Abstract
Cadmium (Cd) is considered one of the most hazardous heavy metals. Therefore, it is important to detect Cd. A polyclonal antibody that recognizes Cd–ethylenediamine-N,N,N′,N′-tetraacetic acid complex was prepared via the injection of BALB/c mice with Cd–2-(4-aminobenzyl)-diethylene triamine pentaacetic acid–keyhole limpet hemocyanin. A simple, inexpensive, and reliable indirect competitive enzyme-linked immunosorbent assay (ELISA) based on the polyclonal antibody was developed for determination of Cd. The assay was specific to Cd with an IC50 value of 2.042 μg mL−1 and the detection limit of 0.0135 μg mL−1. Cross-reactivities (CRs) with Cu2+, Pb2+, Zn2+, Mg2+, Ca2+, Fe2+, and Ni2+ were below 0.001% except for Hg2+ with a CR less than 6%. The recoveries from deionized water were from 92.2% to 116.0%. Results indicated that the ELISA based on the polyclonal antibody would be very promising analytical tools for rapid and sensitive determination of metal ions in the environment.
Introduction
Heavy metal contamination is of serious concern to human health since these substances are nonbiodegradable and retained by the ecological system (Verma & Singh, Citation2005). Among them, cadmium (Cd) is known to be both extremely toxic and ubiquitous in natural environments (Larison, Likens, Fitzpatrick, & Crock, Citation2000). Cd is a natural constituent of rocks rich in phosphate and due to the use of phosphate-based fertilizers in agriculture. Various sources of Cd pollution exist, because it can be broadly distributed in soil, water, and food (Amdur, Doull, & Klaanen, Citation1996). In the polluted area, Cd emitted in the past continues to contaminate the soil and remains a source of contaminated particles (Nawrot et al., Citation2006). Cd is not easily degraded in the biosphere, accumulating in the upper tiers of food chains or via metal containing dust particles (He, Liu, Yang, & Sun, Citation2009). Cd is highly mobile in biological systems and presents potential risks to the environment and to human health (Adriano, Citation2001). The biological half-life of Cd is very long (decades) in the muscles, kidneys, liver, and whole body (Jin et al., Citation2013). Results of both human and animal studies suggested an association between Cd exposure, elevated blood glucose levels, and the development of diabetes and diabetes-related kidney disease (Edwards & Prozialeck, Citation2009). Moreover, there was a dose–response relationship between Cd exposure and prevalence of osteoporosis. Osteoporosis caused by Cd was related to kidney dysfunction (Jin et al., Citation2004). Furthermore, Cd and Cd compounds were proved to be human carcinogens based on findings of a significant association between risk of lung cancer and environmental exposure to Cd (Nawrot et al., Citation2006). Therefore, a convenient and rapid method to detect Cd is urgently needed for farm industry and human health. Traditional instrument analysis methods for Cd are flameless atomic absorption spectrometry (Xu, Wu, Wang, Shang, & Jiang, Citation2013; Yaman, Citation2005), molecular fluorescence spectroscopy (Charles, Dubois, Yunus, & Vander Donckt, Citation2000), electrothermal atomic absorption spectrometry (Wang & Hansen, Citation2002), and inductively coupled plasma atomic emission spectrometric (Beiraghi, Pourghazi, & Amoli-Diva, Citation2014; Zougagh, de Torres, & Cano Pavón, Citation2002). These methods are precise but suffer from the disadvantages of expensive, the need for good professional techniques, and the fact that they are mostly laboratory bound. As an alternative to these traditional instrument intensive methods, immunoassays have significant advantages such as faster speed, less expensive, and ease of use. Immunoassays, which rely on the specific reaction between antibody and antigen, have therefore become a powerful tool to detect trace compounds in many fields such as food inspection (Selvi, Sreenivasa, & Manonmani, Citation2011), environmental analysis (Xu et al., Citation2007), and clinical diagnosis (Lu, Shi, Qin, & Lin, Citation2009; Shiomi et al., Citation2008).
Studies based on antibody and corresponding enzyme-linked immunosorbent assay (ELISA) for Cd determination have been reported (He et al., Citation2009; Liu et al., Citation2009; Zhu et al., Citation2007). Among them, 1-(4-isothiocyanobenzyl)ethylenediamine N,N,N′,N′-tetraacetic acid (ITCBE) is the most commonly used bifunctional chelator. As is known, 2-(4-aminobenzyl)-diethylene triamine pentaacetic acid (DTPA) is much cheaper than ITCBE. To the best of our knowledge, there are no reports on the application of Cd–DTPA–keyhole limpet hemocyanin (Cd–DTPA–KLH) to produce polyclonal antibodies for Cd. This paper described a reliable indirect competitive ELISA based on the polyclonal antibody for Cd determination. This immunoassay used a newly described polyclonal antibody that recognized Cd–ethylenediamine-N,N,N′,N′-tetraacetic acid (Cd–EDTA) complexes but not metal-free EDTA (Khosraviani, Pavlov, Flowers, & Blake, Citation1998; Sasaki, Oguma, Namiki, & Ohmura, Citation2009). Polyclonal antibody was prepared through the injection of BALB/c mice with Cd–DTPA–KLH. The results showed that the indirect competitive ELISA was specific for Cd. Additionally, the proposed ELISA may be a useful tool for monitoring Cd residues in food and environmental samples.
Materials and methods
Chemicals and apparatus
Four BALB/c mice (6–8 weeks old) were provided by Shanghai Shengwang Co., Ltd. (China). DTPA was purchased from Macrocyclics (USA). Bovine serum albumin (BSA), KLH, and goat anti-mouse IgG conjugated with horseradish peroxidase were obtained from Sigma-Aldrich (USA). 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) was purchased from Shanghai Richu Bioscience Co., Ltd. (China). EDTA, 3,3′5,5′-tetramethylbenzidine (TMB), glutaraldehyde, and glycine were purchased from Sinopharm Chemical Reagent (China). Cd (99.999%) and other atomic absorption grade metal ions were purchased from Merck (Germany). Ninety-six-well polystyrene microtiter plates were obtained from Corning-Costar (USA). Buffers and solutions used include coating buffer (0.05 M carbonate buffer, pH 9.6), phosphate-buffered saline (PBS; 0.1 M, pH 7.4), PBS with 0.05% (v v−1) Tween-20 (PBST, pH 7.4), citrate–phosphate buffer (0.01 M citric acid and 0.03 M monosodium phosphate, pH 5.5), substrate solution (4.0 mg urea peroxide added to 10.0 mL of citrate–phosphate buffer containing 0.1 mg mL−1 TMB), and a stop solution (2.0 M sulfuric acid). Deionized water used for making buffers and solutions was collected from a Millipore Water Purification System (Millipore Co., USA). All glasswares were kept in mixed acids (concentrated HCl:concentrated HNO3 = 1:1, v v−1) overnight and thoroughly rinsed with deionized water before use. All chemicals and solvents used were of analytical grade and were used as received.
Preparation of Cd–DTPA–protein conjugates
Immunogen Cd–DTPA–KLH and coating antigen Cd–DTPA–BSA were prepared according to our former report (Xi, Xing, Shi, Wu, & Zhou, Citation2012). Briefly, 5 mg of DTPA dissolved in 5 mL HEPES buffer (0.01 M, pH 7.4) was added into 10 mg of KLH dissolved in 5 mL HEPES buffer (0.01 M, pH 7.4). Then, 1 mL of glutaraldehyde (0.2%) was added for linking DTPA and KLH. Ten hours later, the mixture was purified by an Amicon Ultra-15 device for removing the unreacted low molecular weight reagents. The conjugate was washed three times with HEPES buffer (0.01 M, pH 7.4). Then, 0.3 mL [8.9 mM Cd(NO3)2 in 0.5 M HNO3] was added into the DTPA–protein solution and stirred for 5 h at room temperature. Unreacted ingredients were removed by ultrafiltration. The synthesis of coating antigen Cd–DTPA–BSA was similar to that of Cd–DTPA–KLH. Additional DTPA–BSA conjugate was also prepared as for the preparation of Cd–DTPA–BSA, but Cd(NO3)2 was not added.
Production of polyclonal antibodies
Three BALB/c mice (labeled as R1, R2, and R3, 6–8 weeks old) were immunized by the immunogen with the intraperitoneal injection. Routinely, Cd–DTPA–KLH (100 μg, calculated on protein) diluted with HEPES buffer was emulsified with Freund’s complete adjuvant (1:1 volume ratio) and injected into the peritoneal cavity. After three weeks, a second injection was administered. The immunogen was emulsified with Freund’s complete adjuvant for the first two injections and with Freund’s incomplete adjuvant for the subsequent injections. After two weeks, a third injection of Cd–DTPA–KLH (100 μg, calculated on protein) emulsified with Freund’s incomplete adjuvant (1:1 volume ratio) was administered. Injections third through fifth were conducted on a biweekly interval in similar fashion to the third injection. One week after the fifth immunization, a drop of blood was collected from the tail vein of each mouse. The collected blood was left for 1 h at 37°C. Then, overnight at 4°C and the polyclonal antibody against Cd–DTPA–KLH was isolated via centrifugation (10,000 rpm, 15 min, 4°C). The obtained antisera were stored at −20°C before further use. One BALB/c mouse (labeled as R4, 6–8 weeks old) was unimmunized as the control.
Screening of antisera
Titer of the antiserum from each mouse was tested by indirect noncompetitive ELISA. Ninety-six-well polystyrene microtiter plates were coated with Cd–DTPA–BSA (100 μL per well), which was diluted 1:1000 with carbonate buffer (0.05 M, pH 9.6). After incubated overnight at 4°C, the plates were washed three times with PBST (pH 7.4). The surfaces of the wells were blocked with 5% glycine in PBS (200 μL per well) for 2 h at 37°C. After a washing step, a 100-μL volume of antiserum, serially diluted in PBS was placed into each well, followed by incubation for 1 h at 37°C. After three additional washes with PBST (pH 7.4), 100 μL of goat anti-mouse IgG labeled with horse radish peroxidase (diluted 1:5000 with PBS) was added to each well and then incubated for 1 h at 37°C. The plates were washed three times with PBST (pH 7.4). One hundred microliter of TMB substrate solution (pH 5.5) was added. The reaction was terminated after 10 min with 2.0 M sulfuric acid (50 μL per well), and the absorbance at 450 nm was read with a microtiter plate reader. PBS-diluted serum from a nonimmunized mouse was used as the control.
Optimized working concentration of antibody–antigen
Optimum concentrations of the coating antigen and polyclonal antibody were determined by checkerboard titration. Briefly, the procedure was the same as that of the titer determination, except that in checkerboard titration format, the coating antigen Cd–DTPA–BSA was serially diluted with carbonate buffer solution at concentrations of 1.0, 1.3, 2.0, 2.5, 3.0, 4.0, 5.0, and 10.0 μg mL−1, and the polyclonal antibody was serially diluted in PBS at 1:4000, 1:8000, 1:10,000, 1:12,000, 1:16,000, and 1:32,000. OD 450 nm values between 0.8 and 1.2 were considered optimum working concentrations of the antibody–antigen according to the sensitivity of the microtiter plate reader (He et al., Citation2009; Zhan, Xi, & Zhou, Citation2013). When the OD 450 nm values are closed to 1.0, the microplate reader is sensitive and the data measured can be accurate. So an OD 450 nm value for positive serum close to 1.0 was scored as optimum working conditions of antibody–antigen.
Assay optimization and indirect competitive ELISA
Reaction conditions such as chelating agent, buffer solution, and blocking buffer, which might affect the indirect competitive ELISA were also investigated. Indirect competitive ELISA was established to detect Cd in an aqueous sample. The procedure was the same as that of the working concentration optimization except that (1) microtiter plates were coated with the optimized concentration of Cd–DTPA–BSA and polyclonal antibody; (2) serially diluted Cd standard solutions were mixed with equal volumes of PBS containing EDTA. Then, the mixtures were incubated with equal volumes of diluted polyclonal antibody. And in the indirect competitive ELISA, the antiserum coating the same amount of EDTA to the standard solutions was set to a positive control. The inhibition rate (IR) was calculated by this equation: IR = (ODp − ODc)/ODp × 100%, where ODp is the optical density of positive control and ODc is the optical density of a known concentration of soluble Cd2+. The standard inhibition curve of the indirect competitive ELISA was constructed by IR against the logarithm of the analyte concentration.
Cross-reactivity with other metals
An indirect competitive ELISA was used for the determination of the specificity of the polyclonal antibody. The metal ions Cu2+, Hg2+, Pb2+, Zn2+, Mg2+, Ca2+, Fe2+, and Ni2+ were tested for their abilities to inhibit antibody binding to the immobilized Cd–DTPA–BSA. The cross-reactivity (CR) was obtained from the IC50, which was the concentration of metal that inhibits the color formation (OD 450 nm value) in the indirect competitive ELISA by 50%. And the CR values were calculated as follows: CR% = (IC50 of Cd/IC50 of other metal) × 100% (Qi, Wang, Yang, & Liu, Citation2011).
Spike and recovery studies
A spike sample experiment was performed to estimate the accuracy of the indirect competitive ELISA. Deionized water samples were added with various known concentration of Cd (0.05, 0.50, and 5.00 μg mL−1). Each sample was subsequently analyzed, in triplicate, for Cd content.
Results and discussion
Screening of antisera
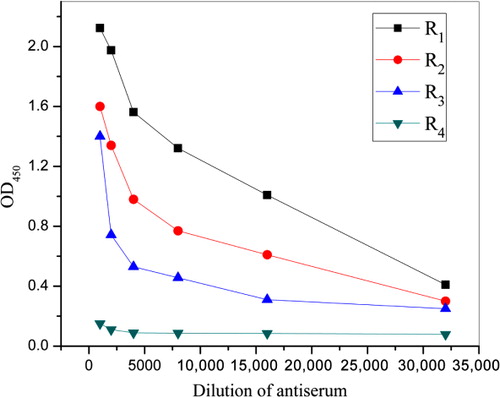
Indirect noncompetitive ELISA format was used to characterize the titers of polyclonal antibodies from the three BALB/c mice. Negative antiserum collected from the forth mouse (labeled as R4) was set to a control. showed the titers of the antisera, and the results indicated that all the polyclonal antibodies displayed high levels of affinity. Among them, the antiserum of mouse R1 demonstrated the highest titer. Therefore, the antiserum of mouse R1 was then selected for further investigations.
Optimized working concentration of antibody–antigen
Working concentrations of antibody–antigen were optimized by checkerboard assays. The data are displayed in . In the checkerboard titration, OD 450 nm values close to 1.0 were considered optimum working concentrations of the antibody–antigen according to the sensitivity of the microtiter plate reader. Out of consideration for decreasing the amount of antigens and antibodies used, the optimum working concentration was selected at OD 450 nm value of 1.088, when the concentration of antigen was 2.0 µg mL−1 and the dilution of polyclonal antibody was 1:10,000, respectively.
Table 1. Optimized working concentration of antibody–antigen.
Assay optimization and indirect competitive ELISA
Optimum concentrations of antigen and polyclonal antibody were first determined by checkerboard assays. In order to obtain the optimum sensitivity for the Cd assay, reaction conditions such as chelating agent, coating buffer, pH, and blocking buffer were then systematically optimized. Comparing the OD 450 nm value (data not show), the optimum parameters for the indirect competitive ELISA were determined to be: chelating agent, 0.5 mM EDTA; coating buffer, 0.05 M carbonate buffer, pH 9.6, and blocking buffer was 5% glycine.
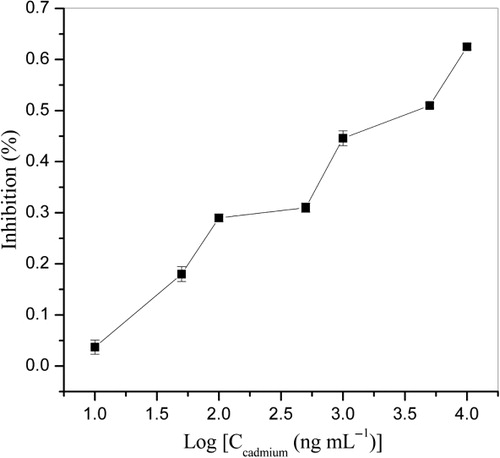
The optimized Cd indirect competitive ELISA used 2.0 μg mL−1 of Cd–DTPA–BSA, polyclonal antibody at a dilution of 1:10,000, Cd standard solution in 0.5 mM EDTA, 0.05 M carbonate buffer (pH 9.6) as coating buffer, and blocking with 5% glycine. Standard indirect competitive ELISA curve is shown in . The IC50 value of the optimized assay was 2.042 μg mL−1, and the lowest detection limit (defined as IC10 value, Yu, Vdovenko, Wang, & Sakharov, Citation2011) was 0.0135 μg mL−1.
CR with other metals
The metal ions Cu2+, Hg2+, Pb2+, Zn2+, Mg2+, Ca2+, Fe2+, and Ni2+ were tested for CR. shows the CRs characterized by optimized indirect competitive ELISA system. CRs by other metal ions were lower than 0.001% except for Hg2+ (5.8%). These results were in accordance with results of previous studies (Jones, Yu, Delehanty, & Blake, Citation2002; Lou, Yang, Zhu, & Liu, Citation2009; Zhan, Xi, & Zhou, Citation2013). Except for a relatively high CR toward Hg2+, no significant CR was observed for other metal chelates tested. These results indicated that the polyclonal antibody was highly specific for Cd.
Table 2. CR of other metals in the indirect competitive ELISA for cadmium.
Spike and recovery studies
Accuracy of the indirect competitive ELISA was tested by spike and recovery tests, and results are shown in . The recoveries of Cd from water samples were in the range of 92.2–116%, indicating that the residues analysis for Cd with the indirect competitive ELISA is suitable for a rapid and convenient determination method.
Table 3. Recovery of cadmium spiked into deionized water.
Conclusions
Cd is one of the most aggressive and persistent elements in natural environment. This work described the production of a highly sensitive polyclonal antibody that can recognize Cd–EDTA complex. Furthermore, an indirect competitive ELISA based on the polyclonal antibody was developed for Cd determination. The low limit of detection of the assay was 0.0135 μg mL−1, while the interference observed from other metal–EDTA complexes was negligible (except for Hg–EDTA with a CR of 5.8%). In addition, the entire procedure is very easy to perform in 96-well polystyrene microtiter plates and permits the analysis of a batch of 20 samples, in triplicate in less than 2 h using the preblocked microplates. The indirect competitive ELISA might be very promising analytical tool for rapid and sensitive determination of Cd in food and in the environment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adriano, D. C. (2001). Trace elements in terrestrial environments: Biogeochemistry, bioavailability, and risks of metals (2nd ed.). New York, NY: Springer-Verlag.
- Amdur, M. O., Doull, J., & Klaanen, C. D. (1996). Casarett and Doll’s Toxicology: The basic science of Poison (4th ed.). New York, NY: Pergamon Press.
- Beiraghi, A., Pourghazi, K., & Amoli-Diva, M. (2014). Thiodiethanethiol modified silica coated magnetic nanoparticles for preconcentration and determination of ultratrace amounts of mercury, lead, and cadmium in environmental and food samples. Analytical Letters, 47, 1210–1223. doi:10.1080/00032719.2013.865206
- Charles, S., Dubois, F., Yunus, S., & Vander Donckt, E. (2000). Determination by fluorescence spectroscopy of cadmium at the subnanomolar level: Application to seawater. Journal of Fluorescence, 10(2), 99–105. doi:10.1023/A:1009430723152
- Edwards, J. R., & Prozialeck, W. C. (2009). Cadmium, diabetes and chronic kidney disease. Toxicology and Applied Pharmacology, 238, 289–293. doi:10.1016/j.taap.2009.03.007
- He, H., Liu, Z. Y., Yang, S. G., & Sun, C. (2009). Preparation of anti-cadmium–EDTA complex polyclonal antibody and its application for determination of cadmium in aqueous solution. Analytical Letters, 42, 409–424. doi:10.1080/00032710802514832
- Jin, L., Zhang, L., Li, Z., Liu, J. M., Ye, R., & Ren, A. (2013). Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a Chinese population. Reproductive Toxicology, 35, 25–31. doi:10.1016/j.reprotox.2012.10.015
- Jin, T., Nordberg, G., Ye, T., Bo, M., Wang, H., Zhu, G., … Bernard, A. (2004). Osteoporosis and renal dysfunction in a general population exposed to cadmium in China. Environmental Research, 96, 353–359. doi:10.1016/j.envres.2004.02.012
- Jones, R. M., Yu, H., Delehanty, J. B., & Blake, D. A. (2002). Monoclonal antibodies that recognize minimal differences in the three-dimensional structures of metal-chelate complexes. Bioconjugate Chemistry, 13, 408–415. doi:10.1021/bc0155418
- Khosraviani, M., Pavlov, A. R., Flowers, G. C., & Blake, D. A. (1998). Detection of heavy metals by immunoassay: Optimization and validation of a rapid, portable assay for ionic cadmium. Environmental Science & Technology, 32(1), 137–142. doi:10.1021/es9703943
- Larison, J. R., Likens, G. E., Fitzpatrick, J. W., & Crock, J. G. (2000). Cadmium toxicity among wildlife in the Colorado Rocky Mountains. Nature, 406, 181–183. doi:10.1038/35018068
- Liu, G., Wang, J., Li, Z., Liang, S., Liu, J., & Wang, X. (2009). Development of direct competitive enzyme-linked immunosorbent assay for the determination cadmium residue in farm produce. Applied Biochemistry and Biotechnology, 159, 708–717. doi:10.1007/s12010-009-8539-6
- Lou, Y., Yang, F., Zhu, X., & Liu, F. (2009). Production of a specific monoclonal antibody against mercury-chelate complexes and its application in antibody-based assays. Food and Agricultural Immunology, 20(1), 23–33. doi:10.1080/09540100802626479
- Lu, Y., Shi, W., Qin, J., & Lin, B. (2009). Low cost, portable detection of gold nanoparticle-labeled microfluidic immunoassay with camera cell phone. Electrophoresis, 30, 579–582. doi:10.1002/elps.200800586
- Nawrot, T., Plusquin, M., Hogervorst, J., Roels, H. A., Celis, H., Thijs, L., … Staessen, J. A. (2006). Environmental exposure to cadmium and risk of cancer: A prospective population-based study. The Lancet Oncology, 7(2), 119–126. doi:10.1016/S1470-2045(06)70545-9
- Qi, H., Wang, R., Yang, G., & Liu, J. (2011). An enzyme-linked immunoassay for the detection of medroxyprogesterone acetate in intestines based on monoclonal antibody. Food and Agricultural Immunology, 22(2), 125–134. doi:10.1080/09540105.2010.549208
- Sasaki, K., Oguma, S., Namiki, Y., & Ohmura, N. (2009). Monoclonal antibody to trivalent chromium chelate complex and its application to measurement of the total chromium concentration. Analytical Chemistry, 81, 4005–4009. doi:10.1021/ac900419c
- Selvi, A. A., Sreenivasa, M. A., & Manonmani, H. K. (2011). Enzyme-linked immunoassay for the detection of glyphosate in food samples using avian antibodies. Food and Agricultural Immunology, 22, 217–288. doi:10.1080/09540105.2011.553799
- Shiomi, K., Hagiwara, Y., Sonoue, K., Segawa, T., Miyashita, K., Maeda, M., … Hino, O. (2008). Sensitive and specific new enzyme-linked immunosorbent assay for N-ERC/mesothelin increases its potential as a useful serum tumor marker for mesothelioma. Clinical Cancer Research, 14, 1431–1437. doi:10.1158/1078-0432.CCR-07-1613
- Verma, N., & Singh, M. (2005). Biosensors for heavy metals. Biometals, 18, 121–129. doi:10.1007/s10534-004-5787-3
- Wang, J., & Hansen, E. H. (2002). Development of an automated sequential injection on-line solvent extraction-back extraction procedure as demonstrated for the determination of cadmium with detection by electrothermal atomic absorption spectrometry. Analytica Chimica Acta, 455, 283–292. doi:10.1016/S0003-2670(02)00026-0
- Xi, T., Xing, H. B., Shi, W. W., Wu, Y. G., & Zhou, P. (2012). Preparation and characterization of artificial antigens for cadmium and lead. Biological Trace Element Research, 150, 411–417. doi:10.1007/s12011-012-9463-0
- Xu, H., Wu, Y., Wang, J., Shang, X., & Jiang, X. (2013). Simultaneous preconcentration of cadmium and lead in water samples with silica gel and determination by flame atomic absorption spectrometry. Journal of Environmental Sciences, 25(supplement 1), S45–S49. doi:10.1016/S1001-0742(14)60624-0
- Xu, T., Jing, H. Y., Li, Q. X., Liu, S. Z., Sheng, W., & Li, J. (2007). Development of an enzyme-linked immunosorbent assay for the detection of pentachlorophenol residues in water samples. Food and Agricultural Immunology, 18, 189–201. doi:10.1080/09540100701780237
- Yaman, M. (2005). The improvement of sensitivity in lead and cadmium determinations using flame atomic absorption spectrometry. Analytical Biochemistry, 339(1), 1–8. doi:10.1016/j.ab.2005.01.009
- Yu, F. Y., Vdovenko, M. M., Wang, J. J., & Sakharov, I. Y. (2011). Comparison of enzyme-linked immunosorbent assays with chemiluminescent and colorimetric detection for the determination of ochratoxin A in food. Journal of Agricultural and Food Chemistry, 59, 809–813. doi:10.1021/jf103261u
- Zhan, X. J., Xi, T., & Zhou, P. (2013). An indirect competitive immunoassay for mercury ion determination using polyclonal antibody against the Hg-GSH complex. Environmental Forensics, 14(2), 103–108. doi:10.1080/15275922.2012.760175
- Zhu, X. X., Xu, L., Lou, Y., Yu, H. N., Li, X., Blake, D. A., & Liu, F. Q. (2007). Preparation of specific monoclonal antibodies (MAbs) against heavy metals: MAbs that recognize chelated cadmium ions. Journal of Agricultural and Food Chemistry, 55, 7648–7653. doi:10.1021/jf071025l
- Zougagh, M., de Torres, A. G., & Cano Pavón, J. M. (2002). Determination of cadmium in water by ICP-AES with on-line adsorption preconcentration using DPTH-gel and TS-gel microcolumns. Talanta, 56, 753–761. doi:10.1016/S0039-9140(01)00605-1