Abstract
The current study aimed to remit the immunoreactivity of glycinin and β-conglycinin through the methods of glycation. The immunoreactivity of the glycated soybean antigen proteins after hydrolyzed was analyzed in vitro. Then in the in vivo trial, 24 crossbred castrated piglets were allocated to four dietary treatments in a complete block design, each treatment with six replicates. From day 29–43, the control groups were fed diets with 4% unglycated glycinin or β-conglycinin, while the treatment groups received diets containing 4% glycated glycinin or β-conglycinin. Generally, the immunoreactivity removal rate increased in all products as time went on both in vitro and in vivo. The immunoreactivity residual rate of unglycated soybean antigen protein was much higher than those of the glycated ones in vitro. Moreover, pepsin and trypsin affected glycated glycinin and glycated β-conglycinin in a different way.
1. Introduction
Because of its high nutritional and functional properties, soybean is widely used in food and feed industry. However, soybean antigen proteins can cause allergic reaction in infant, piglets, calves, and other young animals (Li et al., Citation1991; Sun, Li, Li, Dong, & Wang, Citation2008). Soybean is considered to be one of the major food allergens, which is more prominent in the industrial countries (Wang, Qin, Sun, & Zhao, Citation2014). In recent years, soybean allergy has become a public problem all over the world (Guo, Piao, Cao, & Ou, Citation2008; Wang et al., Citation2014). Glycinin and β-conglycinin are two major antigen proteins in soybean and they accounted for about 65–80% of the total seed protein. Glycinin is a hexamer with a molecular weight of 320–360 kDa. The monomeric subunit structure of glycinin is like A-S-S-B, wherein A is an acidic polypeptide chain, B is a basic polypeptide chain and they two were linked by disulfide bond (-S-S-) (Kitamura & Shibasaki, Citation1975). β-conglycinin is a kind of glycoprotein which containing approximately 4–5% carbohydrates (Mujoo, Trinh, & Ng, Citation2003) and it has a molecular weight of 140–180 kDa which contains α′, α, and β subunits (Maruyama et al., Citation2001). So far, studies on soybean antigen proteins were mainly concentrated in their anti-nutritional effects (Miller et al., Citation1984; Sissons, Smith, Hewitt, & Nyrup, Citation1982), inactivation technique (Qin, Ter Elst, Bosch, & Van der Poel, Citation1996), and the sensitization mechanism (Saito, Kohno, Tsumura, Kugimiya, & Kito, Citation2001; Sun et al., Citation2008). Maillard reaction is a technique which was used in the inactivation of shellfish allergens (Nakamura, Watanabe, Ojima, Ahn, & Saeki, Citation2005), peanut allergens (Gruber, Becker, & Hofmann, Citation2005), cherry allergens (Gruber, Vieths, Wangorsch, Nerkamp, & Hofmann, Citation2004), β-lactoglobulin (Hattori et al., Citation2004), and soybean protein (Usui et al., Citation2004; Wilson, Blaschek, & de Mejia, Citation2005). Although the inactivation mechanism of Maillard reaction is still not entirely clear, this method showed its advantage on reducing the allergic reaction (Usui et al., Citation2004).
Fructo-oligosaccharides (FOSs) are inexpensive mixture of fructose and low polymerization degree by the (DPs degree of polymerization 2–7) oligofructose composition, but it is classified as a special nutritional and functional specific foods. In nutrition, they are a source of dietary fiber, low-energy, suitable for diabetics, and no carcinogenic effects. Through combining with FOS (Maillard reaction), the immunoreactivity of soy protein isolates could be reduced (Jürgen, Silván, Moreno, Olano, & Castillo, Citation2007).
In this study, FOS and soybean antigen proteins were mixed at certain molar mass ratio and temperature and relative humidity (RH) combinations which could reduce the immunoreactivity of soybean antigen proteins. Then the immunoreactivity variations of the glycated soybean antigen proteins hydrolyzed by pepsin or trypsin were measured. In the in vivo part, the immunoreactivity disappearance rate of these two proteins in the digestive tract of piglets and their effects on the growth performance of these animals were evaluated. This study may provide some theoretical foundation for the low immunoreactive glycated soybean antigen proteins production in the feed/food industry.
2. Materials and methods
2.1. Isolation of glycinin and β-conglycinin and glycated with FOS
Glycinin and β-conglycinin were isolated from defatted soy flour according to the method described by Nagano, Hirotsuka, Mori, Kohyama, and Nishinari (Citation1992). Protein content was determined by the Kjeldahl method and purity was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using mini-gel apparatus (Bio-Rad Laboratories, CA, America) followed by Coomassie Brilliant Blue R-250 staining. The gel was scanned by Bio Imaging System with Gene Genius (Syngene, USA), and the purity of glycinin was measured by GeneTools Analysis Software, Version 3.03.03 (Guo et al., Citation2008). Glycinin and β-conglycinin were glycated with FOS according to the Maillard reaction in powder system described by Jürgen et al. (Citation2007).
2.2. Production of polyclonal antibodies
Glycinin and β-conglycinin (over 95% purity) as immunogens were used to produce polyclonal antibodies. The antibodies were purified by binding (affinity) to the pre-coated glycinin or β-conglycinin on the column (1.0 × 10 cm Sepharose 4B column, Amersham Pharmacia Biotech AB).
2.3. Enzymatic hydrolysis of unglycated and glycated glycinin or β-conglycinin in vitro
The unglycated or glycated glycinin and β-conglycinin were hydrolyzed by porcine pepsin (Amresco 1:3000) or trypsin (Amresco 1:250) or cooperation of two enzymes (Boisen & Fernández, Citation1995; Fu, Abbott, & Hatzos, Citation2002) at various hydrolysis time (0.5, 1, 15, 30, 60 min) and enzyme/substrate ratio (1:20, 1:10, 1:1, 10:1; W/W) in vitro. Briefly, 0.2 ml of each protein samples was dissolved in 0.1 M phosphate-buffered saline (PBS; pH = 6.0; pepsin) or 0.2 M PBS (pH = 6.8; trypsin), respectively. Then the pH value was adjusted to 2.0 by 0.2 N HCl. Pepsin in 0.03 M NaCl (pH = 2) or trypisn in 0.05 M PBS (pH = 6.8; 2.5, 5, 50, 500 mg/ml) was added in order to achieve different enzyme/substrate ratio (1:20, 1:10, 1:1, 10:1; W/W). The mixture was placed in an incubator of 37°C and shaken at 100 rpm. The samples were collected at 0.5, 1, 15, 30, or 60 min later.
2.4. Digestion of unglycated and glycated glycinin or β-conglycinin in vivo
Animals were maintained according to the rules of China Animal Care and Use Committee. Twenty-four healthy crossbred piglets (Duroc × Landrace × Yorkshire) with an average initial body weight of 6.7 ± 0.5 kg, weaned at 28 days of age, were allocated to four groups randomly, each group with six replicates (female/male = 1:1). The piglets were fed the diets containing 4% unglycated or glycated (65°C, 75% RH) glycinin or β-conglycinin (; Zhao et al., Citation2008). Unglycated globulins were isolated from defatted soybean powder and glycated globulins were prepared by the Maillard reaction of FOS and unglycated globulins in the optimizing condition.
Table 1. Composition and nutrient levels of the diets (as-fed basis).
All the diets were isonitrogenous and isocaloric to minimize the effect of the replacement of skimmed milk powder and fish meal with the tested proteins. Chromium-sesquioxide (Cr2O3, 0.5%) was added to the diets as the indigestible marker. Piglets were fed supplements without ingredients originating from leguminous products during the suckling period in order to adapt to this routine. The average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (feed-to-gain ratio, F/G) were recorded during the experiment. At 38 days of age, piglets were anesthetized with excess procaine and sacrificed two hour after the morning meal. The digesta in stomach, duodenum, middle jejunum, ileum, and caecum were quickly collected and stored at −80°C until analysis.
2.5. Preparation of diets and gastrointestinal contents
Globulins and their glycated forms were extracted using 10 times of volumes of 0.025 M Tris-HCl (pH 8.6) from the diets and digesta collected from stomach, duodenum, middle jejunum, ileum, and caecum (Zhao et al., Citation2008). Extracts were clarified by centrifuging at 10,000 ×g for 15 min at 4°C and the supernatant was stored at −80°C until required.
2.6. The proteins of gastrointestinal contents stability assays
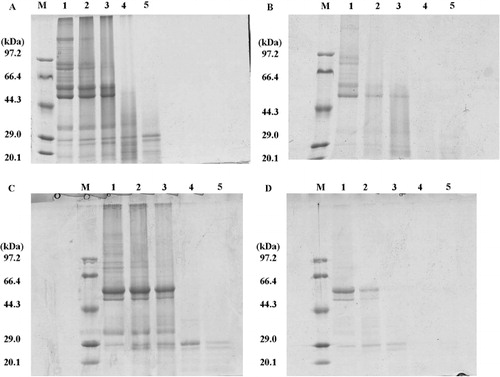
The proteins of gastrointestinal contents in each part were subjected to SDS-PAGE by using mini-gel apparatus (Bio-Rad Laboratories, CA, USA) with a 12% acrylamide separating and a 5% acrylamide stacking gel with slight modification. The samples were dissolved in 1MTris-HCl buffer (containing 2.7 M glycerol, 0.15 M SDS, and 0.15 mM bromophenol blue, pH 6.8). The loading was 10 μg of protein per well for each part of intestinal tract. Molecular weight markers were also loaded in a separate well. Electrophoresis was performed at 100 V for separating gel and at 200 V for stacking gel. Proteins were visualized by CBB staining.
2.7. Indirect competitive enzyme-linked immunosorbent assay (ELISA)
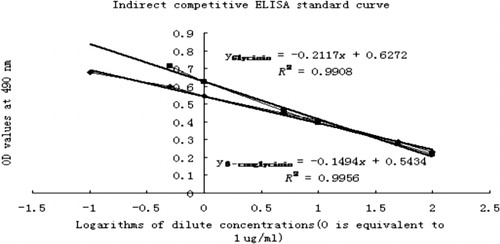
The immunoreactivity of the unglycated and glycated glycinin or β-conglycinin was determined as described by Zhao et al. (Citation2008). The optical density (OD) value at 490 nm was determined using an Auto Microplate Reader (Bio-rad Model 680, America). The standard curve was drawn according to a series of OD values of the purified antigen.
2.8. Determination of indigestible marker
Chromium in the diets and digesta was assayed using the wet-ashing technique. Atomic absorption spectrometry (using a PE spectrophotometer, model AA 7 800) was used to measure the Cr concentration.
Calculation of the disappearance rate of immunoreactive globulins and their glycated forms according to the indicator technique, based on the concentration of immunoreactive globulins and the indigestible marker Cr2O3, the disappearance rate of immunoreactive globulins or their glycated forms were calculated using the following formula:
2.9. Statistical analysis
All data were expressed as mean values ± standard error of the mean (SEM) and analyzed by Duncan's multiple range tests of the general linear model procedure (SPSS Inc., Chicago, USA). Differences were considered statistically significant if P < 0.05.
3. Results
3.1. Quality check of glycinin and β-conglycinin
Determined by the Kjeldahl method and SDS-PAGE analysis, the globulins isolated by the method of Nagano et al. (Citation1992) contain more than 85% glycinin or β-conglycinin.
3.2. Calibration curves
In the process of inhibition ELISA, the calibration curves () show that there was a negative correlation between calibration density and OD value.
3.3. The immunoreactivity of glycated glycinin and β-conglycinin
Determined by indirect competitive ELISA, the immunoreactivity of glycated glycinin and β-conglycinin was lower compared with unglycated forms by 78% and 82%.
3.4. Comparitive immunoreactivity of glycated glycinin or β-conglycinin on the hydrolysis
There was interaction between enzyme and time for the immunoreactivity of glycated glycinin and β-conglycinin hydrolyzed with digestive enzymes shown in (P < 0.001). Regarding to the variation of immunoreactivity, glycated glycinin was more sensitive to pepsin, but glycated β-conglycinin was more liable to trypsin. Immunoreactivity of glycated glycinin hydrolyzed with trypsin and glycated β-conglycinin hydrolyzed with pepsin still existed after 60 min, while the immunoreactivity of glycated glycinin and β-conglycinin nearly vanished after 15–30 min in other hydrolyzed fluid.
Table 2. Immunogenicity of the hydrolyzed unglycated or glycated glycinin and β-conglycinin (E/S = 1:10).
3.5. Effect of glycinin and β-conglycinin on growth performance
The results show that piglets had reduced feed intake during the first two days post-weaning and the feed intake was returned to normal levels two days later. Moreover, there were no significant differences among individuals (P < 0.05). The growth performance data are presented in . Generally, when piglets were fed diets with glycated glycinin or β-conglycinin, ADG (P < 0.001) increased whereas ADFI (P = 0.002) closed. There were no significant differences when piglets fed glycated glycinin compared with glycated β-conglycinin.
Table 3. Effects of unglycosylated or glycosylated glycinin and β-conglycinin on growth performance of piglets.
3.6. Comparative digested stability of different protein in the digestive tract
shows a typical SDS-PAGE analysis of protein degradation in stomach, duodenum, middle jejunum, caecum, and colon. summarizes the digested stability of glycated glycinin and β-conglycinin.
3.7. Immunoreactive glycated glycinin and β-conglycinin in diets and digesta
The content of immunoreactive glycinin (3.6364 mg·ml−1) and β-conlycinin (3.6036 mg/ml) in diets were high (). The immunoreactive glycinin and β-conlycinin significantly decreased as the digesta descended down the digestive tract (P < 0.001). The immunoreactive glycated glycinin and β-conlycinin also decreased. In the duodenum and middle jejunum, the concentration of glycated glycinin and β-conlycinin reduced by over half more than 50%. Little immunoreactive glycated glycinin or β-conlycinin were detected in the digesta from caecum and colon. But they continually decreased in the caecum, and there was no significantly a difference in the colon. The content of the immunoreactive glycated glycinin was significantly lower than glycated β-conlycinin in the digesta of the posterior segments of the digestive tract (P < 0.05).
Table 4. Immunoreactive glycated glycinin and β-conlycinin in experimental diets and digesta.
3.8. Comparison of the disappearance rate of immunoreactive glycated glycinin and β-conglycinin
After calculating the disappearance rate of immunoreactive glycated glycinin and β-conlycinin, the variations of immunoreactive glycated glycinin and β-conlycinin during digestion were clearly displayed compared with unglycated forms (). As the digesta descended down the digestive tract, the disappearance rate of immunoreactive glycated glycinin and β-conlycinin tended to increase. The degradation of immunoreactive globulins in the duodenum and middle jejunum was the fastest. There was a significant difference between the stomach and duodenum (P < 0.001). The disappearance rate of globulins was accessed to 1 in the large intestine. In the digestive tract, the disappeared rate of immunoreactive glycinin was a little higher than β-conlycinin, which indicates that glycated glycinin was more easily degraded than glycated β-conglycinin.
4. Discussion
The immunoreactivity of antigen proteins can be removed through the Maillard reaction (Hattori et al., Citation2000; Kobayashi et al., Citation2001). It was proved that glycation with FOS can effectively modified the structure and properties of 11S and 7S proteins, thereby reduced the antigen activity of these two proteins upon near 90% (Jürgen et al., Citation2007). The dry powder reaction system used in this study was easier and much more efficient compared with the wet reaction system. The results showed that immunoreactivity of glycinin and β-conglycinin was reduced about 80% under the conditions of 65°C, 75% RH, molar mass ratio of 1:14, and the reaction time of 60 h.
The protein glycation reaction can improve the solubility of the protein (Su & Guo, Citation2010), and the solubility of the glycinin-glucose product increased at initiate to 24 h under 50°C, 65% RH (Achouri, Boye, Yaylayan, & Yeboah, Citation2005). With the temperature rising, protein denaturation would happen, thereby the higher of the temperature, the lower of the immunoreactivity. Meanwhile the Maillard reaction could promote the removal of the immunoreactivity of glycated products by moisturizing. Further, the dissolution of protein was significantly low when the reaction temperature was 75°C compared with 65°C, the increased temperature could promote Maillard reaction process and the aggregation. The aggregation is caused by the reaction between an amide group in a protein-bound glutamine and a ε-amino group in a protein-bound lysine side chain, resulting in crosslinks between protein molecules (Babiker et al., Citation1998).
The dry reaction system was selected to prepare the saccharification product, and unglycated globulins were taken as control (Jürgen et al., Citation2007). The immunoreactivity of the unglycated or glycated glycinin and β-conglycinin was compared under the enzymatic hydrolysis.
Generally, after hydrolysis the glycated glycinin and β-conglycinin had much lower immunoreactivity than those of the unglycated proteins; glycated glycinin also appeared to be very stable to trypsin whereas the glycated β-conglycinin was difficult to be degraded by pepsin (). The other substrate ratios were shown the similar results as presentation.
The immunologic activity of specific serum IgE antibodies directed against the carbohydrate part of glycated allergens has been a matter of debate since the discovery of N-glycanspecific IgE (Van Ree, Citation2002). In this study, the immunoreactivity of the hydrolyzed unglycated or glycated glycinin and β-conglycinin was analyzed in vitro. The immunoreactivity of glycated glycinin and β-conglycinin was all lower than those of the unglycated proteins. Significant differences between the gastric and intestinal digestion of soybean glycinin and β-conglycinin were observed, and this may attribute to the substrate specificity of pepsin and trypsin (Lalles et al., Citation1999; Perez, Mills, Lambert, Johnson, & Morgan, Citation2000; Zhao et al., Citation2008). Glycinin (pI = 6.4) was hydrolyzed by pepsin easily, while β-conglycinin (pI = 4.9) was sensitized to trypsin (Astwood, Leach, & Fuchs, Citation1996; Fu et al., Citation2002; Nagano et al., Citation1992; Sangild, Silver, Fowden, Turvey, & Foltman, Citation1994; Wu, Murphy, Johnson, Reuber, & Fratzke, Citation2000). Moreover, it was reported that β-conglycinin had more trypsin cleavage sites than glycinin (Kuipers, Alting, & Gruppen, Citation2007). With the increasing of the enzyme substrate ratio, the hydrolysis time of these proteins was shortening (Fu et al., Citation2002; Moreno, Citation2007; Takagi, Teshima, Okunuki, & Sawada, Citation2003; Venkatachalam, Teuber, Peterson, Roux, & Sathe, Citation2006).
Glycated soy protein would continue to be hydrolyzed under the action of digestive enzymes, and their low immunoreactivity further reduced. But when the hydrolysis time more than 30 min, no significant differences were found in immunoreactivity.
Piglets fed glycated glycinin or β-conglycinin had higher ADG and lower F/G (P < 0.05) whereas there was no significant difference about the ADFI (P < 0.05; ). There might be two reasons for such results. One might be the glycation antigen protein caused the diarrhea occurs decreased, also might be due to the glycation reaction improved the protein quality, so the utilization efficiency increased.
The disappearance rate of immunoreactive glycinin, β-conglycinin, and their glycated forms was shown in Generally, the disappearance rate of immunoreactive of these proteins tended to increase as the digesta descended down the digestive tract. Meanwhile, the immunoreactivity of glycinin or β-conglycinin was further declined and not recovered. The degradation of immunoreactive globulins in the middle jejunum was the fastest. A significant difference between the stomach and middle jejunum was observed too (P < 0.001).
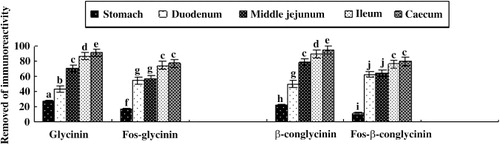
Briefly, the immunoreactivity of unglycated or glycated glycinin and β-conglycinin in the gastrointestinal tracts of piglets was consistent with those got in vitro. From the stomach to the caecum, the immunoreactivity of glycinin and β-conglycinin was decreased continually. However, the immunoreactive disappearance rates of the two proteins were low in the stomach because of the low levels of gastric acid (Effird, Armstrong, & Herman, Citation1982). In the small intestine, trypsin and chymotrypsin played the leading role in the digestion of proteins (Calinescu, Nadeau, Mulhbacher, Fairbrother, & Mateescu, Citation2007; Effird et al., Citation1982; Wu et al., Citation2000).
As can be seen from , the decrease rate of immunoreactivity of the glycated glycinin and β-conglycinin compared with unglycated forms was different in the digestive tract. In the duodenum and middle jejunum, the decrease rate of the immunoreactivity was no significant difference, this might be due to the speed of chyme through the duodenum, the glycated proteins were not decomposed in time, or there was a sugar-binding protein bond broken, the epitome re-exposured to elicit an immune response, but considered enzymatic hydrolysis in vitro, this situation hardly occurred. The difference in caecum and colon was not significant either, this was due to the immune response-glycated protein had been very low.
Glycinin contained more intra-molecular and inter-molecular disulfide bonds which had hindered its combination with trypsin or chymotrypsin (Fukushima, Citation1991; Yamauchi, Yamagishi, & Iwabuchi, Citation1991). In addition, no difference was found about ADFI, but piglets fed glycated glycinin or β-conglycinin had higher ADG and lower F/G. However, the growth performance of glycated group was still lower than those of the group without soy protein (Zhao et al., Citation2008). These results had proved that glycation had a better performance on the immunoreactivity removal of glycinin and β-conglycinin on some extent.
Taken together, glycation had improved the quality of soybean soy protein and reduce the immunoreactivity. The immunoreactivity of the glycated proteins could be further reduced in the hydrolysis of digestive enzymes. The changes of the reduce rate of the immunoreactivity in glycated soy protein antigen in the digestive tract need further study to confirm. Glycation seemed to be an effective method to remove the immunoreactivity of soybean antigen proteins and these low immunoreactive glycated proteins showed their application prospect in the feed/food industry.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Achouri, A., Boye, J. I., Yaylayan, V. A., & Yeboah, F. K. (2005). Functional properties of glycated soy 11S glycinin. Journal of Food Science, 70, C269–C274. doi:10.1111/j.1365-2621.2005.tb07172.x
- Astwood, J. D., Leach, J. N., & Fuchs, R. L. (1996). Stability of food allergens to digestion in vitro. Nature Biotechnology, 14, 1269–1273. doi:10.1038/nbt1096-1269
- Babiker, E. E., Hiroyuki, A., Matsudomi, N., Iwata, H., Ogawa, T., Bando, N., & Kato, A. (1998). Effect of polysaccharide conjugation or transglutaminase treatment on the allergenicity and functional properties of soy protein. Journal of Agricultural and Food Chemistry, 46, 866–871. doi:10.1021/jf9705072
- Boisen, S., & Fernández, J. A. (1995). Prediction of the apparent ileal digestibility of protein and amino acids in feedstuffs and feed mixtures for pigs by in vitro analyses. Animal Feed Science and Technology, 51, 29–43. doi:10.1016/0377-8401(94)00686-4
- Calinescu, C., Nadeau, É., Mulhbacher, J., Fairbrother, J. M., & Mateescu, M. A. (2007). Carboxymethyl high amylose starch for F4 fimbriae gastro-resistant oral formulation. International Journal of Pharmaceutics, 343, 18–25. doi:10.1016/j.ijpharm.2007.04.017
- Effird, R. C., Armstrong, W. D., & Herman, D. L. (1982). The development of digestive capacity in young pigs: Effects of age and weaning system. Journal of Animal Science, 55, 1380–1387.
- Fu, T. J., Abbott, U. R., & Hatzos, C. (2002). Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid – A comparative study. Journal of Agricultural and Food Chemistry, 50, 7154–7160. doi:10.1021/jf020599h
- Fukushima, D. 1991. Recent progress of soybean protein foods: Chemistry, technology and nutrition. Food Review International, 7, 323–351. doi:10.1080/87559129109540915
- Gruber, P., Becker, W. M., & Hofmann, T. (2005). Influence of the Maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin. Journal of Agricultural and Food Chemistry, 53, 2289–2296. doi:10.1021/jf048398w
- Gruber, P., Vieths, S., Wangorsch, A., Nerkamp, J., & Hofmann, T. (2004). Maillard reaction and enzymatic browning affect the allergenicity of Pru av 1, the major allergen from cherry (Prunus avium). Journal of Agricultural and Food Chemistry, 52, 4002–4007. doi:10.1021/jf035458+
- Guo, P. F., Piao, X. S., Cao, Y. H., & Ou, D. Y. (2008). Recombinant soybean protein β-conglycinin α´-subunit expression and induced hypersentivity reaction in rats. International Archives of Allergy and Immunology, 145, 102–110. doi:10.1159/000108135
- Hattori, M., Miyakawa, S., Ohama, Y., Kawamura, H., Yoshida, T., To, K., … Takahashi, K. (2004). Reduced immunogenicity of ß-Lactoglobulin by conjugation with acidic oligosaccharides. Journal of Agricultural and Food Chemistry, 52, 4546–4553. doi:10.1021/jf0353887
- Hattori, M., Nagasawa, K., Ohgata, K., Sone, N., Fukuda, A., Matsuda, H., & Takahashi, K. (2000). Reduced immunogenicity of β-Lactoglobulin by conjugation with carbocymethl dextran. Bioconjugate Chemistry, 11, 84–93. doi:10.1021/bc990096q
- Jürgen, L., Silván, J. M., Moreno, F. J., Olano, A., & Castillo, M. D. (2007). In vitro glycation and antigenicity of soy proteins. Food Research International, 40, 153–160.
- Kitamura, K., & Shibasaki, K. (1975). Isolation and some physicochemical properties of acidic subunits of soybean 11S globulin. Agricultural and Biological Chemistry, 39, 945–951. doi:10.1271/bbb1961.39.945
- Kobayashi, K., Hirano, A., Ohta, A., Yoshida, T., Takahashi, K., & Hattori, M. (2001). Reduced immunogenicity of β-lactoglobulin by conjugation with carboxymethyl dextrans differing in molecular weight. Journal of Agricultural and Food Chemistry, 49, 823–831. doi:10.1021/jf000926q
- Kuipers, B. J. H., Alting, A. C., & Gruppen, H. (2007). Comparison of the aggregation behavior of soy and bovine whey protein hydrolysates. Biotechnology Advances, 25, 606–610. doi:10.1016/j.biotechadv.2007.07.005
- Lalles, J. P., Tukur, H. M., Salgado, P., Mills, E. N. C., Morgan, M. R. A., Quillien, L., … Toullec, R. (1999). Immunochemical studies on gastric and intestinal digestion of soybean glycinin and ß-conglycinin in vivo. Journal of Agricultural and Food Chemistry, 47, 2797–2806. doi:10.1021/jf980882+
- Li, D. F., Nelssen, J. L., Reddy, P. G., Blecha, F., Klemm, R. D., Giesting, D. W., … Goodband, R. D. (1991). Measuring suitability of soybean products for early-weaned pigs with immunological criteria. Journal of Animal Science, 69, 3299–3307.
- Maruyama, N., Adachi, M., Takahashi, K., Yagasaki, K., Kohno, M., Takenaka, Y., … Utsumi, S. (2001). Crystal structures of recombinant and native soybean β-conglycinin β homotrimers. European Journal of Biochemistry, 268, 3595–3604. doi:10.1046/j.1432-1327.2001.02268.x
- Miller, B. G., Newby, T. J., Stokes, C. R., Hampson, D. J., Brown, P. J., & Bourne, F. J. (1984). The importance of dietary antigen in the cause of post weaning diarrhea in pigs. American Journal of Veterinary Research, 45, 1730–1733.
- Moreno, J. F. (2007). Gastrointestinal digestion of food allergens: Effect on their allergenicity. Biomedicine & Pharmacotherapy, 61, 50–60. doi:10.1016/j.biopha.2006.10.005
- Mujoo, R., Trinh, D. T., & Ng, P. K. W. (2003). Characterization of storage proteins in different soybean varieties and their relationship to tofu yield and texture. Food Chemistry, 82, 265–273. doi:10.1016/S0308-8146(02)00547-2
- Nagano, T., Hirotsuka, M., Mori, H., Kohyama, K., & Nishinari, K. (1992). Dynamic viscoelastic study on the gelation of 7S globulin from soybeans. Journal of Agricultural and Food Chemistry, 40, 941–944. doi:10.1021/jf00018a004
- Nakamura, A., Watanabe, K., Ojima, T., Ahn, D. H., & Saeki, H. (2005). Effect of Maillard reaction on allergenicity of scallop tropomyosin. Journal of Agricultural and Food Chemistry, 53, 7559–7564. doi:10.1021/jf0502045
- Perez, M. D., Mills, E. N. C., Lambert, N., Johnson, L. T., & Morgan, M. R. A. (2000). The use of anti-soya globulin antisera in investigating soya digestion in vivo. Journal of the Science of Food and Agriculture, 80, 513–521. doi:10.1002/(SICI)1097-0010(200003)80:4<513::AID-JSFA562>3.0.CO;2-N
- Qin, G. X., Ter Elst, E. R., Bosch, M. W., & Van der Poel, A. F. B. (1996). Thermal processing of whole soya beans: Studies on the inaction of antinutritional factors and effects on ileal digestibility in piglets. Animal Feed Science and Technology, 57, 313–324. doi:10.1016/0377-8401(95)00863-2
- Saito, T., Kohno, M., Tsumura, K., Kugimiya, W., & Kito, M. (2001). Novel method using phytase for separating soybean ß-conglycinin and glycinin. Bioscience Biotechnology and Biochemistry, 65, 884–887. doi:10.1271/bbb.65.884
- Sangild, P. T., Silver, M., Fowden, A. L., Turvey, A., & Foltman, B. (1994). Adrenocortical stimulation of stomach development in the prenatal pig. Biology of the Neonate, 65, 378–389. doi:10.1159/000244067
- Sissons, J. W., Smith, R. H., Hewitt, D., & Nyrup, A. (1982). Prediction of the suitability of soya-bean products for feeding to preruminant calves by an in-vitro immunochemical method. British Journal of Nutrition, 47, 311–318. doi:10.1079/BJN19820040
- Su, Z. G., & Guo, S. T. (2010). Mannose glycosylation of soybean protein (in Chinese). Soybean Science, 29, 486–489.
- Sun, P., Li, D. F., Li, Z. J., Dong, B., & Wang, F. L. (2008). Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. Journal of Nutritional Biochemistry, 19, 627–633. doi:10.1016/j.jnutbio.2007.08.007
- Takagi, K., Teshima, R., Okunuki, H., & Sawada, J. (2003). Comparative study of inn vitro digestibility of food proteins and effect of preheating on the digestion. Biological & Pharmaceutical Bulletin, 26, 969–973. doi:10.1248/bpb.26.969
- Usui, M., Tamura, H., Nakamura, K., Ogawa, T., Muroshita, M., Azakami, H., … Kato, A. (2004). Enhanced bactericidal action and masking of allergen structure of soy protein by attachment of chitosan through Maillard-type protein-polysaccharide conjugation. Molecular Nutrition & Food Research (Food/Nahrung), 48, 69–72.
- Van Ree, R. (2002). Carbohydrate epitopes and their relevance for the diagnosis and treatment of allergic diseases. International Archives of Allergy and Immunology, 129, 189–197. doi:10.1159/000066770
- Venkatachalam, M., Teuber, S. S., Peterson, W. R., Roux, K. H., & Sathe, S. K. (2006). Antigenic stability of pecan [Carya illinoinensis (Wangenh.) K. Koch] proteins: Effects of thermal treatments and in vitro digestion. Journal of Agricultural and Food Chemistry, 54, 1449–1458.
- Wang, T., Qin, G. X., Sun, Z. W., & Zhao, Y. (2014). Advances of research on glycinin and ß-conglycinin: A review of two major soybean allergenic proteins. Critical Reviews in Food Science and Nutrition, 54, 850–862. doi:10.1080/10408398.2011.613534
- Wilson, S., Blaschek, K., & de Mejia, E. G. (2005). Allergenic proteins in soybean: Processing and reduction of P34 allergenicity. Nutritional Review, 63(2), 47–58. doi:10.1111/j.1753-4887.2005.tb00121.x
- Wu, S. W., Murphy, P. A., Johnson, L. A., Reuber, M. A., & Fratzke, A. R. (2000). Simplified process for soybean glycinin and ß-conglycinin fractionation. Journal of Agricultural and Food Chemistry, 48, 2702–2708. doi:10.1021/jf990785w
- Yamauchi, F., Yamagishi, T., & Iwabuchi, S. (1991). Molecular understanding of heat-induced phenomena of soybean protein. Food Review International, 7, 283–322. doi:10.1080/87559129109540914
- Zhao, Y., Qin, G. X., Sun, Z. W., Zhang, X. D., Bao, N., Wang, T., … Sun, L. (2008). Disappearance of immunoreactive glycinin and ß-conglycinin in the digestive tract of piglets. Archives of Animal Nutrition, 4, 322–330. doi:10.1080/17450390802190318