenicol residue. Analytical and Bioanalytical Chemistry, 405, 7541–7544. doi:10.1007/s00216-013-7210-9
- Xing, C. R., Feng, M., Hao, C. L., Xu, L. G., Kuang, H., Wang, L. B., & Xu, C. L. (2013). Visual sensor for the detection of trace Cu(II) ions using an immunochromatographic strip. Immunological Investigations, 42, 221–234. doi:10.3109/08820139.2012.752378
- Xing, C., Kuang, H., Hao, C., Liu, L., Wang, L., & Xu, C. (2014). A silver enhanced and sensitive strip sensor for Cadmium detection. Food and Agricultural Immunology, 25, 287–300. doi:10.1080/09540105.2013.781140
- Xu, J., Yin, W. W., Zhang, Y. Y., Yi, J., Meng, M., Wang, Y. B., … Xi, R. M. (2012). Establishment of magnetic beads-based enzyme immunoassay for detection of chloramphenicol in milk. Food Chemistry, 134, 2526–2531. doi:10.1016/j.foodchem.2012.04.083
- Xu, N., Xu, L., Ma, W., Kuang, H., & Xu, C. (2014). Development and characterisation of an ultrasensitive monoclonal antibody for chloramphenicol. Food and Agricultural Immunology. Advance online publication. doi:10.1080/09540105.2014.950201
- Yan, W. J., Xu, L. G., Xu, C. L., Ma, W., Kuang, H., Wang, L. B., & Kotov, N. A. (2012). Self-assembly of chiral nanoparticle pyramids with strong R/S optical activity. Journal of the American Chemical Society, 134, 15114–15121. doi:10.1021/ja3066336
- Yuan, J., Oliver, R., Aguilar, M.-I., & Wu, Y. (2008). Surface plasmon resonance assay for chloramphenicol. Analytical Chemistry, 80, 8329–8333. doi:10.1021/ac801301p
- Yuan, M., Sheng, W., Zhang, Y., Wang, J., Yang, Y., Zhang, S., … Wang, S. (2012). A gel-based visual immunoassay for non-instrumental detection of chloramphenicol in food samples. Analytica Chimica Acta, 751, 128–134. doi:10.1016/j.aca.2012.08.044
- Zhang, S., Zhang, Z., Shi, W., Eremin, S. A., & Shen, J. (2006). Development of a chemiluminescent ELISA for determining chloramphenicol in chicken muscle. Journal of Agricultural and Food Chemistry, 54, 5718–5722. doi:10.1021/jf060275j
- Zhang, X., Liu, L., Chen, X., Kuang, H., Song, S., & Xu, C. (2013). Immunochromatographic strip development for ultrasensitive analysis of aflatoxin M1. Analytical Methods, 5, 6567–6571. doi:10.1039/c3ay41498a