Abstract
This study aims to investigate the effects of temperature, time, pH, ovalbumin/glucose ratio, and ovalbumin concentration on the potential allergenicity of ovalbumin during the Maillard reaction, and to define the key influential factors. Results indicated that immunoglobulin G (IgG) binding of ovalbumin could either be increased or decreased, depending on the reaction temperature, and the glycation parameters could work collectively. Response surface methodology (RSM) was used to optimize conditions under temperature below denaturation of ovalbumin. The key influential factor was temperature, followed by pH and ovalbumin concentrations. Under the optimized condition, a reduction of immunoglobulin E (IgE) binding to 61.86% was observed. RSM was proved to be a good tool in optimizing the Maillard reaction parameters to reduce potential allergenicity of ovalbumin.
1. Introduction
A hen’s egg is one of the most common offending foods that can trigger an allergic reaction, especially in children. Prevalence of egg allergy was reported to be up to 1.7% in childhood (Eggesbø, Botten, Halvorsen, & Magnus, Citation2001; Rona et al., Citation2007), and a recent large population-based cross-sectional study of 12-month-old Australian infants reported a prevalence of egg allergy of 8.9% (Osborne et al., Citation2011). Currently, strict avoidance of the offending food remains to be the best choice for egg allergic patients. Nevertheless, egg avoidance diet significantly reduces the quality of life for their families, and maintaining a stringent diet may result in limited dietary choices and nutritional inadequacy (Sicherer, Noone, & Muñoz-Furlong, Citation2001). Moreover, avoidance places a constant responsibility on patients and caregivers, making patients vulnerable to unintentional ingestion and anaphylaxis (Bollinger et al., Citation2006). Therefore, considerable efforts have been exerted on developing an approach to produce safe egg products for the egg allergic patients.
Ovalbumin is one of the major allergens in hen eggs, comprising 54% of the total egg white protein. Thus, reduction in ovalbumin allergenicity is to some extent vital for hypoallergenic egg products. Among the common protein preparations and treatment conditions, ovalbumin exhibits the greatest degree of constancy in composition, molecular weight, and solubility. Moreover, ovalbumin has an additional advantage that it is water soluble over the entire titratable pH range. Hence, egg ovalbumin is commonly used as a model protein.
The Maillard reaction is a popular food processing technique, and the effects of Maillard reaction on allergenicity of buckwheat allergens (Yang, Li, Li, & Wang, Citation2013), hazelnut allergens (Iwan et al., Citation2011), peanut allergens (Gruber, Becker, & Hofmann, Citation2005), soy proteins (Wilson, Blaschek, & de Mejia, Citation2005), and shellfish allergens (Nakamura, Watanabe, Ojima, Ahn, & Saeki, Citation2005) have been reported earlier, concluding that glycation could either decrease or increase potential allergenicity, depending on the protein processed. Ovalbumin can potentially undergo Maillard reaction on any of its 20 lysine residues, and it is acetylated at the N-terminal (Narita, & Ishii, Citation1962). However, limited information on the effect of Maillard reaction on the immunoglobulin E (IgE) or G binding ability of ovalbumin is available, and there are contradictory reports. In one work, ovalbumin was dissolved in 0.1 M phosphate buffer (pH 7.2), mixed with glucose at a protein/glucose ratio of 1:0.05 (w/w), freeze-dried, and then stored for 48 or 96 h in a desiccator at 50°C and 0.65 water activity. Results showed that Maillard reaction decreased the IgE binding ability but increased the IgG binding ability of ovalbumin (Jiménez-Saiz, Belloque, Molina, & López-Fandiño, Citation2011). Ma et al. reported that buffer concentration affected the IgG and IgE binding of ovalbumin when it reacted with glucose at 25:21 (w:w) in bicarbonate buffer (pH 9.6), at a temperature below denaturation of ovalbumin (Ma, Chen, Gao, Hu, & Li, Citation2013; Ma, Gao, & Chen, Citation2013). When the buffer concentration was ≥20 mM, IgG binding capacity of glycated ovalbumin was in positive correlation with its IgE binding capacity, while the contrary was found when the buffer concentration was <20 mM.
The Maillard reaction parameters might influence glycation of ovalbumin individually and collectively. However, the traditional multivariable optimisation is usually based on the “one-factor-at-a-time” approach, which is unable to detect interactions among independent variables, and lack of complete information on effects of all determinants (Zhou, Fu, & Li, Citation2015). Response surface methodology (RSM) enables the evaluation of the effects of many independent variables and their interactions based on quantitative data. Today, this statistical method has been successfully applied to optimize the effects of the Maillard reaction parameters on the antigenicity of parvalbumin (Li, Jiang, You, Luo, & Feng, Citation2014), β-lactoglobulin (Li, Luo, Feng, & Liao, Citation2013), α-lactalbumin (Bu, Lu, Zheng, & Luo, Citation2009; Li, Luo, & Feng, Citation2011), and α-lactoglobulin (Bu, Luo, Lu, & Zhang, Citation2010). The Maillard reaction parameters might also affect the allergenic and antigenic potential of ovalbumin during the reaction. Currently, no report on the different Maillard reaction parameters on the IgG/IgE binding capacity of ovalbumin is available, neither is available the key factors influencing its allergenicity during glycation.
This study aims to investigate the effects of different wet-heating conditions of Maillard reaction (temperature, time, pH, ovalbumin/glucose ratio, and ovalbumin concentration) on the potential allergenicity of ovalbumin. RSM was employed to optimize the reaction parameters below denaturation temperature of ovalbumin (78.3°C). Single-factor tests were used to determine the principal influential factors and better ranges of parameters for RSM.
2. Materials and methods
2.1. Materials
Ovalbumin (A5503, purity > 98%) from Sigma–Aldrich (St. Louis, MO, USA) was the antigen used for enzyme-linked immunosorbent assay (ELISA). The antiserum was prepared as previously described (Ma, Gao, et al, Citation2013). All other reagents used were of analytical grade.
2.2. Preparation of ovalbumin/glucose conjugates
A series of 10 mM citrate-phosphate buffer at various pH (2.2, 3.98, 6.03, and 8.08) was prepared, and sodium carbonate-bicarbonate buffer (10 mM) was used to produce a pH of 9.99. Preliminary experiment showed that these two buffers did not show considerable difference in testing IgG binding of ovalbumin. The reaction systems were prepared by dissolving ovalbumin and glucose at the desired concentration based on the experimental design.
2.3. Experimental design for single factor
In this study, ovalbumin and glucose were reacted at varies temperature (60, 70, 80, 90, and 100°C), time (10, 30, 60, 90, and 120 min), pH (2, 4, 6, 8, and 10), glucose/lysine ratio (1, 3, 10, 30, and 100), and ovalbumin concentration (4, 2.4, 0.4, 0.24, and 0.04 mg/mL), respectively. When one factor was evaluated, the other factors were kept constant. Exactly 400 μL of each prepared sample was placed in well-capped flasks and heated in a water bath under the different temperatures and reaction periods. After heating, the samples were rapidly cooled down with ice to prevent further reaction. Each sample treatment was performed at least in triplicate. All the following experiments were performed in triplicate unless mentioned otherwise.
2.4. Indirect ELISA to determine the IgG binding capacity of ovalbumin
An indirect ELISA was performed as previously described to determine the IgG binding ability of ovalbumin (Ma, Chen, et al, Citation2013). The negative control (with sera from non-immunized mice) and positive control (untreated ovalbumin) were included in each plate. The test was performed in triplicate.
IgG binding of ovalbumin was calculated using the following formula:
2.5. Competitive inhibition ELISA to determine the IgE binding capacity of ovalbumin
The IgE binding capacity of the glycated ovalbumin under our optimized condition was defined by competitive inhibition ELISA with egg allergic patients’ sera pool, following the protocol as we previously described (Ma, Gao, et al, Citation2013) with minor modifications: the plate was blocked with 3% glutin, and o-Phenylenediamine was used for color development. A blank without antigen, the negative control with antigen but without antibody, and positive controls, commercial ovalbumin at different concentrations, were included in each plate. Calculations for IgE binding followed these of Jiménez-Saiz et al. (Citation2011). The sera used are described in . Egg allergy was diagnosed on the basis of the detection of the serum IgE specific for egg white using the Allergy Screen (MEDIWISS Analytic GmbH, Moers, Germany) and objective clinical manifestations observed after egg ingestion.
Table 1. The egg allergic patients sera used in our study.
2.6. SDS-PAGE
SDS-PAGE was performed in a PhastSystem Electrophoresis equipment using precast gel 12% Bis-Tris (Bio-Rad, USA), following the manufacturer’s instructions for the electrophoretic and Coomassie staining conditions. Samples were dissolved in 10 mM Tris-HCl buffer (pH 8.0) containing 2.5% SDS and 10 mM EDTA. The mixture was then heated at 95°C for 10 min in the presence of 5% 2-β-mercaptoethanol.
2.7. Experimental design for RSM
RSM, a statistical approach used to represent the effect of interaction between different process variables, has been employed to help optimize the process with a reduced number of experiments. In the present study, we applied RSM to determine the optimum conditions for IgG binding of ovalbumin during the Maillard reaction. The experimental design, as well as graphical and statistical analyses, was created using “Design Expert” software (version 7.0.0, Stat-Ease Inc., Minneapolis, MN, USA).
2.8. Statistical analysis
All analyses were performed in triplicate. Statistical significance was considered at p < 0.05. All statistical evaluations of RSM were performed with the statistical software Design-Expert 7.0.0.
3. Results and discussion
3.1. Single-factor test results for IgG binding
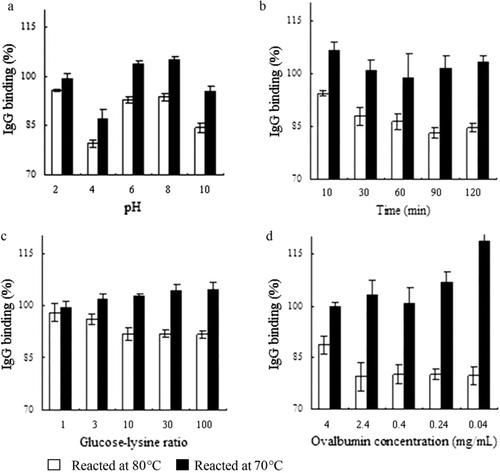
Temperature is a critical factor to consider in the Maillard reaction. IgG binding of ovalbumin after glycation at 60, 70, 80, 90, and 100°C was 101.68 ± 3.44, 102.11 ± 3.99, 95.23 ± 3.26, 88.27 ± 7.72, and 89.41 ± 2.89, respectively. It is clear that when reacted ≤70°C, IgG binding of ovalbumin increased after the Maillard reaction, and the contrary is true when reacted ≥80°C. We then tried the effect of other parameters (pH, time, glucose-lysine ratio, and ovalbumin concentration) reacted at 80°C and 70°C, respectively (). Factors chosen for initial selection were pH 6, time 60 min, glucose-lysine ratio 10:1, and ovalbumin concentration 0.4 mg/mL, and when one factor was changed, the other independent variables were kept constant. The key influential factors when reacted at 80°C are time and pH, while when reacted at 70°C, pH, and ovalbumin concentration were proved to be more influential. These differences in the changes of most influential factors can be explained by the collective (apart from the independent) influence of the Maillard reaction parameters.
3.2. SDS-PAGE analysis for some chosen variables in single-factor test
The SDS-PAGE patterns of untreated ovalbumin and ovalbumin-glucose conjugates are presented in . Under the given conditions, the ovalbumin-glucose mixtures formed smears with MW >150 kDa. This may indicate that some covalently linked ovalbumin-glucose conjugates were produced due to the Maillard reaction. We compared the smears in lanes 2, 4, and 8, and those in lanes 3, 5, and 9 upon different temperature. After heating at 60°C for 1 h, only a faint smear was formed. By contrast, heating at 80°C or 100°C produced more smears, particularly after heating at 100°C. These analyses revealed that the rate and extent of formation of ovalbumin-glucose conjugate was significantly affected by the reacting temperature.
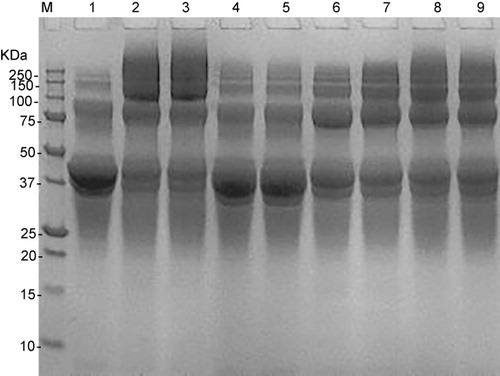
The differences in the smears in lanes 6 and 8, and those in lanes 7 and 9 mean that pH affects ovalbumin-glucose conjugation. When conjugating at pH 7.76, less ovalbumin-glucose conjugation was formed comparing with those conjugated at pH 6. Generally, it is believed that Maillard reaction is favored at high pH, which is different from our results above. However, accelerated Maillard reaction at lower pH system under certain conditions was also reported, such as in the work of Scamana, Nakaia, and Aminlarib (Citation2006).
For the influence of ovalbumin/glucose ratio, it was found that conjugation rate of ovalbumin-glucose increased with glucose/lysine ratio when reacted at 100°C, pH 6 (lanes 2 and 3), and 80°C, and pH 7.76 (lanes 6 and 7). On the other hand, glucose/lysine ratio did not exert an effect when reacted at 60°C, pH 6 (lanes 4 and 5) and 80°C, and pH 6 (lanes 8 and 9), indicating an interdependence of variables. Moreover, although difference in conjugation existed upon pH and ovalbumin/glucose ratio, it was not as much as that imposed by temperature.
All the above analyses of the SDS-PAGE profiles indicated the occurrence of the Maillard reaction in our ovalbumin-glucose mixture, and they also reveal that the processing variables affect the ovalbumin-glucose conjugation.
3.3. Optimization design for RSM
The single-factor experiment above indicated that below denaturation temperature, the most influential factors on the IgG binding of ovalbumin after glycation were (1) temperature, (2) pH, and (3) ovalbumin concentration. Maximum IgG binding was chosen and optimized because in the previous experiments we have made it clear that below denaturation temperature in low ionic strength, human IgE binding of ovalbumin decreased with an increase in rabbit IgG binding, and to directly optimize human IgE binding would need amount of egg allergic patients sera beyond our reserve. After analyzing with RSM Box–Behnken software, a design with three influence factors and three categorical factors were generated by “Design Expert” software (). Then, 17 runs were conducted by varying the initial pH and ovalbumin concentration, and reacting under different temperature (). The 17 sets of experiments were randomly performed in duplicate.
Table 2. Numeric and categorical factors of RSM.
Table 3. Conditions of the Maillard reaction by RSM.
3.3.1. Model fitting
The experimental set and the corresponding experimental data are shown in . Analysis of variance (ANOVA) was performed to calculate the regression coefficient of the response surface equation and verify the validity of RSM. summarizes the ANOVA (F-test). The significance of each coefficient and the interaction strength between each parameter was assessed using the p-value.
Table 4. ANOVA for response surface type III model.
In the present experiment, the “Model F-value” of 54.53 implies that the model is significant. The chance that a “Model F-Value” this large can occur due to noise is only 0.01%. Value of “Prob > F” less than 0.05 indicates that the model terms are significant. Meanwhile, the “Lack of Fit F-value” of 15.47 implies that the lack of fit is significant. There is only a 1.15% chance that a “Lack of Fit F-value” this large could occur due to noise. The “Pred R2” of 0.79 is in reasonable agreement with the “Adj R2” of 0.97, which means that experimental data was well-fit with the response surface model. “Adeq Precision” measures the signal-to-noise ratio. A ratio greater than 4.00 is desirable. Our ratio of 27.30 indicates an adequate signal.
Multivariable linear regression was used to estimate the coefficients on the second-order polynomial equation and the obtained regression coefficients, whose significance is shown in . The equation used is as follows:
Table 5. Regression coefficient of polynomial function of response surface of ovalbumin IgG binding capacity.
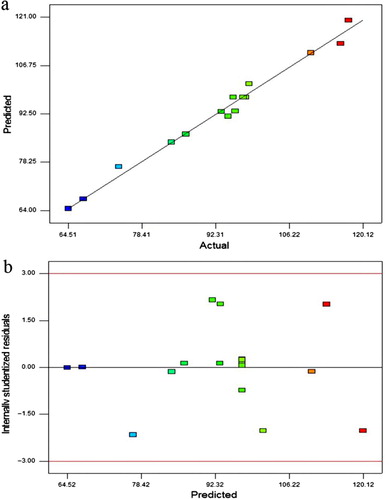
shows the actual values versus the predicted values of the IgG binding (%), indicating an excellent agreement with each other. The residual plot in allows the visual assessment of the distance of each observation from the fitted line. The residuals were randomly scattered in a constant width band. These analyses tell that this model can be used to navigate the design space.
3.3.2. Analysis of response surfaces
Response surfaces were drawn to determine the individual and interactive effects of the test variables on IgG binding of ovalbumin. Response surface graphs were plotted where one variable was kept constant, while the other two were allowed to vary within the experimental range. The relationship between variables can be illustrated by these response surface plots (Lu, Engelmann, Lila, & Erdman, Citation2008).
The two-dimensional graph in shows the interaction of three factors. When the effects of temperature and pH on ovalbumin IgG binding are examined (), it can be seen that at a fixed temperature, IgG binding of ovalbumin increased with initial pH. However, further increase in the initial pH decreased the IgG binding. Thus, an optimal pH value exists. The increase in IgG binding of ovalbumin at 6 < pH < 8 may be caused by the high conjugation rate of glucose, as the results in (lanes 6 and 8, and lanes 7 and 9) indicate that lower pH could accelerate glucose conjugation. However, further increase in pH followed by heating might cause changes in ovalbumin conformation, burying some originally exposed epitopes. The maximum effect of pH was attained at 7~8.
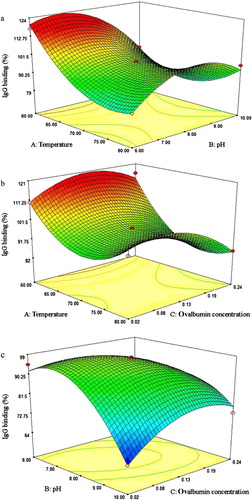
The effect of different temperature and ovalbumin concentration on its IgG binding was also examined (). From these curves, it can be deduced that with the increase of temperature, IgG binding of ovalbumin decreased to the minimum and then increased. The high IgG binding observed at >75°C may have resulted from the conversion of ovalbumin into the thermo-stabilized form S-ovalbumin or the formation of an intermediate between ovalbumin and S-ovalbumin. The formation of S-ovalbumin has been found to occur in vitro at moderate temperature and alter the human IgE binding (Jiménez-Saiz, Pineda-Vadillo, López-Fandiño, & Molina, Citation2012).
Finally, demonstrated that when ovalbumin was lower than 0.13 mg/mL, protein concentration was positively correlated with its IgG binding. However, further increase in ovalbumin concentration reduced the IgG binding. The heat-induced denaturation kinetics of ovalbumin was reported to be independent on ovalbumin concentration (Weijers, Barneveld, Cohen Stuart, & Visschers, Citation2003). Nevertheless, the phenomenon did not occur in this case. This observation might be attributed to the fact that both glucose conjugation and heat denaturation occur in the Maillard reaction, wherein the former can change the denaturation kinetics of ovalbumin, thereby influencing both the conformational and linear epitopes.
As shown in and , IgG binding of ovalbumin initially decreased and then increased with increasing temperature. Moreover, shows that IgG binding of ovalbumin did not decrease with time. The result suggests no direct correlation exists between IgG binding and degree of glucose conjugation, because conjugating of glucose occurs with time. The changes in ovalbumin IgG binding depend largely on the Maillard reaction conditions. In addition, the IgG binding of ovalbumin was reported to be influenced by the conformational changes caused by the Maillard reaction (Ma, Gao, et al, Citation2013; Ma, Chen, et al, Citation2013).
3.3.3. Optimization and verification
Temperature, pH, and ovalbumin concentration exerted different effects on the IgG binding of ovalbumin. The maximum IgG binding of 124.04% was achieved at 60°C, pH 6.87, and ovalbumin concentration of 0.11 mg/mL.
The experimental and predicted values of the responses were compared to check the adequacy of RSM. The experimental values of the maximum IgG binding was 121.77 ± 2.45%, and no significant (p > 0.05) difference was noted between the experimental and theoretically predicted values. The sufficiency of the corresponding RSM was verified based on the observations.
Under the optimized conditions, IgE binding of 61.86 ± 0.68% of the untreated ovalbumin was observed, meaning that our optimization significantly decreased the IgE binding capacity of ovalbumin.
4. Conclusion
This study investigated the effect of Maillard reaction conditions (temperature, time, pH, ovalbumin/glucose ratio, and ovalbumin concentration) on the potential allergenicity of ovalbumin. The better ranges for RSM were chosen according to single-factor experiments. Temperature appeared to be the most influential factor, and the Maillard reaction parameters were found to work collectively in affecting the IgG binding. The optimized conditions reduced more than one third of the IgE binding capacity of ovalbumin, providing a chance of reducing its potential allergenicity in processing at comparatively low temperature.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bollinger, M. E., Dahlquist, L. M., Mudd, K., Sonntag, C., Dillinger, L., & McKenna, K. (2006). The impact of food allergy on the daily activities of children and their families. Annals of Allergy, Asthma and Immunology, 96, 415–421. doi:10.1016/S1081-1206(10)60908-8
- Bu, G. H., Lu, J., Zheng, Z., & Luo, Y. K. (2009). Influence of Maillard reaction conditions on the antigenicity of bovine alpha-lactalbumin using response surface methodology. Journal of the Science of Food and Agriculture, 89, 2428–2434.
- Bu, G. H., Luo, Y. K., Lu, J., & Zhang, Y. (2010). Reduced antigenicity of -lactoglobulin by conjugation with glucose through controlled Maillard reaction conditions. Food and Agricultural Immunology, 21, 143–156.
- Eggesbø, M., Botten, G., Halvorsen, R., & Magnus, P. (2001). The prevalence of allergy to egg: A population-based study in young children. Allergy, 56, 403–411.
- Gruber, P., Becker, W. M., & Hofmann, T. (2005). Influence of the maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin. Journal of Agricultural and Food Chemistry, 53, 2289–2296.
- Iwan, M., Vissers, Y. M., Fiedorowicz, E., Kostyra, H., Kostyra, E., Savelkoul, H. F., & Wichers, H. J. (2011). Impact of Maillard reaction on immunoreactivity and allergenicity of the hazelnut allergen Cor a 11. Journal of Agricultural and Food Chemistry, 59, 7163–7171.
- Jiménez-Saiz, R., Belloque, J., Molina, E., & López-Fandiño, R. (2011). Human immunoglobulin E (IgE) binding to heated and glycated ovalbumin and ovomucoid before and after in vitro digestion. Journal of Agricultural and Food Chemistry, 59, 10044–10051.
- Jiménez-Saiz, R., Pineda-Vadillo, C., López-Fandiño, R., & Molina, E. (2012). Human IgE binding and in vitro digestion of S-OVA. Food Chemistry, 135, 1842–1847.
- Li, Z., Jiang, M. Z., You, J., Luo, Y. K., & Feng, L. G. (2014). Impact of Maillard reaction conditions on the antigenicity of parvalbumin, the major allergen in grass carp. Food and Agricultural Immunology, 25, 486–497.
- Li, Z., Luo, Y. K., & Feng, L. G. (2011). Effects of Maillard reaction conditions on the antigenicity of alpha-lactalbumin and beta-lactoglobulin in whey protein conjugated with maltose. European Food Research and Technology, 233, 387–394. doi:10.1007/s00217-011-1532-7
- Li, Z., Luo, Y. K., Feng, L. G., & Liao, P. (2013). Effect of Maillard reaction conditions on antigenicity of beta-lactoglobulin and the properties of glycated whey protein during simulated gastric digestion. Food and Agricultural Immunology, 24, 433–443.
- Lu, C. H., Engelmann, N. J., Lila, M. A., & Erdman, J. W. Jr. (2008). Optimization of lycopene extraction from tomato cell suspension culture by response surface methodology. Journal of Agricultural and Food Chemistry, 56, 7710–7714.
- Ma, X. J., Chen, H. B., Gao, J. Y., Hu, C. Q., & Li, X. (2013). Conformation affects the potential allergenicity of ovalbumin after heating and glycation. Food Additive and Contaminants Part A- Chemistry Analysis Control Exposure & Risk Assessment, 30, 1684–1692. doi:10.1080/19440049.2013.822105
- Ma, X. J., Gao, J. Y., & Chen, H. B. (2013). Combined effect of glycation and sodium carbonate–bicarbonate buffer concentration on IgG binding, IgE binding and conformation of ovalbumin. Journal of the Science of Food and Agriculture, 93, 3209–3215.
- Nakamura, A., Watanabe, K., Ojima, T., Ahn, D. H., & Saeki, H. (2005). Effect of maillard reaction on allergenicity of scallop tropomyosin. Journal of Agricultural and Food Chemistry, 53, 7559–7564.
- Narita, K., & Ishii, J. (1962). N-Terminal sequence of ovalbumin. Journal of Biochemistry, 52, 367–373.
- Osborne, N. J., Koplin, J. J., Martin, P. E., Gurrin, L. C., Lowe, A. J., Matheson, M. C., … Allen, K. J. (2011). Prevalence of challenge-proven IgE mediated food allergy using population-based sampling and predetermined challenge criteria in infants. Journal of Allergy and Clinical Immunology, 127, 668–676.e1–2. doi:10.1016/j.jaci.2011.01.039
- Rona, R. J., Keil, T., Summers, C., Gislason, D., Zuidmeer, L., Sodergren, E., … Madsen, C. (2007). The prevalence of food allergy: A meta-analysis. Journal of Allergy and Clinical Immunology, 120, 638–646. doi:10.1016/j.jaci.2007.05.026
- Scamana, C., Nakaia, S., & Aminlarib, M. (2006). Effect of pH, temperature and sodium bisulfite or cysteine on the level of Maillard-based conjugation of lysozyme with dextran, galactomannan and mannan. Food Chemistry, 99, 368–380. doi:10.1016/j.foodchem.2005.08.003
- Sicherer, S. H., Noone, S. A., & Muñoz-Furlong, A. (2001). The impact of childhood food allergy on quality of life. Annals of Allergy, Asthma and Immunology, 87, 461–464. doi:10.1016/S1081-1206(10)62258-2
- Weijers, M., Barneveld, P. A., Cohen Stuart, M. A., & Visschers, R. W. (2003). Heat-induced denaturation and aggregation of ovalbumin at neutral pH described by irreversible first-order kinetics. Protein Science, 12, 2693–2703. doi:10.1110/ps.03242803
- Wilson, S., Blaschek, K., & de Mejia, E. (2005). Allergic proteins in soybean: processing and reduction of P34 allergenicity. Nutrition Reviews, 63, 47–58.
- Yang, Z. H., Li, C., Li, Y. Y., & Wang, Z. H. (2013). Effects of Maillard reaction on allergenicity of buckwheat allergen Fag t 3 during thermal processing. Journal of the Science of Food and Agriculture, 93, 1510–1515. doi:10.1002/jsfa.5928
- Zhou, G., Fu, L., & Li, X. (2015). Optimisation of ultrasound-assisted extraction conditions for maximal recovery of active monacolins and removal of toxic citrinin from red yeast rice by a full factorial design coupled with response surface methodology. Food Chemistry, 170, 186–192.