Abstract
A dual-label time-resolved fluoroimmunoassay (TRFIA) was introduced for the simultaneous quantification of chlortetracycline (CTC) and doxycycline (DOX) in edible animal tissues. Europium- and samarium-labelled antibodies were used, because lanthanides have higher stabilities and narrower emission spectra than most fluorescent dyes. The limits of detection for simultaneous determination of CTC and DOX were 0.03 ng ml−1 and 0.04 ng ml−1, respectively. The average recoveries and the intra- and inter-assay coefficients of variation were 85.8–102.4%, 4.3–8.4% and 5.5–8.9%, respectively, and 85.3–101.6%, 4.5–9.2% and 5.3–9.6% for DOX, respectively. The proposed TRFIA for spiked samples was confirmed by high-performance liquid chromatography with a high correlation coefficient (R2) of 0.9933–0.9969. Therefore, the TRFIA may be an alternative sensitive and fast quantitative method for the high-throughput simultaneous screening of CTC or DOX in edible animal tissues.
Introduction
Chlortetracycline (CTC) and doxycycline (DOX) belong to the tetracycline antibiotics family, which are broad-spectrum antibiotic with the ability to interfere with the production pathways of essential proteins for bacteria. In order to prevent illegal abuse of CTC and DOX, which results in drug tolerance, a maximum residue limit (MRL) for tetracyclines is 100 ng g−1 in muscle, 300 ng g−1 in liver, 600 ng g−1 in kidney and 200 ng g−1 in egg (Animal Husbandry and Veterinary Bureau of Ministry of Agriculture, Citation2003; EMEA/MRLs/803/01–FINAL, Citation2001). The analytical methods most often used for the detection of CTC and DOX in tissues and other animal products are high-performance liquid chromatography (HPLC; Huan, Yanfei, Dongmei, Yulian, & Zonghui, Citation2011; Konstantina, Victoria, & Ioannis, Citation2008; Liu et al., Citation2013), HPLC–UV (Yuan, Wang, Yates, & Peterson, Citation2010), high-performance thin-layer chromatography–fluorescence detection and electrospray ionisation mass spectrometry (Chen & Schwack, Citation2013) and liquid chromatography–mass spectrometry (Aga et al., Citation2005; Andersen et al., Citation2005; Granelli & Branzell, Citation2007; Lindsey, Meyer, & Thurman, Citation2001; Schneider, Darwish, & Freenma, Citation2007). Although the instrumental methods are highly sensitive and specific, expensive equipment, highly skilled personnel and extensive sample clean-up are required. Therefore they are time-consuming and expensive for the inspection of large amount of samples, which restricts the extensive application in routine foods of animal origin safety monitoring.
Immunoassays can provide a simple, rapid, sensitive and inexpensive screening alternative for detection of veterinary drug residues. A number of researchers have established the enzyme-linked immunosorbent assay (ELISA; Everest, Cobb, Courboin, & Jackman, Citation1994; Gao, Zhao, Zhang, Wang, & Wang, Citation2013; Le, Yi, Zhao, & Wei, Citation2011; Le, Zhao, Wei, & Bi, Citation2012; Le et al., Citation2009; Pastor-Navarro, Morais, Maquieira, & Puchades, Citation2007; Zhang, Lu, Liu, Zhao, & Xi, Citation2007; Zhao et al., Citation2008), immunochromatography assay (ICA; Le, Yi, et al., Citation2011; Le, Yu, et al., Citation2011) for determination of CTC or DOX. ELISA and ICA methods are used more frequently because of their high sensitivities, portability and suitability for large-scale uses, but these methods are imprecise and positively biased because of matrix effects (Zhang et al., Citation2011). As an ultrasensitive and selective immunoassay technology, time-resolved fluoroimmunoassay (TRFIA) has the potential to overcome these drawbacks. TRFIA is a practical, time-and cost-efficient method with relatively simple protocols that require relatively small sample volumes (Zhang et al., Citation2011). Unlike previous methods, TRFIA of lanthanide chelates has been shown to be one of the most successful non-isotopic detection techniques and has been used in numerous applications in the biomedical sciences (Le, Yan, Liu, & Wei, Citation2013; Wei et al., Citation2013; Zhang et al., Citation2011).
In this study, for the first time, a highly sensitive and cost-effective dual-label TRFIA assay was developed for the simultaneous measurement of CTC and DOX in edible tissues of animals using goat anti-mouse antibodies and goat anti-rabbit antibodies labelled with samarium (Sm3+) and europium (Eu3+), respectively. In addition, the correlation between the TRFIA and HPLC analysis on detecting CTC and DOX in edible animal tissues was shown in this study.
Materials and methods
Reagents and materials
CTC, DOX, other tetracyclines, oxytetracycline, tetracycline, demeclocycline, 4-epi-doxycycline, 4-epi-oxytetracycline, 4-epi-chlortetracycline, 4-epi-tetracycline, goat anti-mouse antibody, goat anti-rabbit antibody and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sephadex G-50 was purchased from Pharmacia (Piscataway, NJ, USA). Anti-CTC monoclonal antibodies (anti-CTC McAb), anti-DOX polyclonal antibodies (anti-DOX PcAb), CTC-ovalbumin (CTC-OVA) and DOX-para-aminobenzoic-acid-OVA (DOX-PABA-OVA) conjugates were obtained from our laboratory (Le, Yi, et al., Citation2011; Le et al., Citation2009). N 1-[p-isothiocyanato-benzyl]-diethylene-triamine-N1, N2, N3-tetraacetate-Eu3+ (DTTA-Eu3+) and N1-[p-isothiocyanato-benzyl]-diethylene-triamine-N1,N2,N3-tetraacetate-Sm3+ (DTTA-Sm3+) were obtained from PerkinElmer Life and Analytical Sciences (PerkinElmer, Waltham, MA, USA). Other chemicals and reagents used were of analytical grade.
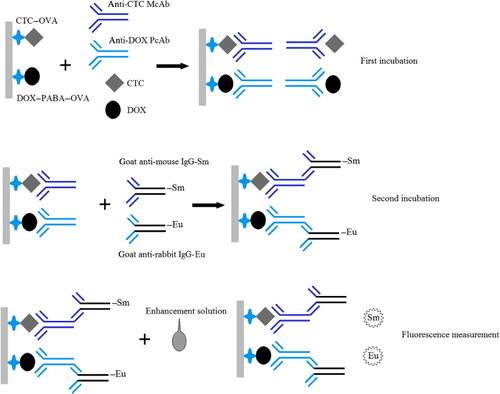
Labelling of antibodies with Eu3+ and Sm3+ chelates
The labelling procedure was performed using Sm3+ or Eu3+ labelling kits according to the manufacturer’s instructions. Briefly, 2 ml of goat anti-mouse antibodies (10 mg ml−1 dissolved in 50 mmol l−1 phosphate-buffered saline, pH 7.0) were purified and concentrated using a centrifuge tube with a membrane (Sephadex, G-50) before labelling. The purified antibody was dissolved in Sm chelates labelling buffer to a final concentration of 1 mg ml−1. Then 0.2 mg of DDTA-Sm3+ was added. The mixture was incubated at room temperature overnight. The free Sm3+ chelates and aggregated goat anti-mouse antibodies were removed from labelled proteins using gel filtration on a Sephadex G-50 column (1.5 cm × 40 cm). The absorbance of the eluate was measured at 280 nm. The fractions from the first peak with the highest Sm3+ counts were pooled and characterised. Goat anti-rabbit antibodies were labelled with europium by the same procedure except Sm3+-DTTA was replaced by Eu3+-DTTA. The labelled IgG was preserved by rapid freezing and drying in a high vacuum after dilution with elution buffer containing 0.2% bovine serum albumin (BSA) as a stabiliser, and then stored at –20°C.
Immobilisation of time-resolved fluoroimmunoassay procedures
The dual-label system is shown in . The coating antigens (CTC–OVA and DOX–PABA–OVA) were diluted to final concentration of 1 mg l−1 with coating buffer (0.1 mol/L carbonate containing 0.05% NaN3 and 0.9% NaCl, at pH 7.8). Then 100 µL of the diluted CTC–OVA and DOX–PABA–OVA was pipetted into 96-well microtitre plate and incubated for 14 h at room temperature. After three rinses, 200 µl of blocking solution was added to block the coated surface for 2 h at 37°C. After the blocking solution was removed, 50 µl of test sample extract, or a series of standards, was then pipetted into the coated micro-titration well, and then 25 µl each of anti-CTC McAb and anti-DOX PcAb was added into each well. The plates were incubated at 37°C for 1 h with agitation. After three rinses, 50 µl each of the Eu3+-labelled goat anti-rabbit antibodies and Sm3+-labelled goat anti-mouse antibodies were added, and the plate was incubated at 37°C for 0.5 h with agitation. The plate was then rinsed six times and 200 µl of enhancement solution (per litre: 15 µmol β-naphthoyltrifluoroacetone, 50 µmol tri-n-octylphosphine oxide and 1 ml Triton X-100) was added into each well. The plates were agitated for 5 min to ensure maximum development of fluorescence. The fluorescence intensity (cps) of Sm3+ and Eu3+ was measured with an AutoDELFIA1235 instrument (Waltham, MA, USA) and software designed in our laboratory (). The fluorescence readings of control and test wells were represented as B0 and B. Calibration curves were plotted and concentrations in unknown samples were measured using Multicalc software (Wallac Oy, Finland), using a spline algorithm on the logarithmically transformed data. The CTC and DOX contents of the samples were calculated using these standard curves.
Sample pre-treatment
Samples including chicken muscle, liver and egg were obtained from a local supermarket (Chongqing, China), and the contents of CTC and DOX were determined by HPLC. Samples without detectable CTC and DOX determined by HPLC were used as a negative control. Testing sample (2 ± 0.005 g) was homogenised, mixed with 4 ml of ethyl acetate, vortexed for 2 min and centrifuged at 2000 rpm for 10 min. The supernatants were collected and the debris was treated one more time as above. The final supernatants were evaporated at 60°C in water bath with a gentle nitrogen flow. The muscle and livers extractions were resuspended in 1 ml of 10 mol l−1 PBS (pH7.4) for TRFIA analysis. The sample pre-treated for HPLC analysis was carried out as reported in a previous study (Le, Yi, et al., Citation2011; Le et al., Citation2012).
Validation of the TRFIA
The limit of detection (LOD), recovery and precision were evaluated by spiked samples experiment. Twenty different tissue samples, which had previously been proven by HPLC to be free of CTC and DOX, were purchased from a local supermarket. Each tissue sample was assayed using TRFIA to determine the LOD. LOD was based on the mean value of 20 blank samples plus three times the mean standard deviation. To study the recovery, chicken liver, muscle and egg samples were spiked with different concentrations of CTC/DOX at the levels of 0.5, 1 and 2 times of MRLs, according to the standards in China. The liver samples were fortified by CTC/DOX at final concentrations of 150, 300 and 600 ng g−1 in sample buffer, and the muscle and egg samples at the levels of 50, 100, 200 and 400 ng g−1. Recovery and precision of the assay were determined by TRFIA. The intra-variations between five replicates and inter-variations from five independent experiments were determined for the sample recovery, according to the standard curve, by TRFIA.
Results and discussion
Assay specificity
The specificity of anti-CTC McAb and anti-DOX PcAb to various tetracyclines and their related derivatives was tested using the TRFIA, which was determined by measuring their IC50 values (50% inhibition levels) in the TRFIA. The concentrations of the competitive compounds of CTC, DOX, tetracycline, oxytetracycline, demeclocycline, 4-epi-tetracycline, 4-epi-doxycycline, 4-epi-chlortetracycline and 4-epi-oxytetracycline were 10000, 1000, 100, 10, 1, 0.1 and 0.01 ng ml−1 in each well, respectively. The cross-reactivity was calculated by the formula [IC50 value of CTC (or DOX)/IC50 value of interferent] × 100. As shown in , the anti-CTC McAb showed cross-reactivities towords 4-epi-doxycycline (67.81%) and the anti-DOX PcAb showed cross-reactivities towards 4-epi-chlortetracycline (75.23%), but did not generate significant cross-reactivity (<0.01) with the other antibiotics listed above. CTC and DOX did not interfere with the detection of the others during simultaneous detection. These results prove that anti-DOX PcAb and anti-CTC McAb had high affinities and specificities for CTC and DOX.
Table 1. Cross-reactivities of various tetracyclines by the TRFIA based on the anti-CTC McAb and anti-DOX PcAb.
Standard curves for the TRFIA
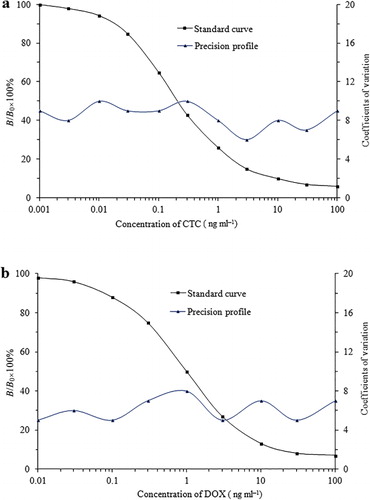
The standard curves were obtained from 10 separate assays. The standard calibration curves plotted by CTC/DOX TRFIA analysis () showed that the working concentrations ranged from 0.01 to 100 ng ml−1. The calibration curve equations were y = −26.525x + 48.986 (R2 = 0.9586; ) for CTC and y = −27.734x + 50.718 (R2 = 0. 0.9441; ) for DOX, where y = B/B0, B0 corresponds to the fluorescence count (cps) at zero concentration, and x is the concentration (ng ml−1). The IC50 of the method for CTC was 0.92 ng ml−1, and the one for DOX 1.06 ng ml−1. These results are much better than we achieved in previous studies (Le, Yi, et al., Citation2011; Le et al., Citation2009; Le et al., Citation2012). Within-assay precision (n = 10) using standards was <10% for CTC in the range 0.001−100 ng ml−1 and <8% for DOX in the range 0.01−100 ng ml−1.
Validation of the TRFIA
CTC/DOX-negative chicken muscle, liver and egg samples (determined by HPLC) were spiked with different concentrations of CTC/DOX and simultaneously analysed by TRFIA as described above. Based on the results from 20 different blank samples, the LOD of the method for CTC was 0.03 ng ml−1, and the one for DOX 0.04 ng ml−1 in various biological matrices.
The recovery and reproducibility were evaluated by spiked samples experiment. The reproducibility of the immunoassay method was evaluated by intra-and inter-assay coefficients of variation (CVs). To study the recovery, chicken liver, muscle and egg samples were spiked with different concentrations of CTC/DOX at the levels of 0.5, 1 and 2 times of MRLs, according to the standards in China. The liver samples were fortified by CTC/DOX at final concentrations of 150, 300 and 600 ng g−1, and the muscle and egg samples at the levels of 50, 100, 200 and 400 ng g−1. The results, shown in , indicated that the recovery ranged from 85.8 to 102.4% for CTC and from 85.3 to 101.6% for DOX. The intra-assay variation was 4.3–8.4% for CTC and 4.5–9.2% for DOX, while the inter-assay variation was 5.5–8.9% for CTC and 5.3–9.6% for DOX (). Thus, the recoveries and reproducibility of the proposed assay in our cases were acceptable for the detection of CTC and DOX residues.
Table 2. Recovery and reproducibility of the TRFIA for CTC and DOX spiked into chicken egg, muscle and liver samples (n = 5).
Validation of TRFIA with HPLC
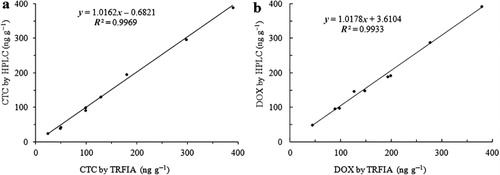
To validate the applicability of this TRFIA method, the samples were spiked with CTC at different concentration (chicken muscle spiked at 25, 50 and 100 ng g−1; egg spiked at 50, 300 and 150 ng g−1; chicken liver spiked at 100, 200 and 400 ng g−1) and spiked with DOX at different concentration (chicken muscle spiked at 50, 100 and 150 ng g−1; egg spiked at 100, 200 and 400 ng g−1; chicken liver spiked at 150, 200 and 300 ng g−1). The spiked samples were pre-treated as described above for TRFIA. The comparison of the TRFIA with the HPLC was shown in . For the quantitative analyses, a side-by-side comparison of TRFIA with HPLC method showed high correlation for samples spiked with CTC (R2 = 0.9969; ) and samples spiked with DOX (R2 = 0.9933; ). Thus, dual-label TRFIA is a reliable method for the simultaneous determination of CTC and DOX residues in food samples.
Conclusions
In this study, we established a simple and sensitive dual-label TRFIA method for the simultaneous determination of CTC and DOX levels in chicken egg, muscle and liver. The proposed format presents advantages of rapidity, simplicity and cost-effectiveness. At optimal assay conditions, the IC50 values of the TRFIA for CTC and DOX were 0.92 and 1.06 ng ml−1. LODs of the assay for CTC and DOX were 0.03 and 0.04 ng ml−1. The IC50 and detection limit of the assay were lower than those in published papers. The TRFIA was also validated by HPLC with good correlation. The proposed TRFIA could be a feasible quantitative/screening method for CTC and DOX analysis in edible animal tissues.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aga, D. S., O’Connor, S., Ensley, S., Payero, J. O., Snow, D., & Tarkalson, D. (2005). Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry, 53, 7165–7171. doi:10.1021/jf050415+
- Andersen, W. C., Roybal, J. E., Gonzales, S. A., Turnipseed, S. B., Pfenning, A. P., & Kuck, L. R. (2005). Determination of tetracycline residues in shrimp and whole milk using liquid chromatography with ultraviolet detection and residue confirmation by mass spectrometry. Analytica Chimica Acta, 529(1–2), 145–150. doi:10.1016/j.aca.2004.08.012
- Animal Husbandry and Veterinary Bureau of Ministry of Agriculture. (2003). Announcement of ministry of agriculture maximum residue levels of veterinary drug in foodstuffs of animal origin. Chinese Journal of Veterinary Drug, 4, 15–20.
- Chen, Y., & Schwack, W. (2013). Planar chromatography mediated screening of tetracycline and fluoroquinolone antibiotics in milk by fluorescence and mass selective detection. Journal of Chromatography A, 1312, 143–151. doi:10.1016/j.chroma.2013.09.006
- EMEA/MRL/803/01 FINAL. (2001). Retrieved from http://www.emea.eu.int/pdfs/vet/mrls/080301en.pdf
- Everest, S. J., Cobb, A. L., Courboin, G. M., & Jackman, R. (1994). Development of an ELISA for the detection of chlortetracycline, oxytetracycline and tetracycline residues. Food and Agricultural Immunology, 6(1), 55–61. doi:10.1080/09540109409354813
- Gao, F., Zhao, G. X., Zhang, H. C., Wang, P., & Wang, J. P. (2013). Production of monoclonal antibody against doxycycline for immunoassay of seven tetracyclines in bovine muscle and milk. Journal of Environmental Science and Health, Part B, 48(2), 92–100. doi:10.1080/03601234.2013.726856
- Granelli, K., & Branzell, C. (2007). Rapid multi-residue screening of antibiotics in muscle and kidney by liquid chromatography-electrospray ionisation-tandem mass spectrometry. Analytica Chimica Acta, 586, 289–295. doi:10.1016/j.aca.2006.12.014
- Huan, Y., Yanfei, T., Dongmei, C., Yulian, W., & Zonghui, Y. (2011). Development of an HPLC-UV method for the simultaneous determination of tetracyclines in muscle and liver of porcine, chicken and bovine with accelerated solvent extraction. Food Chemistry, 124, 1131–1138. doi:10.1016/j.foodchem.2010.07.024
- Konstantina, I. N., Victoria, F. S., & Ioannis, N. P. (2008). Development and validation of an HPLC method for the determination of seven tetracycline antibiotics residues in chicken muscle and egg yolk according to 2002/657/EC. Journal of Liquid Chromatography & Related Technologies, 31, 2141–2158. doi:10.1080/10826070802225445
- Le, T., Yan, P. F., Liu, J., & Wei, S. (2013). Simultaneous detection of sulfamethazine and sulfaquinoxaline using a dual-label time-resolved fluorescence immunoassay. Food Additives and Contaminants, 30, 1264–1269. doi:10.1080/19440049.2013.801084
- Le, T., Yi, S. H., Zhao, Z. W., & Wei, W. (2011). Rapid and sensitive enzyme-linked immunosorbent assay and immunochromatographic assay for detection of chlortetracycline residue in animal edible tissues. Food Additives and Contaminants, 28, 1516–1523. doi:10.1080/19440049.2011.589037
- Le, T., Yu, H., Guo, Y., Babacar, N., Shen, Y., & Bi, D. (2009). Development of an indirect competitive ELISA for the detection of doxycycline residue in animal edible tissues. Food and Agricultural Immunology, 20(2), 111–124. doi:10.1080/09540100902849740
- Le, T., Yu, H., Wang, X., Babacar, N., Guo, Y., & Bi, D. (2011). Development and validation of an immunochromatographic test strip for rapid detection of doxycycline residues in swine muscle and liver. Food and Agricultural Immunology, 22, 235–246. doi:10.1080/09540105.2011.556713
- Le, T., Zhao, Z., Wei, W., & Bi, D. (2012). Development of a highly sensitive and specific monoclonal antibody-based enzyme-linked immunosorbent assay for determination of doxycycline in chicken muscle, liver and egg. Food Chemistry, 134, 2442–2446. doi:10.1016/j.foodchem.2012.04.030
- Lindsey, M. E., Meyer, M., & Thurman, E. M. (2001). Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Analytical Chemistry, 73, 4640–4646. doi:10.1021/ac010514w
- Liu, Y., Yang, H., Yang, S., Hu, Q., Cheng, H., Liu, H., & Qiu, Y. (2013). High-performance liquid chromatography using pressurized liquid extraction for the determination of seven tetracyclines in egg, fish and shrimp. Journal of Chromatography B, 917–918, 11–17.
- Pastor-Navarro, N., Morais, S., Maquieira, A., & Puchades, R. (2007). Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues application to honey samples. Analytica Chimica Acta, 594, 211–218.
- Schneider, M. J., Darwish, A. M., & Freenma, D. W. (2007). Confirmatory assay for the determination of tetracycline, oxytetracycline, chlortetracycline and its isomers in muscle and kidney using liquid chromatography-mass spectrometry. Analytica Chimica Acta, 586, 269–274. doi:10.1016/j.aca.2006.09.025
- Wei, S., Le, T., Chen, Y., Xu, J., He, H. Q., Niu, X. D., & Luo, J. H. (2013). Time-resolved fluoroimmunoassay for quantitative determination of tylosin and tilmicosin in edible animal tissues. Chinese Science Bulletin, 58, 1838–1842. doi:10.1007/s11434-013-5749-7
- Yuan, S., Wang, Q., Yates, S. R., & Peterson, N. G. (2010). Development of an efficient extraction method for oxytetracycline in animal manure for high performance liquid chromatography analysis. Journal of Environmental Science Health B, 45, 612–620.
- Zhang, J., Gao, L., Zhou, B., Zhu, L., Zhang, Y., & Huang, B. (2011). Simultaneous detection of deoxynivalenol and zearalenone by dual-label time-resolved fluorescence immunoassay. Journal of the Science of Food and Agriculture, 91, 193–197.
- Zhang, Y., Lu, S., Liu, W., Zhao, C., & Xi, R. (2007). Preparation of anti-tetracycline antibodies and development of an indirect heterologous competitive enzyme-linked immunosorbent assay to detect residue of tetracycline in milk. Journal of Agriculture and Food Chemistry, 55, 211–218.
- Zhao, C. B., Peng, D. P., Wang, Y. L., Huang, L. L., Chen, D. M., Tao, Y. F., & Yuan, Z. H. (2008). Preparation and validation of the polyclonal antibodies for detection of chlortetracycline residues. Food and Agricultural Immunology, 19, 163–174.