Abstract
To explore the mechanism for amelioration effect of bovine casein glycomacropeptide on oxazolone-induced ulcerative colitis in mice. BALB/c mice were divided into four groups as follows: (1) healthy control, (2) ulcerative colitis model control, (3) casein glycomacropeptide-supplemented ulcerative colitis model (CGMP group), and (4) sulfasalazine-supplemented ulcerative colitis model (SASP group). At the end of the administration period, serum IL-1β, IL-2, IL-4, IL-5, IFN-γ, TNF-α, and IL-10 were measured by cytometric bead array and enzyme-linked immunosorbent assay, and mitogen-activated protein kinase p38 and NF-κB p65 were determined by Western blotting. The results demonstrated the inactivation of NF-κB and MAPK signaling pathways and the downregulation of the serum levels of IL-1β, IL-5, IFN-γ, and TNF-α and upregulation of IL-10 production. These changes may be associated with amelioration of oxazolone-induced ulcerative colitis by bovine casein glycomacropeptide.
Keywords:
Introduction
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD), which develops in the rectum and the colon. Although the exact mechanism of the pathogenesis of UC remains unclear, several studies have reported that environmental factors, especially enteric microflora, as well as genetic and immune factors, play an important role in the pathogenesis of UC (Farrell & Peppercorn, Citation2002). Dysfunction of the mucosal immune system has been implicated in the UC development (Ordás, Eckmann, Talamini, Baumgart, & Sandborn, Citation2012; Sartor, Citation2006). Interestingly, CGMP has been shown to inhibit the excessive apoptosis in colon epithelial cells of mice with UC through FasR/FasL, NF-κB P65, and TGF-β1 pathways (Chen, Wang, Zhu, Yan, & Pang, Citation2014).
Prevention and treatment of UC have been intensively studied recently. The management of UC comprises three basic treatments: drugs, agents, and chemicals. However, specific treatment should be tailored according to UC severity of the patients and the extent of colonic involvement (Ordás et al., Citation2012). Topical or oral administration of mesalazine or corticosteroids, as well as their combination, can induce remission in patients with mild to moderate UC. However, if the patients are resistant to the above treatments or develop steroid dependency, they need subcutaneous injection of adalimumab, an anti-TNF-α monoclonal antibody agent. The patients with acute severe UC should have a series of examination and receive intravenous corticosteroids in hospital. If the response is poor, patients should receive infliximab or ciclosporin, or even have surgery (for example, colectomy). Interestingly, several recent studies have indicated that probiotics as dietary supplement could be a promising strategy to control UC (Kruis et al., Citation2004; Miele et al., Citation2009; Mardini & Grigorian, Citation2014). Besides, a number of researches had proved that some peptide can alleviate the severity of UC and may be potentially useful in the treatment of UC (Azuma et al., Citation2014; Kim et al., Citation2013; Lin, Zamora, Takahashi, & Lui, Citation2007).
A polypeptide fraction containing sialic acid was isolated from the κ-casein fraction of bovine milk (Delfour, Jollès, Alais, & Jollès, Citation1965). The cleavage of the Phe–Met bond at position 105–106 of milk κ-casein by chymosin produces two fragments: insoluble paracasein (residues 1–105) and soluble polypeptide (residues 106–169); the latter is known as caseinomacropeptide (CMP). Approximately, 30–50% of CMP exists in the glycosylated form, i.e., casein glycomacropeptide (CGMP). CGMP has no ultraviolet absorption at 280 nm because it contains no aromatic amino acid. CGMP can serve as a dietary supplement to alleviate the symptoms of phenylketonuric patients. Moreover, CGMP has several other bioactivities, such as inhibition of pathogen proliferation, enhancement of probiotic proliferation, and regulation of immune responses (Bruck et al., Citation2003; Oh, Worobo, Kim, Rheem, & Kim, Citation2000; Otani & Hata, Citation1995). Previous studies have demonstrated the immunoregulatory function of CGMP (Requena et al., Citation2009).
In this study, we sought to understand the anti-inflammatory mechanism of CGMP in oxazolone-induced UC in mice. We employed sulfasalazine (SASP) as a positive control for UC treatment, since SASP has been known to have special affinity to intestinal mucosa and improve intestinal mucosa recovery. Serum IL-1β, IL-2, IL-4, IL-5, IFN-γ, TNF-α, and IL-10 were measured, and MAPK p-p38 and NF-κB p65 were determined at the end of the experimental treatments. We found that CGMP had significant effect on regulating the immune system, microflora, and inflammation in the intestine and contributing to the protection of intestinal tract.
Material and methods
Mice
BALB/c mice (SPF, male, 25 ± 2 g) and basal diet were purchased from the experimental animal center of the Military Academy of Medical Sciences of the Chinese People’s Liberation Army. Individually ventilated cages were used in the experiments.
Reagents
CGMP was obtained from Tatua Co-Operative Dairy Company Limited (Tatuanui, Morrinsville, New Zealand) and achieved 71% purity. Oxazolone was purchased from Sigma-Aldrich (St. Louis, MO, USA). SASP was obtained from Sunve Pharmaceutical (Shanghai, China). A mouse-specific Th1/Th2 Cytokine Kit (BD Biosciences, San Jose, CA, USA) was used for cytometric bead array (CBA) analysis. The IL-1β and IL-10 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R& D Systems (Minneapolis, MN, USA). A nuclear/cytoplasmic protein extraction kit was obtained from KeyGen Biotech (Nanjing, China). The BCA protein assay kit was obtained from Biosynthesis Biotechnology (Beijing, China). Rabbit anti-mouse p-p38 polyclonal antibody, mouse anti-NF-κB p65 monoclonal antibody, and mouse anti-Histone H1 monoclonal antibody were all purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Rabbit anti-mouse β-actin monoclonal antibody, HRP-labeled goat anti-mouse IgG, and HRP-labeled goat anti-rabbit IgG were obtained from the Zhongshan Golden Bridge Biotechnology (Beijing, China). Western blot stripping buffer and an enhanced chemiluminescence detection kit were obtained from Sun Biomedical Technology (Beijing, China).
Ulcerative colitis model
BALB/c mice (n = 40) were provided with the basal diet for one week to acclimatize to the diet. Mice were randomly grouped (n = 10/group) as follows: healthy control, UC model control, CGMP-supplemented UC model (CGMP group), and SASP-supplemented UC model (SASP group). The SASP group served as a positive control to evaluate the anti-inflammatory effect of CGMP on oxazolone-induced UC. The abdominal hair was shaved from the mice in each group. The shaved area of the mice from the UC control, CGMP, and SASP groups was rubbed with a cotton swab dipped in 3% oxazolone (dissolved in 50% ethanol) once a day for 2 days. In parallel, the shaved area of the mice in the healthy control group was treated with 50% ethanol alone. All the mice were then under food restriction for 24 h. About 150 μL of 1% oxazolone (dissolved in 50% alcohol) was administrated via gastric cannula into the small intestine using a silicone rubber tube, which was inserted approximately 4 cm into the anus of the mice supplemented with CGMP and SASP. The UC control group received 150 μL of 50% alcohol in parallel. Every day, the excreta stick to the anus of the UC control, CGMP, and SASP groups were inspected to determine the symptoms of diarrhea.
CGMP and SASP supplementation
Shortly after treatment with oxazolone, CGMP (50 mg/kg/d) or SASP (40 mg/kg/d) was given to the mice orally as a single bolus of supplement to the basal diet for five consecutive days. The healthy and UC control groups were fed with the basal diet alone.
Serum and colon tissue collection
Mouse peripheral blood was collected from the ophthalmic venous plexus 4 h after final supplementation of CGMP and SASP. The blood samples were kept at 4° C overnight and the serum was collected by centrifugation at 1000×g for 15 min. After blood collection, the mice in all the experimental groups were sacrificed to collect the colon. The colon was homogenized to extract nuclear and cytoplasmic proteins. Protein concentrations of the extracted samples were measured with the BCA protein assay kit.
Cytometric bead array
Serum (50 μL) was mixed with equal volume of capture beads (a mixture of IL-2, IL-4, IL-5, IFN-γ, and TNF-α capture beads) and PE-conjugated detection antibodies (a mixture of specific antibodies against the above cytokines) and incubated at room temperature for 2 h. The incubated mixture was then washed with 1 mL of washing solution and centrifuged at 200×g for 5 min. The bead pellet was resuspended in 300 μL of washing solution and the cytokine levels were determined using a FACS Calibur flow cytometer (BD Biosciences). The data were analyzed with BD CBA data analysis software (Fulton, McDade, Smith, Kienker, & Kettman, Citation1997).
Enzyme-linked immunosorbent assay
Serum IL-1β and IL-10 levels were determined using the IL-1 and IL-10 ELISA kits according to the instructions provided by manufacturer (Nakahama et al., Citation2011; Liesz et al., Citation2011).
Western blotting
MAPK p-p38 and NF-κB p65 protein levels were determined by Western blotting (Lee et al., Citation2014). The protein quantification was performed using the Quantity One gel image analysis software.
Statistical analysis
All experimental data were processed with SPSS 19.0 software for one-way analysis of variance (ANOVA). The data shown are means ± standard deviation (SD).
Results
Effect of CGMP on oxazolone-induced ulcerative colitis in mice
During the experimental period, the mice in the healthy control group behaved normally. By contrast, the mice in the UC control group had severe diarrhea as described previously (Chen et al., Citation2014; Jia & Chen, Citation2010; Wang & Chen, Citation2012). The mice in the CGMP and SASP groups showed similar diarrheal symptoms compared to the mice in the UC control group within the first 3 days upon supplementation. However, the diarrheal severity was dramatically decreased starting from the fourth day after CGMP and SASP supplementation. These results validate our experimental approach and indicate the amelioration effect of CGMP on oxazolone-induced UC mouse model.
Effect of experimental treatments on the serum IL-2, IL-4, IL-5, IFN-γ, TNF-α, IL-1β, and IL-10 Levels
To understand the mechanism of amelioration effect of CGMP in UC, we measured the serum cytokine levels in the mice from all the four experimental groups. IL-2, IL-4, IL-5, IFN-γ, and TNF-α protein levels were determined by CBA (). The representative data showed no distinct difference in the PE fluorescence intensity of serum IL-2 or IL-4 among the four experimental groups, indicating the constant production of the two cytokines in serum under all experimental conditions. The fluorescence intensities of serum IL-5 and IFN-γ increased in the UC control but remained unchanged in the SASP and CGMP groups compared to the healthy control group. The fluorescence intensity of serum TNF-α in the CGMP and SASP groups was higher than that of the healthy control group but lower than that of the UC control group.
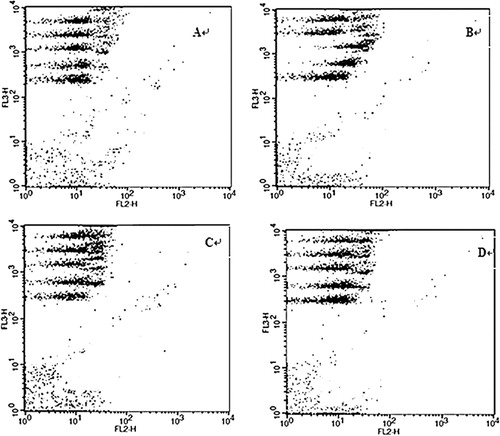
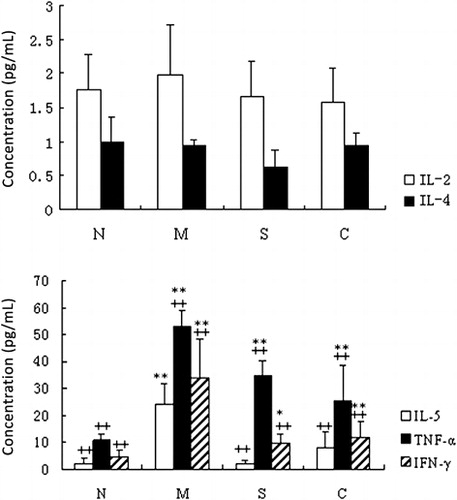
The serum levels of IL-2, IL-4, IL-5, IFN-γ, and TNF-α were further quantified in all the experimental groups (). There was no significant difference (P > 0.05) in the levels of serum IL-2 or IL-4 among the four groups (, upper panel). Serum IL-5 concentration was significantly (P < 0.01) higher in the UC control group than that of the healthy control group and was significantly lower (P < 0.01) in the CGMP and SASP groups than that of the UC control group (, lower panel). Serum TNF-α concentration of the UC control group was significantly higher than that of the healthy control group (P < 0.01). Both CGMP and SASP groups had significantly decreased levels of TNF-α compared to the UC control group (P < 0.01). The concentration of IFN-γ in the UC control group was significantly higher than the other three experimental groups (P < 0.01).
The serum levels of IL-1β and IL-10 mice from all the experimental groups were determined by ELISA (). The IL-1β concentration was significantly increased in the UC control group compared to the other three groups (P < 0.05). By contrast, the IL-10 concentration was significantly decreased in the UC control compared to the other three groups (P < 0.05). Serum IL-1β and IL-10 concentrations showed no significant difference among the healthy control, SASP, and CGMP groups (P > 0.05).
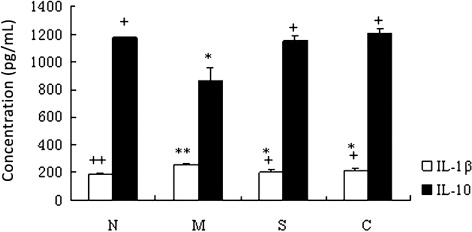
Taken all together, our results indicated that CGMP suppressed the cytokine production of IL-5, IFN-γ, TNF-α, IL-1β and enhanced the IL-10 secretion in the UC mouse model, suggesting that the changes of those cytokine production are likely correlate with the amelioration effect of CGMP on UC.
Effect of experimental treatments on NF-κB p65 and MAPK p-p38 expression in mice with ulcerative colitis
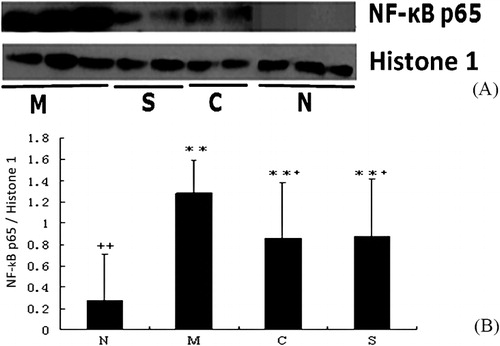
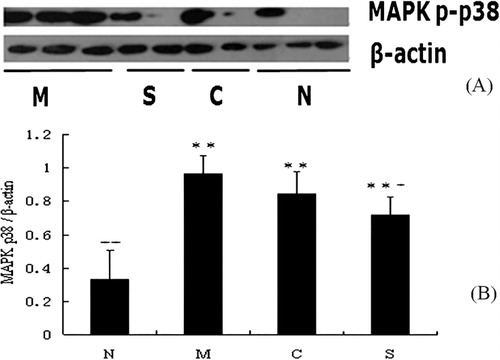
To explore the signaling pathways associated with the amelioration effect of CGMP on UC, we measured the expression levels of NF-κB p65 and MAPK p-p38 by analyzing the colonic tissue extracts using western blotting ( and ). We observed a decrease in the expression of NF-κB p65 and MAPK p-p38 in the CGMP and SASP groups compared to the UC control group. The relative expression levels of NF-κB p65 and MAPK p-p38 were obtained after normalization to the internal controls Histone 1 and β-actin, respectively ( and ). The expression of NF-κB p65 was significantly increased in the UC control than the healthy control group (P < 0.01). Both CGMP and SASP supplementation significantly inhibited the expression of NF-κB p65 compared to the UC control group (P < 0.05), although the expression levels of NF-κB p65 in these two groups were significantly higher than that in the healthy control group (P < 0.01). Likewise, the expression of MAPK p-p38 was also significantly higher in the UC control group than the healthy control group (P < 0.01). SASP supplementation significantly reduced the expression of MAPK p-p38 compared to the UC control group (P < 0.05). CGMP supplementation also showed a decrease tendency (P > 0.05) of MAPK p-p38 expression compared to the UC control. Thus, we concluded that CGMP-induced amelioration effect on UC could be involved in the inactivation of NF-κB and MAPK signaling pathways.
Discussion
General IBD drugs including salicylates, steroid hormones, and immune inhibitors have high remission rate, which is 70% for Crohn’s disease and 80% for UC. However, the current therapeutic treatment can only give rise to a 50% reduction in recurrence rate. Almost two-thirds of Crohn’s disease patients and one-third of UC patients need surgery eventually. Another promising approach for UC treatment is nutritional management. In 2005, Chinese Guidelines and Rules for the Nutritional Management of Crohn’s Disease were issued by the Society of Parenteral and Enteral Nutrition of Chinese Medical Association, the Society of Gastroenterology, the Society of Surgery, and the Society of Nursing. However, with regards to the individual patient, more accurate and effective instructions for nutritional management remain to be established. Our laboratory has explored the methodologies to increase the utilization of raw milk and established several technologies to isolate and purify CGMP, as well as evaluate the function of CGMP-related bioactive peptides, leading to a solid foundation for the industrialization of CGMP and CGMP-related bioactive peptides. In other to provide more insights of the application of CGMP, it is necessary to better understand the mechanism by which CGMP improves and treats experimentally induced UC mouse model.
Many studies have showed that the balance of Th1/Th2 is closely associated with a variety of autoimmune diseases including IBD (Akitake et al., Citation2010; Nicholson & Kuchroo, Citation1996). Th1 cells drive the cellular immunity, while Th2 cells drive the humoral immunity. The differentiation and polarization of T cell subsets is controlled by a variety of transcription factors, which play an essential role in gene expression and regulation of Th1/Th2 cytokines (Agnello et al., Citation2003; Asnagli & Murphy, Citation2001; Sullivan, Juedes, Szabo, Von Herrath, & Glimcher, Citation2003). To examine the changes of serum cytokine levels in the experimental UC mouse models after CGMP supplementation, we employed a multi-factor detection technique – CBA, which consists of a mixture of five different capture beads and fluorescent detection antibodies against IL-2, IL-4, IL-5, IFN-γ, and TNF-α. We observed that CGMP significantly inhibited the production of IL-5, IFN-γ, and TNF-α which were induced in the UC models, suggesting an anti-inflammatory effect of CGMP on UC.
Several previous studies have demonstrated that several key transcription factors, such as STAT6, GATA3, JunB, and c-maf, can induce and enhance the production of Th2-cytokines (Glimcher & Murphy, Citation2000; Li, Tournier, Davis, & Flavell, Citation1999; Zheng & Flavell, Citation1997). Recent studies have also reported that NF-κB p65 is highly expressed in UC patients, suggesting that NF-κB could be a therapeutic target for IBD (Andresen et al., Citation2005). In this present study, we found that oral administration of bovine CGMP significantly inhibited NF-κB p65 expression. Thus, the contribution of the oral administration of bovine CGMP to the recovery from UC is probably associated with the inhibition of NF-κB p65 transcription factor. Two hypotheses can be proposed to explain the inhibitory effect of CGMP on NF-κB p65. One is the direct binding of CGMP to p65, which potentially inhibits the binding of p65 to the response element in DNA resulting in the inhibition of p65 activity. The other possibility is that CGMP regulates the upstream molecules in NF-κB signaling pathway, leading to the reduced activity of NF-κB p65 in the nucleus (Kamura et al., Citation1998; Maine, Mao, Komarck, & Burstein, Citation2007). Many studies have shown that IKK is a key mediator in degradation of IκB and activation of NF-κB signaling pathway. IKK activation promotes the phosphorylation of IκB, which is in turn recognized by E3RS IκB, and then leads to ubiquitination of IκB. The ubiquitinated IκB is degraded by proteasome and then result in the nuclear translocation of p65, which binds to the target gene and activates gene transcription. Thus, we speculate that CGMP probably mediate IKK activity to inhibit the phosphorylation of IκB and the translocation of p65.
It is known that the MAPK signaling pathway is involved in the activation of the NF-κB signaling pathway. Rho protein, a member of G protein family, activates the MAPK and further activates the NF-κB signaling pathway (Hippenstiel, Soeth, & Kellas, Citation2000). Although there was no significant difference in the expression of MAPK p38 on the CGMP group compared to the UC control, there was a decrease tendency of its expression level upon CGMP supplementation. Moreover, we measured the serum levels of IL-1β and TNF-α, which are known to be closely regulated by the MAPK p38 and NF-κB signaling pathways (Nemoto, Didonato, & Lin, Citation1998). We found that both of IL-1β and TNF-α were significantly decreased in the CGMP group compared to the UC group. By contrast, we observed that the serum level of an anti-inflammatory cytokine IL-10 was significantly increased in the CGMP group compared to the UC group. Our observation is consistent with previous reports that IL-10 controls intestinal inflammation by regulating NF-κB activity and could serve as a novel therapeutic target for IBD (Tamada et al., Citation2006). Thus, we speculate that CGMP may exert its amelioration effect on UC by inhibiting the activation of both NF-κB and MAPK pathways.
In conclusion, the mechanism for amelioration effect of bovine casein CGMP on oxazolone-induced UC mouse model could be attributed to the CGMP-mediated inactivation of NF-κB and MAPK signaling pathways, leading to the enhanced secretion of IL-10 and decreased production of IL-1β, IL-5, IFN-γ, and TNF-α. In addition, our data have suggested that CGMP might act as a transcription factor that plays a key role in regulating cytokine gene expression to alleviate the severity of UC. Not only do these findings unveil new insights into the application of bovine bioactive peptides on modulation, improvement, and treatment of IBD, they also provide several potential biomarkers for IBD therapy, such as IL-5, IFN-γ, TNF-α, IL-1β, and IL-10.
Acknowledgments
We thank our national financial support. We would like to acknowledge Dr Yi Yao in Yale University School of Medicine for kindly editing and proofreading this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agnello, D., Lankford, C. S. R., Bream, J., Morinobu, A., Gadina, M., O’Shea, J. J., & Frucht, D. M. (2003). Cytokine and transcription factors that regulate T helper cell differentiation: New players and new insights. Journal of Clinical Immunology, 23(3), 147–161. doi:10.1023/A:1023381027062
- Akitake, R., Nakase, H., Tamaoki, M., Ueno, S., Mikami, S., & Chiba, T. (2010). Modulation of Th1/Th2 balance by infliximab rescues postoperative occurrence of small-intestinal inflammation associated with ulcerative colitis. Digestive Diseases and Sciences, 55, 1781–1784. doi:10.1007/s10620-009-0910-5
- Andresen, L., Jorgensen, V. L., Perner, A., Hansen, A., Eugen-Olsen, J., & Rask-Madsen, J. (2005). Activation of nuclear factor κB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut, 54, 503–509. doi:10.1136/gut.2003.034165
- Asnagli, H., & Murphy, K. M. (2001). Stability and commitment in T helper cell development. Current Opinion in Immunology, 13, 242–247. doi:10.1016/S0952-7915(00)00210-7
- Azuma, K., Osaki, T., Tsuka, T., Imagawa, T., Okamoto, Y., & Minami, S. (2014). Effects of fish scale collagen peptide on an experimental ulcerative colitis mouse model. PharmaNutrition, 2, 161–168. doi:10.1016/j.phanu.2014.10.001
- Bruck, W. M., Kelleher, S. L., Gibson, G. R., Nielsen, K. E., Chatterton, D. E. W., & Lonnerdal, B. (2003). rRNA probes used to quantify the effects of glycomacropeptide and alpha-lactalbumin supplementation on the predominant groups of intestinal bacteria of infant rhesus monkeys challenged with enteropathogenic Escherichia coli. Journal of Pediatric Gastroenterology and Nutrition, 37, 273–280. doi:10.1097/00005176-200309000-00014
- Chen, Q., Wang, H., Zhu, C., Yan, Y., & Pang, G. (2014). Anti-apoptotic effects of milk-derived casein glycomacropeptide on mice with ulcerative colitis. Food and Agricultural Immunology, 25, 453–466. doi:10.1080/09540105.2013.823912
- Delfour, A., Jollès, J., Alais, C., & Jollès, P. (1965). Caseino-glycopeptides: Characterization of a methionine residue and of the N-terminal sequence. Biochemical and Biophysical Research Communications, 19, 452–455. doi:10.1016/0006-291X(65)90145-2
- Farrell, R. J., & Peppercorn, M. A. (2002). Ulcerative colitis. The Lancet, 359, 331–340. doi:10.1016/S0140-6736(02)07499-8
- Fulton, R. J., McDade, R. L., Smith, P. L., Kienker, L. J., & Kettman, J. R., Jr. (1997). Advanced multiplexed analysis with the FlowMetrix system. Clinical Chemistry, 43, 1749–1756.
- Glimcher, L. H., & Murphy, K. M. (2000). Lineage commitment in the immune system: The T helper lymphocyte grows up. Genes & Development, 14, 1697–1711. doi:10.1101/gad.14.14.1693
- Hippenstiel, S., Soeth, S., & Kellas, B. (2000). Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood, 95, 3044–3051.
- Jia, Y.-C., & Chen, Q.-S. (2010). Improvement of oxazolone-induced ulcerative colitis in mice by bovine casein glycomacropeptide. Food Science, 31, 365–368.
- Kamura, T., Sato, S., Haque, D., Liu, L., Kaelin, W. G., Jr., Conaway, R. C., & Conaway, J. W. (1998). The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes & Development, 12, 3872–3881. doi:10.1101/gad.12.24.3872
- Kim, S. D., Kwon, S., Lee, S. K., Kook, M., Lee, H. Y., Song, K.-D., … Bae, Y.-S. (2013). The immune-stimulating peptide WKYMVm has therapeutic effects against ulcerative colitis. Experimental & Molecular Medicine, 45(9), e40. doi:10.1038/emm.2013.77
- Kruis, W., Frič, P., Pokrotnieks, J., Lukáš, M., Fixa, B., Kaščák, M., … Schulze, J. (2004). Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut, 53, 1617–1623. doi:10.1136/gut.2003.037747
- Lee, S.-J., Shin, J.-S., Choi, H.-E., Lee, K.-G., Cho, Y.-W., An, H.-J., … Lee, K.-T.(2014). Chloroform fraction of Solanum tuberosum L. cv Jayoung epidermis suppresses LPS-induced inflammatory responses in macrophages and DSS-induced colitis in mice. Food and Chemical Toxicology, 63, 53–61. doi:10.1016/j.fct.2013.10.040
- Li, B., Tournier, C., Davis, R. J., & Flavell, R. A. (1999). Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. The EMBO Journal, 18, 420–432. doi:10.1093/emboj/18.2.420
- Liesz, A., Zhou, W., Mracsko, E., Karcher, S., Bauer, H., Schwarting, S., … Veltkamp, R. (2011). Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain, 134, 704–720. doi:10.1093/brain/awr008
- Lin, X., Zamora, P. O., Takahashi, K., & Lui, Y. (2007). Alleviation of experimental ulcerative colitis with the synthetic peptide, F2A4-K-NS (fibratide). Digestive Diseases and Sciences, 52, 2054–2062. doi:10.1007/s10620-006-9641-z
- Maine, G. N., Mao, X., Komarck, C. M., & Burstein, E. (2007). COMMD1 promotes the ubiquitination of NF-κB subunits through a cullin-containing ubiquitin ligase. The EMBO Journal, 26, 436–447. doi:10.1038/sj.emboj.7601489
- Mardini, H. E., & Grigorian, A. Y. (2014). Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: A meta-analysis. Inflammatory Bowel Diseases, 20, 1562–1567. doi:10.1097/MIB.0000000000000084
- Miele, E., Pascarella, F., Giannetti, E., Quaglietta, L., Baldassano, R. N., & Staiano, A. (2009). Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. The American Journal of Gastroenterology, 104, 437–443. doi:10.1038/ajg.2008.118
- Nakahama, T., Kimura, A., Nguyen, N. T., Chinen, I., Hanieh, H., Nohara, K., … Kishimoto, T. (2011). Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proceedings of the National Academy of Sciences of United States of America, 108, 14222–14227. doi:10.1073/pnas.1111786108
- Nemoto, S., Didonato, J. A., & Lin, A. (1998). Coordinate regulation of IĸB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB-Inducing Kinase. Molecular and Cellular Biology, 18, 7336–7343.
- Nicholson, L. B., & Kuchroo, V. K. (1996). Manipulation of the Th1/Th2 balance in autoimmune disease. Current Opinion in Immunology, 8, 837–842. doi:10.1016/S0952-7915(96)80013-6
- Oh, S., Worobo, R. W., Kim, B.-C., Rheem, S., & Kim, S. (2000). Detection of the cholera toxin-binding activity of κ-casein macropeptide and optimization of its production by the response surface methodology. Bioscience, Biotechnology, and Biochemistry, 64, 516–522. doi:10.1271/bbb.64.516
- Ordás, I., Eckmann, L., Talamini, M., Baumgart, D. C., & Sandborn, W. J. (2012). Ulcerative colitis. The Lancet, 380, 1606–1619. doi:10.1016/S0140-6736(12)60150-0
- Otani, H., & Hata, I. (1995). Inhibition of proliferative responses of mouse spleen lymphocytes and rabbit Peyer’s patch cells by bovine milk caseins and their digests. Journal of Dairy Research, 62, 339–348. doi:10.1017/S0022029900031034
- Requena, P., Daddaoua, A., Guadix, E., Zarzuelo, A., Suárez, M. D., de Medina, F. S., & Martínez-Augustin, O. (2009). Bovine glycomacropeptide induces cytokine production in human monocytes through the stimulation of the MAPK and the NF-κB signal transduction pathways. British Journal of Pharmacology, 157, 1232–1240. doi:10.1111/j.1476-5381.2009.00195.x
- Sartor, R. B. (2006). Mechanisms of Disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nature Clinical Practice Gastroenterology & Hepatology, 3, 390–407. doi:10.1038/ncpgasthep0528
- Sullivan, B. M., Juedes, A., Szabo, S. J., Von Herrath, M., & Glimcher, L. H. (2003). Antigen-driven effector CD8 T cell function regulated by T-bet. Proceedings of the National Academy of Sciences of United States of America, 100, 15818–15823. doi:10.1073/pnas.2636938100
- Tamada, S., Asai, T., Kuwabara, N., Iwai, T., Uchida, J., Teramoto, K., … Miura, K. (2006). Molecular mechanisms and therapeutic strategies of chronic renal injury: The role of nuclear factor kappaB activation in the development of renal fibrosis. Journal of Pharmacological Sciences, 100(1), 17–21. doi:10.1254/jphs.FMJ05003X4
- Wang, H., & Chen, Q.-S. (2012). Milk-derived casein glycomacropeptide inhibits ulcerative colitis in mice through apoptosis resistance. Food Science, 33, 230–234.
- Zheng, W., & Flavell, R. A. (1997). The transciption factor GATA-3 is necessary and sufficent for Th2 cytokine gene expression in CD4+ T cells. Cell, 89, 587–596. doi:10.1016/S0092-8674(00)80240-8
