ABSTRACT
EMSA Eritin which consists of red rice, soybean, and coconut water extract has been widely reported as having antioxidant activity. This study aimed to analyse the effect of EMSA Eritin on nuclear factor (NF)-κB activation and pro-inflammatory cytokines IL-17 after radiation exposure. The experiments were carried out in the irradiated mouse model (6 Gy). Oral administration of EMSA Eritin 1.04, 3.125, and 9.375 mg/g BW for 14 days was compared with injections of 4.16 mg Erythropoietin-αTM (EPO). This study shows an increase in NF-κB activation and IL-17 production in irradiated mice compared to normal mice (p ≤ .05). EPO injection could not prevent these changes. Oral administration of EMSA Eritin suppressed NF-κB activation, and also decreased production of pro-inflammatory IL-17 significantly (p ≤ .05) compared with irradiated mice and irradiated mice treated with EPO.
Introduction
Radiation is one of the standard cancer treatments. Radiotherapy usually causes small side effects such as fatigue, red skin, irritation, dry mouth, nausea, vomiting, and diarrhoea. However, radiation can also cause acute side effects due to normal cell damage. Tissues damaged by irradiation usually show primary signs of inflammation (Kim & McBride, Citation2010). Inflammation is characterized in the acute phase by free radical generation which leads to the formation of reactive oxygen species (ROS), increased the accumulation of fluid, leukocytes, and inflammatory mediators such as cytokines and growth factors with associated inflammatory infiltrates (Kim & McBride, Citation2010; Schaue, Kachikwu, & McBride, Citation2012).
The inflammatory response is regulated by the expression of pro- and anti-inflammatory mediators (Arican, Aral, Sasmaz, & Ciragil, Citation2005; Lawrence, Citation2009; Qu et al., Citation2013). Aggarwal, Shishodia, Sandur, Pandeya, and Sethi (Citation2006) mentions that chronic inflammation is related to increased levels of pro-inflammatory cytokines, chemokines, adhesion molecules, and inflammatory enzymes. Radiation-induced damage in normal tissue is also affected by several of the immune system mechanisms associated with wound healing (Schaue & McBride, Citation2010). Th17 cells are critical players in chronic inflammation (Mangan et al., Citation2006) mediated by IL-17 cytokines. IL-17 affects several inflammatory responses which involve recruitment of immune cells (Park et al., Citation2005) and induction pro-inflammatory factors such as IL-1, IL-6, and TNF (Numasaki et al., Citation2003). Inflammation response is mainly mediated by nuclear factor (NF)-κB activation because it has the ability to induce the transcription of pro-inflammatory genes (Bonizzi & Karin, Citation2004; Liang, Zhou, & Shen, Citation2004; Tak & Firestein, Citation2014).
These side effects of radiotherapy, if not promptly treated, will cause complications of the other chronic inflammation diseases. EMSA Eritin polyherbal is a supplement that consists of a combination of red rice, soybean extract, and coconut water. These herbal compounds have long been known have antioxidant activity because they contain natural compounds such as pantothenic acid, biotin, riboflavin, folic acid, thiamine B1, vitamin C, isoflavones, anthocyanins, genistein, cytokinin, nicotinic acid, pyridoxine, daidzein, glycitein, and some phenolic acids. All of these natural compounds have a function as radio-protective agents that can increase the activity of antioxidant enzymes (Wei, Zhang, Wang, & Lebwohl, Citation2002; Yong, Ge, & Tan, Citation2009). Red rice extract is proved to have antioxidant activity because it can increase antioxidant enzymes such as superoxide dismutase (SOD) and catalase (Walter & Marchesan, Citation2011). Arora, Byrem, Nair, and Strasburg (Citation2000) explain that isoflavone from soybean can inhibit the diffusion of free radicals and decrease the kinetic reactions of free radicals. Kinetin contained in coconut water can reduce the formation of ROS and stimulate the synthesis of SOD and GSH-Px (Liu, Zhang, & Yang, Citation2011).
Antioxidants are substances that the body needs to neutralize free radicals and prevent the damage caused by free radicals on normal cells, proteins, and fats. Antioxidants in the human body can counteract the destruction caused by free radical reactions that can lead to chronic inflammation. Based on the assumption that EMSA Eritin polyherbal can be used to inhibit the inflammatory responses induced by radiation, this research aimed to analyse the effects of EMSA Eritin polyherbal to NF-κB activation and pro-inflammatory cytokines IL-17 in the immune system.
Methods
Mice
Seven to eight weeks old normal mice were maintained in pathogen-free chamber. The experimental method was approved by Ethical Clearance from the Research Ethics Committee (Animal Care and Use Committee) Brawijaya University No. 255-KEP-UB. Mice were divided into six treatment groups, they are control, Rad 6 Gy, Rad 6 Gy + erythropoietin (EPO), Rad 6 Gy + D1, Rad 6 Gy + D2, and Rad 6 Gy + D3. The control group consisted of healthy mice not exposed to radiation. Rad 6 Gy group consisted of irradiated mice without EPO or EMSA Eritin treatments. Rad 6 Gy + EPO group consisted of irradiated mice injected with EPO 4.16 mg twice a week. Rad 6 Gy + D1, D2, or D3 were irradiated mice to which EMSA Eritin was orally administered at doses of 1.04 mg/g BW (D1), 3.125 mg/g BW (D2), or 9.375 mg/g BW (D3), respectively, for 14 days.
Radiation exposure (total body irradiation)
Irradiation was carried out in the Radiology Laboratory, Saiful Anwar Hospital, Malang. The radiation dose used was 6 Gy. Instrument used was an Exposure Instrument Gammacell-40 137 Ce irradiator (Atomic Energy of Canada Ltd.) with a dose rate of 0.76 Gy/min.
Oral administration of EMSA Eritin after radiation
Mice were given EMSA Eritin orally beginning 5 days after irradiation and continuing daily for 14 days. The EMSA Eritin concentrations used in this study were 1.04 mg/g BW (D1), 3.125 mg/g BW (D2), and 9.375 mg/g BW (D3). Intraperitoneal injection of EPO was applied as the control treatment at 4.16 mg, twice a week.
Cell isolation
Mice were killed by cervical dislocation and spleen and bone marrow were isolated and washed with phosphate-buffered saline (PBS) in Petri dishes. Cells were isolated from the spleens by crushing in PBS. Bone marrow cells were isolated from femoral bone marrow obtained by removing the lower and upper ends of the femoral bones and the cells isolated by flushing with 1 ml PBS. Homogenates were centrifuged, 2500 rpm, 10°C, for 5 min. The supernatant was discarded whilst the pellet resuspended in 1 ml of medium.
Fluorescence-activated cell sorting analysis
Spleen cell suspensions were divided into two microtubes (A and B), bone marrow cell suspensions were put into one microtube (C). Cells were centrifuged at a speed of 2500 rpm for 5 min, 10°C. Th supernatants were discarded and the pellets were stained with antibodies. The combinations of dye and antibody used were as follows: dye A: FITC-conjugated to rat anti-mouse CD4, PE-conjugated to rat anti-mouse CD8, and PE/Cy5-conjugated to rat anti-mouse NF-κB; dye B: FITC-conjugated to rat anti-mouse CD4, PE-conjugated to rat anti-mouse CD8, and PE/Cy5-conjugated to rat anti-mouse IL-17; dye C: PE-conjugated to rat anti-mouse Gr1 and PE/Cy5-conjugated to rat anti-mouse IL-17.
Cells were stained with extracellular antibodies then incubated for 20 min at 4°C. After that, cells were mixed with 50 µl cytofix/cytoperm and incubated for 20 min at 4°C. Washperm (500 µl) was added and then the samples were centrifuged, 2500 rpm, 10°C for 5 min. Pellets were then stained with intracellular antibodies and incubated for 20 min at 4°C. Cells that had been incubated following this immunocytochemistry procedure were suspended in 500 µl PBS, transferred into flow cytometry cuvettes and then analysed by flowcytometer.
To avoid non-specific binding fetal bovine serum 5% in PBS was added to both the stain solution and wash buffer. A surplus of antibody can increase the level of non-specific binding, leading to a reduction in the identification of positive cells. In this case the staining followed the manufacturer's protocol, so that there is no excess of antibody. Third, dead cells are notorious for non-specifically binding. This is partially due to DNA, to solve this problem samples were gated and analysed only in the live cell without adding DNAse.
Data analysis
Data were analysed by using BD cellQuest PRO™ software then tabulated and analysed statistically using parametric one-way ANOVA with a significance of 0.05% and followed by Tukey's test (SPSS version 16 for Windows).
Results
EMSA Eritin can suppress NF-κB activation in immunocompetent cell
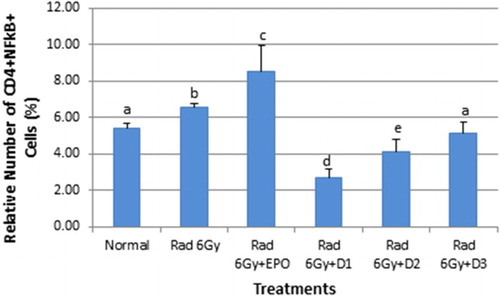
The role of NF-κB in the expression of pro-inflammatory genes including cytokines, chemokines, and adhesion molecules has been extensively reviewed. This study proved that EMSA Eritin can suppress NF-κB both in CD4+ and CD8+ T cells. The number of NF-κB in CD4+ T cells in normal mice was 5.39% and in irradiated mice were 6.64%. They were significantly different (p ≤ .05). Irradiated mice that injected with EPO, 4.16 mg twice a week, had 8.54% NF-κB in CD4+ T cells which is higher than both normal mice and irradiated mice. Oral administration of EMSA Eritin in irradiated mice suppressed the activation of NF-κB in CD4+ T cells in a dose-dependent manner: 2.73% in D1 (1.04 mg/g BW), 4.10% in D2 (3.125 mg/g BW), and 5.14% in D3 (9.375 mg/g BW) () significantly different from irradiated mice and irradiated mice treated with EPO (p ≤ .05).
Figure 1. The oral administration of EMSA Eritin to irradiated mice can suppress the activation of NF-κB in CD4T cells. Controls non-irradiated mice; irradiated mice (6 Gy); test mice; irradiated plus post-irradiation injection of EPO 4.16 mg twice a week; oral administration of EMSA Eritin, 1.04 mg/g BW (D1), 3.125 mg/g BW (D2), and 9.375 mg/g BW (D3) daily for 14 days. NF-κB in CD4+ T cells (CD4+NF-κB+) is presented as percentages of total cell counts. The data are mean ± SD in each group with p ≤ .05.
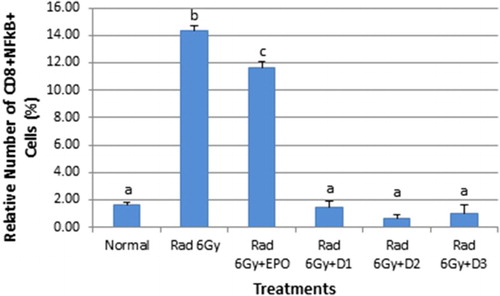
EMSA Eritin also had an impact on NF-κB in CD8+ T cells as shown in . Normal mice had 1.61% NF-κB in CD8+ T cells whilst irradiated mice had 14.38% (p ≤ .05). EPO injections given to irradiated mice reduced the level NF-κB in CD8+ T cells (11.64%, p ≤ .05). Oral administration of EMSA Eritin to groups D1 (1.45%), D2 (0.69%), and D3 (1.05%) had a greater effect on NF-κB in CD8+ T cells reducing the level of NF-κB in CD8+ T cells to values equivalent to the normal condition (not significantly different p > .05) but significantly different from irradiated mice and irradiated mice treated with EPO (p ≤ .05).
EMSA Eritin can decrease production of pro-inflammatory cytokines IL-17
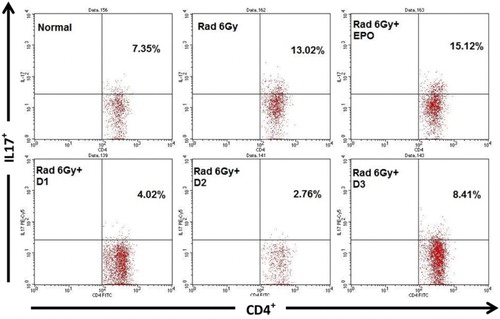
One of the factors which activates NF-κB is IL-17 cytokine; hence, the levels of IL-17 were also measured in this study. The quantity of IL-17 cytokine secreted by CD4+ T cells in normal mice was 7.35% which was significantly increased (13.02%, p ≤ .05) in the irradiated mouse model. Irradiated mice injected with EPO did not show a decrease in IL-17 (15.12%). Irradiated mice given EMSA Eritin 1.04 mg/g BW (D1), 3.125 mg/g BW (D2), and 9.375 mg/g BW (D3) produced 4.02, 2.76, and 8.41%, respectively, significantly lower than in irradiated mice and EPO treated-mice (p ≤ .05) ().
Figure 3. The effect of oral administration of EMSA Eritin on the production of IL-17 by CD4T cells in irradiated mice. Data analysis using flow cytometry. IL-17 produced by CD4+ T cells (CD4+IL-17+) presented as relative number. The data are mean ± SD in each group with p ≤ .05.
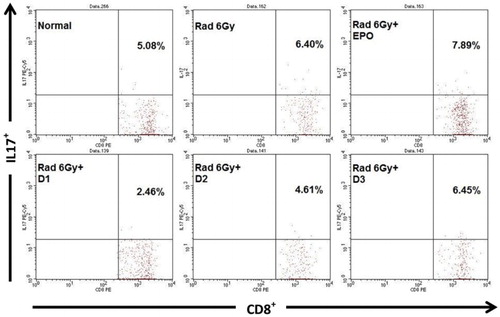
CD8+ T cells also produce IL-17 in normal mice the level was 5.08% whilst in irradiated mice the level was 6.40%, which is significantly greater (p ≤ .05). The results in treated irradiated mice showed that EPO injections could not decrease the level of IL-17 cytokines, 7.89% which is higher than normal mice (p ≤ .05). In contrast oral administration of EMSA Eritin in irradiated mice groups D1 and D2 decreased the level of IL-17 cytokine, 2.46 and 4.61%, respectively, but D3 the highest concentration did not decrease IL-17(p > .05) ().
Figure 4. The effect of oral administration of EMSA Eritin in irradiated mice on the production of IL-17 cytokines by CD8T cells. IL-17 produced by CD8+ T cells (CD8+IL-17+) was presented as relative number. The data are mean ± SD in each group with p ≤ .05.
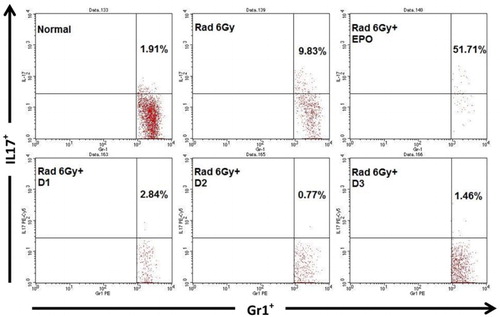
IL-17 is also produced by granulocyte cells (Gr1+). IL-17 secreted by Gr1+, in normal mice was 1.91%, in irradiated mice the level was nine times higher, 9.83% (p ≤ .05). Surprisingly, in irradiated mice injected with EPO, the IL-17 levels were very high, 51.71%. Irradiated mice given EMSA Eritin show that the treatment decreased IL-17 levels produced by Gr1+, 2.84, 0.77, and 1.46%, respectively. These results did not differ significantly from the levels found in normal mice (p > .05) (). This result indicates that orally administered EMSA Eritin can reduce the inflammation produced by irradiation.
Discussion
This study demonstrated that irradiation increased NF-κB activation and the production of Il-17, a pro-inflammatory cytokine. EPO, usually given to patients after radiotherapy treatment to stimulate the production of red blood cell by bone marrow could not decrease inflammatory mediators such as NF-κB and IL-17, indicating EPO does not work as an anti-inflammatory agent especially after irradiation. Inflammation after radiation exposure caused an increase in NF-κB produced by CD4+ and CD8+ T lymphocytes cells. These findings agree with those of Lawrence (Citation2009) who showed that the NF-κB pathway has a role in the expression of pro-inflammatory genes including cytokines, chemokines, and adhesion molecules. Ogawa et al. (Citation2003) mentions that ROS formation occurred immediately after irradiation and continued for several hours, resulting in oxidative DNA damage. ROS activates the NF-κB pathway (Jialal, Devaraj, & Kaul, Citation2001), which leads to many inflammatory events.
Furthermore, inflammation after irradiation also occurs as a result of IL-17 cytokine production by immunocompetent cells. IL-17 is known to play an important role in the inflammatory response (Jin & Dong, Citation2013; Turner-Brannen et al., Citation2011). Nembrini, Marsland, and Kopf (Citation2009) explain that T cell produce IL-17 cytokine during lung inflammation. IL-17 is widely known as a potent mediator of pro-inflammatory cytokines, chemokines, and acute phase response elements (Gaffen, Citation2004; Lubberts, Citation2008; Onishi & Gaffen, Citation2010). IL-17 is expressed by activated CD4+ T cells, mainly in the memory subset and also by innate immune cells such as macrophages, dendritic cells, and NK cells (Korn, Bettelli, Oukka, & Kuchroo, Citation2009). The expression of IL-17 is increased in several chronic inflammatory diseases (Aarvak, Chabaud, Miossec, & Natvig, Citation1999; Albanesi et al., Citation2000; Lenarczyk et al., Citation2000). IL-17 is a potent inducer of pro-inflammatory cytokines such as TNF-α and IL-1β (Koenders et al., Citation2006; Lubberts, Citation2008). The decrease in NF-κB may be related to IL-17 cytokines because Hata et al. (Citation2002) mentions that IL-17 stimulates inflammatory responses via the NF-κB pathway. Turner-Brannen et al. (Citation2011) suggests that downstream responses induced by IL-17 include transcriptional responses and the direct activation of NF-κB. Thus, decreases in NF-κB, a pro-inflammatory transcription factor, and IL-17 by EMSA Eritin may decrease inflammation after radiotherapy.
EMSA Eritin consists of red rice, soybean, and coconut water extract. Rice has several compounds with antioxidant activity including the phenolic compounds, γ-oryzanol, tocotrienols, and tocopherols (Iqbal, Bhanger, & Anwar, Citation2005). The main additional phenolics in red rice are anthocyanins, cyanidin-3-O-β-d-glucoside, and peonidin-3-O-β-d-glucoside (Chen et al., Citation2006; Hu, Zawistowski, Ling, & Kitts, Citation2003; Oki et al., Citation2002; Yawadio, Tanimori, & Morita, Citation2007; Zhang et al., Citation2006). The relative concentration of total phenolics in red rice is associated with its antioxidant activity (Goffman & Bergman, Citation2004; Itani, Tatemoto, Okamoto, Fujii, & Muto, Citation2002; Zhang et al., Citation2006) and its ability to reduce oxidative stress (Hu et al., Citation2003; Ling, Cheng, Ma, & Wang, Citation2001). In vitro studies have shown that the phenolic compounds in red rice associated with anticarcinogenic, antimutagenic, and antimetastatic activity because of their ability to directly protect damaged DNA (Chen et al., Citation2006; Hu et al., Citation2003). Chawla et al. (Citation2006) mention that the anthocyanins in the red rice pigment are useful as radio protectors.
Soybean produce natural antioxidants such as isoflavones (Saija et al., Citation1995). The main isoflavones in soybean are genistein (4′,5′,7-tryhydroxyisoflavone), daidzein (4′,7-dihydroxyisoflavone), derived from β-glycosides, gensitin and daidzin, glycitein (7,4′-dihydroxy-6-methoxy-isoflavone), and glycosides (Wang & Murphy, Citation1994). Isoflavones are potential candidates as radiation protective agents because they are effective antioxidants and they also increase the activity of antioxidant enzymes (Wei et al., Citation2002). Glycosides are maintained by plant in an inactive form. Flavonoid and isoflavone compounds containing phenolic groups act as antioxidants and prevent damage due to of free radical via one of two mechanisms: they can donate hydrogen ions (Arora, Nair, & Strasburg, Citation1998; Saija et al., Citation1995), or act directly as a free radical scavengers (Arora et al., Citation1998; Nijveldt et al., Citation2001), DNA repair and cell renewal stimulates the activity of immune cells (Zhou & Mi, Citation2005). Genistein is a great antioxidant because it can reduce the levels of free radicals arising from lipid peroxidation products (Filipe et al., Citation2005; Kruk, Aboul-Enein, Michalskam, Lichszteld, & Kladna, Citation2005). Wei et al. (Citation2002) mentions that genistein can also act indirectly as an anti-inflammatory agent because it provides protection from ROS.
Coconut water contains vitamin C, folate, magnesium, and kinetin each of which may play a role in the inflammation process. It was recently shown that vitamin C prevents oxidative damage during ischemic reperfusion in rats and humans (Mora, Iwata, & von Andrian, Citation2008). Folate deficiency can exacerbate cardiovascular disease by augmenting pro-inflammatory signals in the monocyte-macrophage lineage (Kolb & Petrie, Citation2013). Magnesium deficiency can lead to systemic inflammation. Malpuech-Brugère et al. (Citation2000) and Blache et al. (Citation2006) explain that in a rat model magnesium deficiency results an increase in IL-6 cytokine, fibrinogen, and a decrease in plasma albumin which lead to inflammatory responses. Liu et al. (Citation2011) explain about kinetin in coconut water can stimulate the synthesis of SOD and GSH-Px and reduce the formation of ROS.
Conclusion
EMSA Eritin polyherbal which consists of red rice, soybean, and coconut water extract can be used to reduce the inflammatory response after radiotherapy. EMSA Eritin was able to decrease the activation of NF-κB in CD4+ and CD8+ T cells and the production of IL-17 pro-inflammatory cytokine by CD4+ T cells and Gr1+ in irradiated mice. It was more effective in its action than post-irradiation treatment with EPO. Although confusingly production of IL-17 by CD8+ T cells was only shown to be reduced by the two lower concentration (D1 and D2) of EMSA Eritin polyherbal tested
However, this study has proved that EMSA Eritin is a potential alternative of radio-protective agent that suppresses the inflammatory response after irradiation. Although this study provides information about the possible use of EMSA Eritin as a radio-protective agent that suppresses the inflammatory response, further studies are still necessary to establish its radio-protective mechanism.
Acknowledgments
We wish to thank K. Qonitatul, I.C. Yuyun, and P. Bambang for experimental help in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aarvak, T., Chabaud, M., Miossec, P., & Natvig, J. B. (1999). IL-17 is produced by some pro-inflammatory Th1/Th0 cells but not by Th2 cells. Journal of Immunology, 162(3), 1246–1251. Retrieved from http://www.jimmunol.org/content/162/3/1246
- Aggarwal, B. B., Shishodia, S., Sandur, S. K., Pandeya, M. K., & Sethi, G. (2006). Inflammation and cancer: How hot is the link? Biochemical Pharmacology, 72, 1605–1621. doi:10.1016/j.bcp.2006.06.029
- Albanesi, C., Scarponi, C., Cavani, A., Federici, M., Nasorri, F., & Girolomoni, G. (2000). Interleukin-17 is produced by both Th1 and Th2 Lymphocytes, and modulates interferon Gamma- and Interleukin-4-induced activation of human Keratinocytes. Journal of Investigative Dermatology, 115(1), 81–87. doi:10.1046/j.1523-1747.2000.00041.x
- Arican, O., Aral, M., Sasmaz, S., & Ciragil, P. (2005). Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators of Inflammation, 5, 273–279. doi:10.1155/MI.2005.273
- Arora, A., Byrem, T. M., Nair, M. G., & Strasburg, G. M. (2000). Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Archives Biochemistry Biophysics, 373, 102–109. doi:10.1006/abbi.1999.1525
- Arora, A., Nair, M. G., & Strasburg, G. M. (1998). Structure–activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radical Biology & Medicine, 24(9), 1355–1363. doi:10.1016/S0891-5849(97)00458-9
- Blache, D., Devaux, S. Joubert, O. Loreau, N. Schneider, M. Durand, P., … Berthelot, A. (2006). Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radical Biology and Medicine, 41, 277–284. doi:10.1016/j.freeradbiomed.2006.04.008
- Bonizzi, G., & Karin, M. (2004). The two NF-kB activation pathways and their role in innate and adaptive immunity. Trends in Immunology, 25, 280–288. doi:10.1016/j.it.2004.03.008
- Chawla, R., Arora, R. Singh, S. Sagar, R. K. Sharma, R. K. Kumar, R., … Qazi, G. N. (2006). Podophyllum hexandrum offers radioprotection by modulating free radical flux: Role of Aryl-Tetralin Lignans. eCAM, 3, 503–511. doi:10.1093/ecam/nel037
- Chen, P., Kuo, W. Chiang, C. Chiou, H. Hsieh, Y., & Chu, S. (2006). Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and uPA expression. Chemico-Biological Interactions, 163, 218–229. doi:10.1016/j.cbi.2006.08.003
- Filipe, P., Silva, J. N., Haigle, J., Freitas, J. P., Fernandes, A., Santus, R., & Morliere, P., (2005). Contrasting action of flavonoids on phototoxic effects induced in human skin fibroblasts by UVA alone or UVA plus Cyamemazine, a Phototoxic Neuroleptic. Photochemistry Photobiololgy Science, 4, 420–428. doi:10.1039/B416811A
- Gaffen, S. L. (2004). Biology of recently discovered cytokines: Interleukin-17 a unique inflammatory cytokine with roles in bone biology and arthritis. Arthritis Research & Therapy, 6, 240–247. doi:10.1186/ar1444
- Goffman, F. D., & Bergman, C. J., (2004). Rice kernel phenolic content and its relationship with antiradical efficiency. Journal of the Science of Food and Agriculture, 84, 1235–1240. doi:10.1002/jsfa.1780
- Hata, K., Andoh, A., Shimada, M., Fujino, S., Bamba, S., Araki, Y., … Bamba, T., (2002). IL-17 stimulates inflammatory responses Via NF-κB and MAP Kinase pathways in human colonic myofibroblasts. American Journal of Physiology - Gastrointestinal and Liver Physiology, 282, G1035–G1044. doi:10.1152/ajpgi.00494.2001
- Hu, C., Zawistowski, J., Ling, W., & Kitts, D. D. (2003). Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and Nitric Oxide in chemical and biological model systems. Journal of Agricultural and Food Chemistry, 51, 5271–5277. doi:10.1021/jf034466n
- Iqbal, S., Bhanger, M. I., & Anwar, F. (2005). Antioxidant properties and components of some commercially available varieties of rice Bran in Pakistan. Food Chemistry, 93, 265–272. doi:10.1016/j.foodchem.2004.09.024
- Itani, T., Tatemoto, H., Okamoto, M., Fujii, K., & Muto, N. (2002). A comparative study on antioxidative activity and polyphenol content of colored kernel rice. Journal of the Japanese Society for Food Science and Technology, 49, 540–543. doi:10.3136/nskkk.49.540
- Jialal, I., Devaraj, S., & Kaul, N. (2001). The effect of a-tocopherol on monocyte proatherogenic activity. The Journal of Nutrition, 131(2), 389S–394S. Retrieved from http://jn.nutrition.org/content/131/2/389S.long
- Jin, W., & Dong, C. (2013). IL-17 Cytokines in immunity and inflammation. Emerging Microbes and Infections, 2, 1–5. doi:10.1038/emi.2013.58
- Kim, K., & McBride, W. H. (2010). Modifying radiation damage. Current Drug Targets, 11, 1352–1365. doi:10.2174/1389450111009011352
- Koenders, M. I., Lubberts, E., van de Loo, F. A. Oppers-Walgreen, B., van den Bersselaar, L. Helsen, M. M., … van den Berg, W. B. (2006). Interleukin-17 acts independently of TNF-Alpha under arthritic conditions. The Journal of Immunology, 176, 6262–6269. doi:10.4049/jimmunol.176.10.6262
- Kolb, A. F., & Petrie, L. (2013). Folate deficiency enhances the inflammatory response of macrophages. Molecular Immunology, 54(2), 164–172. doi:10.1016/j.molimm.2012.11.012
- Korn, T., Bettelli, E., Oukka, M., & Kuchroo, V. K. (2009). IL-17 and Th17 cells. Annual Review of Immunology, 27, 485–517. doi:10.1146/annurev.immunol.021908.132710
- Kruk, I., Aboul-Enein, H. Y., Michalskam, T., Lichszteld, K., & Kladna, A. (2005). Scavenging of reactive Oxygen species by the plant Phenols Genistein and Oleuropein. Luminescence, 20, 81–89. doi:10.1002/bio.808
- Lawrence, T. (2009). The Nuclear factor NF-kB pathway in inflammation. Inflammation Biology Group, 1–10. doi:10.1101/cshperspect.a001651
- Lenarczyk, A., Helsloot, J., Farmer, K., Peters, L., Sturgess, A., & Kirkham, B. (2000). Antigen induced IL-17 response in the peripheral blood mononuclear cells (PBMC) of healthy controls. Clinical and Experimental Immunology, 122(1), 41–48. doi:10.1046/j.1365-2249.2000.01328.x
- Liang, Y., Zhou, Y., & Shen, P. (2004). NF-κB and its regulation on the immune system. Chinese Society of Immunology, 1(5), 343–350. Retrieved from http://www.cmi.ustc.edu.cn/1/5/343
- Ling, W. H., Cheng, Q. X., Ma, J., and Wang, T. (2001). Red and black rice decrease Atheroscletoric plaque formation and increase antioxidant status in rabbits. Journal of Nutrition, 131, 1421–1426. Retrieved from http://jn.nutrition.org/content/131/5/1421
- Liu, Y., Zhang, Z., & Yang, X. (2011). Kinetin protects against lipid peroxidation and improves antioxidant status in cultured astrocytes and mouse brain exposed to D-galactose. African Journal of Biotechnology, 10(55), 11721–11727. doi:10.5897/AJB11.289
- Lubberts, E. (2008). IL-17/Th17 targeting: On the road to prevent chronic destructive arthritis? Cytokine, 41, 84–91. doi:10.1016/j.cyto.2007.09.014
- Malpuech-Brugère, C., Nowacki, W., Daveau, M., Gueux, E., Linard, C., Rock, E., … Rayssiguier, Y. (2000). Inflammatory response following acute magnesium deficiency in the rat. Biochimica et Biophysica Acta, 501, 91–98. doi:10.1016/S0925-4439(00)00018-1
- Mangan, P. R., Harrington, L. E. O'Quinn, D. B. Helms, W. S. Bullard, D. C., Elson, D. C. C. O., … Weaver, C. T. (2006). Transforming growth factor-beta induces development of the T(H)17 lineage. Nature, 441(7090), 231–234. doi:10.1038/nature04754
- Mora, J. R., Iwata, M., & von Andrian, U. H. (2008). Vitamin effects on the immune system: Vitamins A and D take centre stage. Nature Reviews Immunology, 8(9), 685–698. doi:10.1038/nri2378
- Nembrini, C., Marsland, B. J., & Kopf, M. (2009). IL-17–producing T cells in lung immunity and inflammation. Journal of Allergy and Clinical Immunology, 123(5), 986–994. doi:10.1016/j.jaci.2009.03.033
- Nijveldt, R. J., van Nood, E. van Hoorn, D. E., Boelens, P. G., van Norren, K., & van Leeuwen, P. A. (2001). Flavonoids: A review of probable mechanism of action and potential applications. The American Journal of Clinical Nutrition, 74, 418–425. Retrieved from http://ajcn.nutrition.org/content/74/4/418
- Numasaki, M., Fukushi, J., Ono, M., Narula, S. K., Zavodny, P. J., Kudo, T., … Lotze, M. T. (2003). Interleukin-17 promotes angiogenesis and tumor growth. Blood, 101(7), 2620–2627. doi:10.1182/blood-2002-05-1461
- Ogawa, Y., Kobayashi, T., Nishioka, A., Kariya, S., Hamasato, S., Seguchi, H., &Yoshida, S. (2003). Radiation-induced reactive oxygen species formation prior to Oxidative DNA damage in human peripheral T cells. International Journal of Molecular Medicine, 11(2), 149–152. doi:10.3892/ijmm.11.2.149
- Oki, T., Masuda, M., Kobayashi, M., Nishiba, Y., Furuta, S., Suda, I., & Sato, T. (2002). Polymeric procyanidins as radical-scavenging components in red-hulled rice. Journal of Agricultural and Food Chemistry, 50(26), 7524–7529. doi:10.1021/jf025841z
- Onishi, R. M., & Gaffen, S. L. (2010). Interleukin-17 and its target genes: Mechanisms of Interleukin-17 function in disease. Immunology, 129, 311–321. doi:10.1111/j.1365-2567.2009.03240.x
- Park, H., Li, Z., Yang, X. O., Chang, S. H., Nurieva, R., Wang, Y. H., … Dong, C. (2005). A distinct lineage of CD4 T cells regulates tissue inflammation by producing Interleukin 17. Nature Immunology, 6(11), 1133–1141. doi:10.1038/ni1261
- Qu, N., Xu, M., Mizoguchi, I., Furusawa, J., Kaneko, K., Watanabe, K., … Yoshimoto, T. (2013). Pivotal roles of T-helper 17-related Cytokines, IL-17, IL-22, and IL-23, in Inflammatory Diseases. Clinical and Developmental Immunology, 1–13. doi:10.1155/2013/968549
- Saija, A., Scalese, M., Lanza, M., Marzullo, D., Bonina, F., & Castelli, F. (1995). Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radical Biology and Medicine19(4), 481–486. doi:10.1016/0891-5849(94)00240-K
- Schaue, D., Kachikwu, E.L., & McBride, W. H. (2012). Cytokines in radiobiological responses: A review. Radiation Research, 178, 505–523. doi:10.1667/RR3031.1
- Schaue, D., & McBride, W. H. (2010). Links between innate immunity and normal tissue radiobiology. Radiation Research, 173(4), 406–417. doi:10.1667/RR1931.1
- Tak, P. P., & Firestein, G. S. (2014). NF-κB: A key role in inflammatory diseases. Journal of Clinical Investigation, 107(1), 7–11. doi:10.1172/JCI11830
- Turner-Brannen, E., Choi, K. G., Arsenault, R., El-Gabalawy, H., Napper, S., & Mookherjee, N. (2011). Inflammatory Cytokines IL-32 and IL-17 have common signaling intermediates despite differential dependence on TNF-receptor 1. The Journal of Immunology, 186, 7127–7135. doi:10.4049/jimmunol.1002306
- Walter, M., & Marchesan, E. (2011). Phenolic compounds and antioxidant activity of rice. Brazilian Archives of Biology and Technology, 54(1), 371–377. doi:10.1590/S1516-89132011000200020
- Wang, H., & Murphy, P. A. (1994). Isoflavone content in commercial soybeans foods. Journal of Agricultural and Food Chemistry, 42(8), 1666–1673. doi:10.1021/jf00044a016
- Wei, H., Zhang, X., Wang, Y., & Lebwohl, M. (2002). Inhibition of ultraviolet light-induced oxidative events in the skin and internal organs of hairless mice by Isoflavone Genistein. Cancer Letters, 185(1), 21–29. doi:10.1016/S0304-3835(02)00240-9
- Yawadio, R., Tanimori, S., & Morita, N. (2007). Identification of phenolic compounds isolated from pigmented Pices and their aldose Reductase inhibitory activities. Food Chemistry, 101(4), 1616–1625. doi:10.1016/j.foodchem.2006.04.016
- Yong, J. W. H., Ge, L., & Tan, Y. F. (2009). The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules, 14, 5144–5164. doi:10.3390/molecules14125144
- Zhang, M., Guo, B., Zhang, R., Chi, J., We, Z., Xu, Z., … Tang, X. (2006). Separation, purification and identification of antioxidant compositions in black rice. Agricultural Sciences in China, 5(6), 431–440. doi:10.1016/S1671-2927(06)60073-4
- Zhou, Y., & Mi, M. (2005). Genistein stimulates hematopoiesis and increases survival in irradiated mice. Journal Radiation Research, 46(4), 425–433. doi:10.1269/jrr.46.425