Abstract
The present study was conducted to determine the effect of Diplazium esculentum on Th1 and Th2 cytokine modulation in Swiss albino mice that were administered orally with different doses of boiled D. esculentum (BDE), daily within a span of 180 days. After the treatment, serum was collected. Splenocytes were also cultured in vitro with different concentrations of BDE, and culture supernatant was collected. Both serum and culture supernatant were used for cytokine determination by enzyme-linked immunosorbent assay (ELISA) for different Th1 (IL-2 and IFN-γ) and Th2 (IL-4 and IL-10) cytokines. Results indicated significant decreases (p < 0.05, p < 0.01, and p < 0.001) in both Th1 and Th2 cytokine concentrations when compared with their respective controls. These results suggest that the intake of D. esculentum, even after cooking, may evoke immune dysfunction by altering Th1 and Th2 cytokine balance, may induce severe immunosuppression, and may be considered as alarming.
1. Introduction
Wild edible plants are very commonly used as food among the indigenous people throughout the world. There are possibilities of having several toxic substances in these plants, which upon ingestion may produce several harmful effects on human health. But, to date, little information is available that describes the toxicological impacts of these plants on human health. One such popular wild edible plant is Diplazium esculentum (Koenig ex Retz.) Sw. (Athyriaceae), the most commonly consumed fern throughout Asia and Oceania. The newly emerging coiled fronds are consumed after cooking as a seasonal vegetable during the monsoon season, which continues for almost five months in the tropical regions where this plant grows abundantly. Our previous study of this fern showed that it possesses trace amounts of flavonoids and phenolic compounds which may confer the antioxidant and free radical scavenging activities of this plant (Roy, Hazra, Mandal, & Chaudhuri, Citation2013). Another study indicated that the methanol and chloroform extracts of D. esculentum possess significant antimicrobial and cytotoxic activity (Akter, Hossain, Ara & Akhtar, Citation2014). Moreover, phytochemical analysis indicated the presence of different phytochemicals in D. esculentum such as alkaloids, anthraquinones, anthranol glycosides, cardiac glycosides, cyanidins, saponins, leucoanthocyanins, phytosterols, diterpenes, and triterpenes. Some of these phytochemicals not only provide the antioxidant activity to this plant but also aid in other important pharmacological effects in relation to human health (Tongco, Villaber, Aguda, & Razal, Citation2014).
To date, very few studies have been conducted so far to assess the pharmacological and toxicological impacts of this fern on animal health. Interestingly, this fern is rejected as food by cattle and insets. We observed that this fern grows abundantly in the marshy land and also in the wet shabby places where lot of insects are available. But interestingly, we have never found any insect consuming the leafy portion and all the leaves are intact throughout the season (Roy, Tamang, Dey, & Chaudhuri, Citation2013). On the basis of our observation, we have planned to perform the experiments using inbreed mouse as this is the standard convention to use inbreed strains of mouse for performing the immunological experiments.
We previously demonstrated that D. esculentum, even boiled, possesses potent hemolytic activity and it affects some of the innate and cell-mediated immune responses (Roy, Tamang, Dey et al., Citation2013). Boiled preparation of this fern has been shown to possess spermicidal and antifertility activities in Swiss albino mouse (Roy, Tamang, & Chaudhuri, Citation2013). The study conducted on rabbits and guinea pigs demonstrated systemic toxicity and several pathological effects of this fern. Young fronds of D. esculentum collected from the high-altitude area of Harsil–Gangotri of North India has been found to have moderate level of ptaquiloside (Pta), a nor-sesquiterpenoid glycoside which is clastogenic, mutagenic and carcinogenic that cause enzootic bovine hematuria in hill cattle in India and elsewhere (Somvanshi et al., Citation2006). Pta was found in D. esculentum sample that was prepared by freeze- and shade-drying method. Moreover, the frozen- and shade-dried crude D. esculentum have already been shown to cause mild pathological effects in rats, and induce mortality and moderate pathological effects in guinea pigs (Gangwar, Citation2004). Pta is considered as the causative agent for the location of tumors in the urinary bladder of ruminants and the ileum of rats (Smith et al., Citation1994). However, the effect of this fern on Th1/Th2 cytokine modulation has not yet been studied.
T-cells play a critical role in the pathogenesis of various diseases through the production of a variety of cytokines. Modulation of cytokine secretion by herbal immunomodulators may offer novel approaches in the treatment of a variety of inflammatory diseases (Spelman et al., Citation2006). Among the cells of the immune system, T-cells play a major role in the inflammatory response. Key regulators of this response are a subset of T-cells called CD4-positive T-helper (Th) cells, which can further be differentiated into two subtypes, Th1 and Th2 cells, by the different cytokines produced by these cells (Pacifico et al., Citation2006).
In the present study, we have investigated the effect of the boiled plant material (boiled D. esculentum; BDE) on the serum Th1 (IL-2 and IFN-γ) and Th2 (IL-4 and IL-10) cytokine levels of Swiss albino mice that were treated with different doses of BDE within a span of 180 days. We have also investigated the effect of BDE on Th1 and Th2 cytokine production by Concanavalin A (con A)-induced splenocytes of Swiss albino mouse. Con A is a stimulator of lymphocyte blast formation and possesses mitogenic activity (Kitao & Hattori, Citation1977). The present study was conducted with the cooked plant material only, because the local population consumes this fern regularly after cooking, not as raw material. Therefore, the focus of the present study was to find out whether the cooked D. esculentum possess any immunomodulatory activity by altering the normal Th1/Th2 cytokine homeostasis, and thereby to find out the presence of any heat resistant toxic compound in this edible fern.
2. Materials and methods
2.1. Preparation of the plant material
Young D. esculentum plants were collected during June–August from different areas of North Bengal University campus and the adjoining markets of Darjeeling, West Bengal, India. These were identified by Prof. A. P. Das, Plant Taxonomy Laboratory, Department of Botany, University of North Bengal, where a voucher specimen (Accession No. 9602) was also deposited.
Young frond of D. esculentum (100 g) was washed carefully by tap water, then cut into small pieces, and boiled with 1000 ml of distilled water for 30 min. The boiled plant material was then finely mixed by a mixer and dried in an incubator at 60°C until completely dried. This dried plant material (BDE) was then kept at 4°C for future use.
2.2. Chemicals
Minimum essential medium (MEM), penicillin-streptomycin solution, nystatin, Con A, and trypan blue were procured from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Enzyme-linked immunosorbent assay (ELISA) kits for IL-2, IFN-γ, IL-4 and IL-10 were procured from RayBiotech, Inc., Norcross, USA.
2.3. Animals and surgical procedures
Both male and female Swiss albino mice (25 ± 2 g of body weight [bw]) of 6–8 weeks of age were used for the studies. They were housed in polypropylene cages, with dust free paddy husk as bedding material. They were maintained in the animal house, Department of Zoology, University of North Bengal with food and water ad libitum under a constant 12 h dark/light cycle at an environmental temperature of 25 ± 2°C. Goat (Capra bengalensis) blood was collected aseptically from the jugular vein and serum was collected in aliquots and stored at –20°C until further use. All the experiments were performed after obtaining the approval from the Animal Ethical Committee (Registration No. 840/ac/04/CPCSEA).
2.4. In vivo experiments
2.4.1. Dosage
One hundred twenty (120) Swiss albino mice were divided in to five sets (S 1–5) and each set was subdivided into four groups (G1–4). Therefore, each group contained six mice. All the animals were fed orally with the help of a syringe specially designed by us. Group 1 (G1) of all the sets were considered as control where 0.4 ml of distilled water was given. Group 2 (G2), Group 3 (G3), and Group 4 (G4) of all the sets were fed with 0.4 ml of BDE at the dose of 80, 160, and 320 mg/kg bw, respectively. In this way, all groups of S1 were treated daily for 15 d, S2 daily for 45 d, S3 daily for 90 d, S4 daily for 135 d, and S5 daily for 180 d.
We assume that the average maximum amount of cooked D. esculentum consumed by a 60 kg weighed individual is about 20 g/d. Keeping this ratio in mind, we formulate different doses for an average weighed adult mouse (25 g), namely 80 mg/kg bw, i.e. 2 mg/mouse/d; 160 mg/kg bw, i.e. 4 mg/mouse/d; and 320 mg/kg bw, i.e. 8 mg/mouse/d.
2.4.2. Collection of serum
Mice from each group were sacrificed after proper anesthesia (chloroform and ether in 2:1 ratio) 24 h after the last dose, blood was collected from the heart and serum was separated and stored at –20°C for future use. These serum samples were further used to determine the concentration of different cytokines.
2.5. Ex vivo experiment
2.5.1. Primary culture of splenocytes
Spleen was aseptically removed from mouse and cell suspension was prepared in MEM [containing penicillin-streptomycin (50 U/ml) and nystatin (50 U/ml)]. The cell number was adjusted to 2 × 106 cells per ml and 1 ml of the cell suspension was added in six-well culture plates. Each well was then supplemented with 10% goat serum (Chaudhuri & Chakravarty, Citation1983). Five microliters of Con A (5 µg/ml) was also added to stimulate cytokine production. Finally, 100 µl of different concentrations (0–200 µg/ml) of BDE (suspended in MEM) were added to each well. The whole set-up was incubated for 48 h at 37°C in an incubator having 5% CO2 and 90% humidity. Supernatants of cell cultures were collected after 48 h and used for cytokine estimation.
2.6. Estimation of cytokine production
Previously collected serum samples and splenocyte culture supernatants were used to determine the concentrations of Th1 (IL-2 and IFN-γ) and Th2 (IL-4 and IL-10) cytokines by ELISA according to the manufacturer’s instructions (RayBiotech, Inc., USA). Briefly, a 96-well flat bottom plate was coated with the captured antibody specific to each cytokine. One hundred microliters of serially diluted specific standards for each cytokine and 100 µl of the serum/cell culture supernatants (samples) were pipetted into the wells. The specific cytokine present in the sample was bound to the wells by the immobilized antibody. The wells were washed and biotinylated anti-mouse detection antibody specific for each cytokine was added. After washing away the unbound biotinylated antibody, HRP-conjugated streptavidin was pipetted to the wells. The wells were again washed, a TMB substrate solution was added to the wells, and the color was developed in proportion to the amount of specific cytokine bound. Finally, the stop solution was added which changed the color from blue to yellow, and the intensity of the color was measured at 450 nm.
2.7. Statistical analysis
Data have been presented as mean ± SD of three observations. Statistical analysis was performed using KyPlot version 2.0 beta 15 (32 bit). Differences in mean ± SD among different groups were statistically analyzed using one way analysis of variance (ANOVA) followed by Dunnett’s test. p < 0.05 was considered significant.
3. Results
3.1. Effect of BDE on serum concentration levels of Th1 and Th2 cytokines
Significant decreases (p < 0.05, p < 0.01, and p < 0.001) were observed in both Th1 and Th2 cytokine concentrations in mice that were treated with different doses of BDE for 90, 135, and 180 d, when compared with their respective control groups (). After 15 and 45 d of treatment with different doses of BDE, the concentrations of IL-2, IL-4, and IL-10 did not decrease significantly, though after 45 d of treatment with BDE at 160 and 320 mg/kg bw, the concentrations of IFN-γ have been shown to decrease significantly (p < 0.05) when compared with their respective controls (). After 180 d of treatment at with 80, 160 and 320 mg/kg bw of BDE, the concentration of all the cytokines decreased significantly when compared to their respective controls (p < 0.001; ).
Table 1. Serum concentrations of different Th1 (IL-2 and IFN-γ) and Th2 (IL-4 and IL-10) cytokines of mice that are treated with different doses of BDE for 15 (S1) and 45 (S2) days.
Table 2. Serum concentrations of different Th1 (IL-2 and IFN-γ) and Th2 (IL-4 and IL-10) cytokines of mice that are treated with different doses of BDE for 90 (S3), 135 (S4) and 180 (S5) days.
3.2. Effect of BDE on Th1 and Th2 cytokine production from primary cultured splenocytes
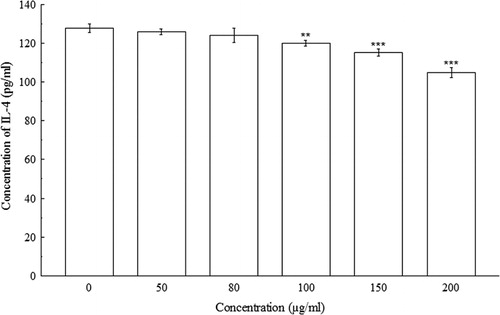
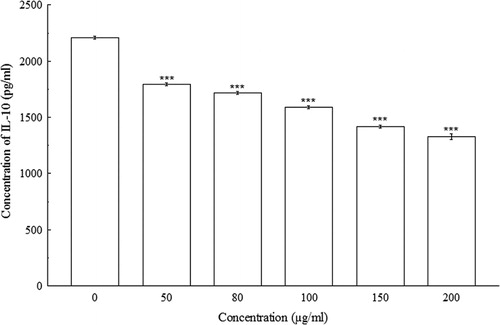
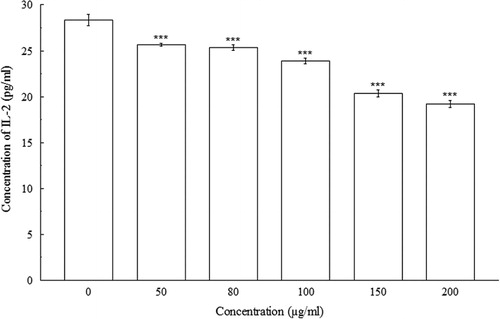
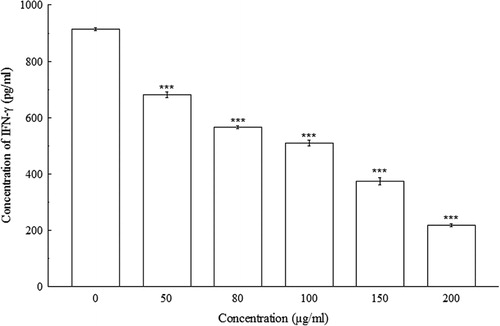
indicated significant concentration-dependent IL-2 decreases (p < 0.001) in Con A-induced splenocytes. At 0 µg/ml, the concentration of IL-2 in splenocyte culture supernatant was 28.33 ± 0.58 pg/ml, whereas, at 200 µg/ml, the concentration of IL-2 decreased to 19.17 ± 0.38 pg/ml. The amount of IFN-γ, IL-4, and IL-10 were also decreased significantly (p < 0.01 and p < 0.001) in a concentration-dependent manner when compared with their respective controls. At 0 µg/ml of BDE, the concentrations of IFN-γ, IL-4, and IL-10 were 913.33 ± 5.77 pg/ml, 127.76 ± 2.21 pg/ml, and 2208.33 ± 14.43 pg/ml, respectively, whereas, at 200 µg/ml, the concentrations of IFN-γ, IL-4, and IL-10 has been shown to decrease to 216.67 ± 5.77 pg/ml, 104.79 ± 2.45 pg/ml, and 1325 ± 25 pg/ml, respectively (–).
4. Discussion
Only few of the pharmacological activities of D. esculentum have been reported so far, and among them little is known about the effect of D. esculentum on the immune system. We have previously reported that the sub-acute, sub-chronic, and chronic oral administration of boiled aqueous preparation of D. esculentum causes immunosuppression and hemolysis in Swiss albino mouse (Roy, Tamang, Dey et al., Citation2013). In continuation of our previous investigations, this work was aimed to investigate the effects of boiled aqueous preparations of D. esculentum both in vivo and in vitro on Th1 (IL-2 and IFN-γ) and Th2 (IL-4 and IL-10) cytokine concentration in mouse.
IL-2 is a representative cytokine produced by activated T-cells that leads to T-cell proliferation and participates in the regulation of other immune cells, including B cells, macrophages and natural killer (NK) cells (Park et al., Citation2007). IFN-γ is a proinflammatory mediator expressed by various cells, including Th1, NK, and NKT cells. IFN-γ is an important immune-activating cytokine that can prime macrophages for activation and induce inflammatory responses, such as those observed in delayed-type hypersensitivity and granulomatous lesions (Pacifico et al., Citation2006). IFN-γ orchestrates leukocyte attraction and directs the growth, maturation, and differentiation of much type of cells in addition to enhancing NK cell activity. As intrinsic factors, IL-2, IL-12, and several other cytokines are known to be the primary cytokines along with the production of IFN-γ by NK cells (Kang, Ahn, Oh, & Kim, Citation2014). IL-4 is produced by activated T lymphocytes and mast cells, and can exert both pro- and anti-inflammatory effects (Kleemann, Zadelaar, & Kooistra, Citation2008). One of the most potent homeostatic regulators of inflammation is the anti-inflammatory cytokine IL-10, which potently inhibits TNF-α production from macrophages together with the other pro-inflammatory cytokines including IL-1, IL-6, GM-CSF, and many chemokines (Brennan et al., Citation2008). Sub-acute, sub-chronic, and chronic oral administration of BDE showed to reduce the body weight and relative spleen weight, as well as suppress the humoral immune response in Swiss albino mouse. Moreover, BDE is also reported to decrease the number of the peritoneal macrophages in mouse (Roy, Tamang, Dey et al., Citation2013). Significant dose-dependent reduction in the level of Th1 (IL-2 and IFN-γ) and Th2 (IL-4 and IL-10) cytokine production by T cells in BDE-treated mouse indicates severe immunosuppression in these mice.
Study revealed that D. esculentum collected from high altitude area of Harsil–Gangotri had high quantity of Pta (Somvanshi et al., Citation2006). Shade- and freeze-dried samples of D. esculentum showed absence of fern toxin Pta but the presence of moderate amount of pterosin B in the freeze-dried samples identified by HPLC method (Gangwar, Citation2004). During metabolism, Pta undergoes a series of reactions and produces a reactive aglycone dienone intermediate, the inactive pterosin B and DNA adducts. Pta is activated at alkaline pH, which is considered as the reason for the location of tumors in the urinary bladder of ruminants and the ileum of rats (Smith et al., Citation1994). Feeding of frozen- and shade-dried samples of D. esculentum to rats and guinea pigs showed decreased body weight, increased spontaneous and decreased forced motor activity. Hematological and biochemical studies in rats and guinea pigs fed with frozen- and shade-dried D. esculentum showed significant alterations in the values of blood glucose and total leukocyte count, increase in serum glutamic oxaloacetic transaminase and serum dehydrogenases. Feeding of frozen dried sample of D. esculentum induced mortality rate in guinea pigs (Gangwar, Citation2004). All these studies done so far were on the freeze- or shade-dried samples of D. esculentum, and its effect on rabbits and guinea pigs. But, there is little information available regarding the effect of boiled preparation of D. esculentum on different laboratory animals, such as mouse, rabbits or guinea pigs. We performed the present experiment using mouse as this is the standard convention to use inbreed strains of mouse for performing the immunological experiments. We performed the study using boiled preparations of D. esculentum, to find out the possible effect of temperature on some toxic compounds present, if any, in D. esculentum, which may alter the Th1 and Th2 cytokine balance. It was found that BDE caused significant dose-dependent reduction in cytokine concentration in both in vivo and in vitro models.
As Pta is a heat labile compound, boiling may probably reduce its toxicity. As BDE caused significant reduction in Th1 and Th2 cytokine concentration in Swiss albino mouse, it is clearly evident that there may be some compound besides Pta that can withstand heat and provide toxicity. Study revealed that Diplazium sammatii, a related edible fern, contains moderate amount of tannins which inhibit protein availability through denaturation (Bassey, Etuk, Ibe, & Ndon, Citation2001). Tannins are heat resistant compounds that can withstand high temperature during boiling. As D. esculentum and D. sammatii are of same genus, we can assume that tannins may also be present in boiled preparation of D. esculentum, and may be one of the causes of immunosuppression. Thus, the cytokine inhibitory effects observed in our study could be related to tannins and other heat stable compounds. Standard tannins (tannic acid) may be applied in the splenocyte cultures to reduce speculation in future studies.
The secreted cytokines of type 1 CD4+ T helper cells (Th1), such as IL-2 and IFN-γ are considered as proinflammatory, whereas Th2 cytokines such as IL-4 and IL-10 can counteract Th1 cytokine production and activity (Kleemann et al., Citation2008). IFN-γ enhances Th1 generation but inhibits Th2 generation, whereas Th2 cells and their cytokine, IL-4, promotes Th2 generation but inhibits Th1 generation. In physiological condition, Th0 cells differentiate to Th1 and Th2 cells proportionally and keep their amount in a relative dynamic balance. Whenever this balance is disturbed, diseases will occur (Guo et al., Citation2014). It has been demonstrated that Th1/Th2 balance plays important roles in anti-tumor immunity in which Th1 cells produce IL-2 and IFN-γ that are essential for inducing cellular and tumor immunity, whereas Th2 cells producing IL-4 and IL-6 are associated with suppression of cytolytic activity (Nakamori et al., Citation2003; Nishimura et al., Citation1999). Under aberrant conditions, a Th1/Th2 imbalance occurs and various cytokines are thought to cause autoimmune diseases, such as autoimmune diabetes, rheumatoid arthritis and Crohn’s disease (Abbas, Murphy, & Sher, Citation1996). Findings from the present study indicate that D. esculentum, when given in chronic dose, can induce Th1/Th2 imbalance, resulting in severe immunosuppression, which may directly or indirectly induce several metabolic diseases and age-related degenerative disorders, as well as may increase the risk of infection in the people who regularly consumes this fern; induce a state of immunodeficiency as an unwanted consequence, and therefore, may also be related to the growth of tumors.
5. Conclusion
D. esculentum is extensively used as a palatable food throughout Asia, Oceania, and many other places throughout the globe, including the northern part of West Bengal, India, where we reside. The possible consequences of the immunosuppressive effects of D. esculentum on human health may be alarming. Considering the findings of the present study, it may be concluded that D. esculentum, even boiled, possesses potent immunosuppressive activities as evident by Th1/Th2 cytokine imbalance. This is the first report on the assessment of the Th1/Th2 cytokine imbalance in the body due to the intake of the edible D. esculentum, and thereby to make people aware about the hazards of its regular consumption which might be helpful in advancing the existing knowledge of this fern in relation to human health.
Acknowledgment
We gratefully acknowledge the financial support (Vide Letter F.No.37-464/2009 (SR) dt.11.01.2010) received from the University Grants Commission (UGC), New Delhi, India, to carry out this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abbas, A. K., Murphy, K. M., & Sher, A. (1996). Functional diversity of helper T lymphocytes. Nature, 383, 787–793. doi:10.1038/383787a0
- Akter, S., Hossain M. M., Ara, I., & Akhtar, P. (2014). Investigation of in vitro antioxidant, antimicrobial and cytotoxic activity of Diplazium esculentum (Retz). Sw. International Journal of Advances in Pharmacy, Biology and Chemistry, 3, 723–733. Retrieved from http://www.ijapbc.com/files/32-3369.pdf
- Bassey, M. E., Etuk, E. U. I., Ibe, M. M., & Ndon, B. A. (2001). Diplazium sammatii: Athyraceae (‘Nyama Idim’): Age-related nutritional and antinutritional analysis. Plant Foods for Human Nutrition, 56(1), 7–12. doi:10.1023/A:1008185513685
- Brennan, F. M., Green, P., Amjadi, P., Robertshaw, H. J., Alvarez-Iglesias, M., & Takata, M. (2008). Interleukin-10 regulates TNF-α-converting enzyme (TACE/ADAM-17) involving a TIMP-3 dependent and independent mechanism. European Journal of Immunology, 38, 1106–1117. doi:10.1002/eji.200737821
- Chaudhuri, T. K., & Chakravarty, A. K. (1983). Goat serum as a substitute for fetal calf serum in in vitro culture of murine lymphocytes. Indian Journal of Experimental Biology, 21, 494–496. Retrieved from http://www.niscair.res.in/sciencecommunication/researchjournals/rejour/ijeb/ijeb0.asp
- Gangwar, N. K. (2004). Studies on the pathological effects of linguda (Diplazium esculentum, Retz.) in laboratory rats and guinea pigs. Indian Journal of Veterinary Pathology, 28, 149–150. Retrieved from http://www.indianjournals.com/ijor.aspx?target=ijor:ijvp&volume=28&issue=2&article=thesis-abs-005
- Guo, H.-W., Yun, C.-X., Hou, G.-H., Du, J., Huang, X., Lu, Y., … Deng, J. (2014). Mangiferin attenuates Th1/Th2 cytokine imbalance in an ovalbumin-induced asthmatic mouse model. PLoS ONE, 9(6), e100394. doi:10.1371/journal.pone.0100394.t002
- Kang, H.-B., Ahn, K.-S., Oh, S.-R., & Kim, J. W. (2014). Genkwadaphnin induces IFN-γ via PKD1/NF-kB/STAT1 dependent pathway in NK-92 cells. PLoS ONE, 9(12), e115146. doi:10.1371/journal.pone.0115146
- Kitao, T., & Hattori, K. (1977). Concanavalin A as a carrier of daunomycin. Nature, 265, 81–82. doi:10.1038/265081a0
- Kleemann, R., Zadelaar, S., & Kooistra, T. (2008). Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovascular Research, 79, 360–376. doi:10.1093/cvr/cvn120
- Nakamori, M., Iwahashi, M., Nakamura, M., Ueda, K., Zhang, X., & Yamaue, H. (2003). Intensification of antitumor effect by T helper 1-dominant adoptive immunogene therapy for advanced orthotopic colon cancer. Clinical Cancer Research, 9, 2357–2365. Retrieved from http://clincancerres.aacrjournals.org/content/9/6/2357.long
- Nishimura, T., Iwakabe, K., Sekimoto, M., Ohmi, Y., Yahata, T., Nakui, M., … Ohta, A. (1999). Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. Journal of Experimental Medicine, 190, 617–628. doi:10.1084/jem.190.5.617
- Pacifico, L., Di Renzo, L., Anania, C., Osborn, J. F., Ippoliti, F., Schiavo, E., & Chiesa, C. (2006). Increased T-helper interferon-γ-secreting cells in obese children. European Journal of Endocrinology, 154, 691–697. doi:10.1530/eje.1.02138
- Park, K.-R., Lee, J.-H., Choi, C. Y., Liu, K.-H., Seog, D.-H., Kim, Y. H., … Yea, S. S. (2007). Suppression of interleukin-2 gene expression by isoeugenol is mediated through down-regulation of NF-AT and NF-κB. International Immunopharmacology, 7, 1251–1258. doi:10.1016/j.intimp.2007.05.015
- Roy, S., Hazra, B., Mandal, N., & Chaudhuri, T. K. (2013). Assessment of the antioxidant and free radical scavenging activities of methanolic extract of Diplazium esculentum. International Journal of Food Properties, 16, 1351–1370. doi:10.1080/10942912.2011.587382
- Roy, S., Tamang, S., & Chaudhuri, T. K. (2013). Sperm viability assessment using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction assay of Swiss albino mice treated with Diplazium esculentum. Asian Journal of Pharmaceutical and Health Sciences, 3, 684–689. Retrieved from http://ajphs.com/wp-content/uploads/2013/05/04_AJPHS_V3_I2_Apr-Jun_2013_246.pdf
- Roy, S., Tamang, S., Dey, P., & Chaudhuri, T. K. (2013). Assessment of the immunosuppressive and hemolytic activities of an edible fern, Diplazium esculentum. Immunopharmacology and Immunotoxicology, 35, 365–372. doi:10.3109/08923973.2013.775588
- Smith, B. L., Seawright, A. A., Ng, J. C., Hertle, A. T., Thomson, J. A., & Bostock, P. D. (1994). Concentration of ptaquiloside, a major carcinogen in bracken fern (Pteridium spp.) from eastern Australia and from cultivated worldwide collection held in Sydney, Australia. Natural Toxins, 2, 347–353. doi:10.1002/nt.2620020602
- Somvanshi, R., Lauren, D. R., Smith, B. L., Dawra, R. K., Sharma, O. P., Sharma, V. K., … Gangwar, N. K. (2006). Estimation of the fern toxin, ptaquiloside, in certain Indian ferns other than bracken. Current Science, 91, 1547–1552. Retrieved from http://www.iisc.ernet.in/currsci/dec102006/1547.pdf
- Spelman, K., Burns, J. J., Nichols, D., Winters, N., Ottersberg, S., & Tenborg, M. (2006). Modulation of cytokine expression by traditional medicines: A review of herbal immunomodulators. Alternative Medicine Review, 11, 128–150. Retrieved from http://www.nutraxin.com.tr/pdf/AstragalusSpecies/Astragalus_02.pdf
- Tongco, J. V. V., Villaber, R. A. P., Aguda, R. M., & Razal, R. A. (2014). Nutritional and phytochemical screening, and total phenolic and flavonoid content of Diplazium esculentum (Retz.) Sw. from Philippines. Journal of Chemical and Pharmaceutical Research, 6, 238–242. Retrieved from http://jocpr.com/vol6-iss8-2014/JCPR-2014-6-8-238-242.pdf