ABSTRACT
A sensitive gold nanoparticle immunochromatographic strip assay and indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) were developed for the detection of ractopamine (RCT), a beta-adrenergic agonist. The immunogen and coating antigen were synthesized by the carbodiimide method and conjugated with keyhole limpet hemocyanin and ovalbumin, respectively. The highly sensitive and specific monoclonal antibody was prepared for RCT, with a 50% inhibition concentration of 0.05 ng/ml and had no cross-reactivity with other beta-adrenergic agonists. An ultrasensitive and rapid immunochromatographic strip assay was developed with an RCT cutoff value of 2 ng/ml. Both developed methods can be used for RCT detection in swine urine.
Introduction
Ractopamine (RCT) is a beta-adrenergic agonist that is added to cattle feed to reduce fat deposition and increase muscle production (Zhang, Gong, Zhang, Xi, Li, Chen, et al., Citation2014). It has been reported that RCT increases feed efficiency, reduces costs, and improves cattle marketing (Wang et al., Citation2015). However, RCT residues have negative effects on human health, leading to metabolic disturbances, arrhythmias, and muscle tremors (Hu, Liu, Li, & Li, Citation2010). Consequently, RCT has been officially banned in the European Union, Japan, and China (Dong, Citation2012). Therefore, it is important to develop a rapid and sensitive RCT detection method.
The current RCT detection methods include electrochemical and fluorescence detection (Liu, Chen, et al., Citation2011; Liu, Lin, et al., Citation2011; Rajkumar, Li, & Chen, Citation2013; Wang, Yong, He, Du, Ma, Dan, et al., Citation2013), gas chromatography (Fang et al., Citation2011), liquid chromatography-mass spectrometry (Amelin, Korolev, & Tret'yakov, Citation2015; Dong, Xi, Xia, Ding, Li, Zhang, et al., Citation2011; Liu, Yan, & Zhang, Citation2014; Liu, Ning, Qu, Peng, Dong, Gao, et al., Citation2012; Wei et al., Citation2014), ultra-performance liquid chromatography-tandem mass spectrometry (Jing-Kai, Teh-Ia, Lie-Chwen, & Tung-Hu, Citation2014), high-performance liquid chromatography (Li, Zhang, Deng, Yang, Liu, Xiao, et al., Citation2010), capillary electrophoresis (Anurukvorakun, Buchberger, Himmelsbach, Klampel, & Suntornsuk, Citation2010), and immunoassays (Cao et al., Citation2013; Mei, Xue, Chao, Bo, Wen, Heng Yi, et al., Citation2014; Sairi & Arrigan, Citation2015). Even though these methods are very sensitive, they require complex and time-consuming pretreatment steps and the instruments are costly.
Immunoassays, which have been widely used for RCT detection, have a high-throughput and are specific, user-friendly, and quick. Immunoassays include enzyme-linked immunosorbent assay (ELISA), fluorescence immunoassay (FLA), time-resolved fluoroimmunoassay, and immunomagnetic-proximity ligation assay (Gao et al., Citation2014; Jiang, Zhu, & Liu, Citation2014; Jing, Xintian, Jingli, Donghui, & Zhenhua, Citation2014; Radu, Aciu, Constantin, Leauta, Dermengiu, Hostiuc, et al., Citation2012; Yue, Wu, Li, & Xu, Citation2009). In this study, we produced an ultrasensitive and specific monoclonal antibody (mAb) based on a hapten containing an RCT fragment. Additionally, we developed both an immunochromatographic strip assay and ELISA for RCT detection.
Materials and methods
Chemicals
Ractopamine hydrochloride, trimethylamine, sodium triacetoxyborohydride [NaBH(OAc)3], octopamine, ethyl levulinate, ovalbumin (OVA), keyhole limpet hemocyanin (KLH; MW: 5,000,000 Da), 2-methylpropyl carbonochloridate, N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), incomplete Freund's adjuvant (FIA), complete Freund's adjuvant (FCA), enzyme immunoassay-grade horseradish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin, 3,3′,5,5′-tetramethylbenzidine (TMB), Tween-20, HRP and gelatin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell culture reagents (e.g. polyethylene glycol solution, HAT supplement, HT supplement, and 1640 cell culture) were obtained from Life Technologies Corporation (Shanghai, China). The materials of creating test strips were purchased from JieYi Biotech. Co., Ltd. (Shanghai, China), including glass fiber membrane for the sample pad, NC membrane used as immobilizing the coating antigen, H5076 filter paper used for the absorbent pad and Ahlstrom 8964 for the conjugated pad.
Solutions
Coating buffer (CB; 0.05 M carbonate bicarbonate, pH 9.6), blocking buffer (0.2% gelatin in coating buffer), washing buffer (PBST: 0.05% Tween-20 (v/v) in 0.01 M PBS), and stop solution (2 M sulfuric acid) were prepared. The substrate buffer consisted of solutions A (citric acid, H2O2, and Na2HPO4) and B (0.06% v/v TMB in glycol) at 5:1 (v/v) ratio. Swine urine was obtained from a local farm (Wuxi, China).
Antigen preparation
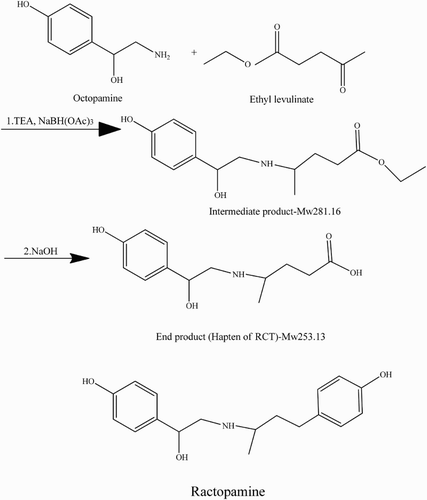
The RCT hapten was synthesized by the reductive amination reaction with octopamine (Liu, Kuang, Peng, Wang, & Xu, Citation2013) (). The immunogen and coating antigens were RCT hapten-EDC-KLH and RCT hapten-EDC-OVA, respectively. RCT was linked to the proteins by an active ester method (Suryoprabowo, Liu, Peng, Kuang, & Xu, Citation2014). Firstly, 10 mg RCT hapten was dissolved in 800 μl of dimethylformamide (DMF) and 1 ml of 0.1 M 2-[N-morpholino] ethanesulfonic acid, pH 4.7. Subsequently, 22.6 mg of EDC and 13.6 mg of NHS were added. The solution was stirred overnight at room temperature and in the dark (solution 1). KLH (11 mg) and OVA (14.8 mg) were mixed with 2 ml of 0.1 M CB, pH 9.6 (solution 2 and solution 3, respectively). Solution 1 was divided in half and added to solutions 2 and 3. The two solutions were stirred overnight at room temperature and dialyzed against 0.01 M PBS for 3 days to remove unbound RCT. Finally, UV and polyacrylamide gel electrophoreses were used to confirm the presence of antigens, which were subsequently stored at −20°C.
RCT mAb preparation
Female BALB/c mice (6–8 weeks of age) were subcutaneously immunized with immunogen RCT hapten-KLH mixed with FCA at 100 μg per mouse. FIA was incorporated to the subsequent boost injection (50 µg/mouse), which was performed on day 28 following the first immunization and repeated every 4 weeks thereafter (Li, Ji, Chen, Liu, Xu, Peng, et al., Citation2008; Xing & Kuang, Citation2013). Following the third injection, mice blood sera were analyzed by indirect competitive (ic)-ELISA. The mouse with the highest inhibition and titer was intraperitoneally injected immunogen RCT hapten-KLH with 25 µg. After 21 days, the mouse splenocytes were fused with Sp 2/0 myeloma cells for hybridoma cell production. Hybridoma cells were selected by ELISA and subcloned three times. The selected hybridoma cell culture was injected into mice for the production of ascites (Deng et al., Citation2012). Antibodies were purified by caprylic acid and ammonium sulfate precipitation, dialyzed against PBS for 3 days, and stored at −20°C.
ELISA
We used the checkerboard method to optimize the concentration of antibody and antigen. In this experiment, 100 μl coating antigen (RCT-OVA) was added to each well in microtiter plates and diluted with coating solution. The coated plates were incubated either at 37°C for 2 h or at 4°C for 12 h and washed 3 times with PBST. Blocking buffer (200 μl/well) was added to the plates, which were subsequently incubated at 37°C for 2 h. The blocked plates were washed twice with washing buffer. RCT standard (50 μl), which was diluted with swine urine, and antibody (50 μl) were added into each well, and the plates were incubated at 37°C for 30 min. Following three washes, HRP (100 μl), previously diluted with antibody dilution buffer (PBST with gelatin) at 1:3000, was added and incubated at 37°C for 30 min. Finally, TMB substrate solution freshly prepared was added (100 μl/well). The plates were washed four times and incubated at 37°C for 15 min. Following the addition of stop solution (50 μl/well), absorbance was measured at 450 nm in a microplate reader (purchased from BioTek) (Guan, Guo, Liu, & Kong, Citation2015). Standard mAb curves were generated by plotting B/B0 against log[RCT]. Antibody sensitivity was determined by 50% inhibition concentration (IC50).
The ELISA conditions were optimized to improve its sensitivity. The effects of pH (5.0, 6.0, 7.4, 8.6, 9.6), methanol concentrations (0%, 10%, 20%, and 30%, v/v), and NaCl concentrations (0%, 0.4%, 0.8%, 1.6%, and 3.2%) were evaluated.
Cross-reactivity
Clenbuterol, salbutamol, phenylethanolamine A, arformoterol, and formoterol were used to assess cross-reactivity (CR) with RCT by ic-ELISA. CR was calculated by the following formula, CR (%) = (IC50 of RCT/IC50 of the tested analogues)×100%.
Preparation of colloidal gold-labeled mAb
The preparation of colloidal gold-labeled mAb has been previously described (Sun et al., Citation2012). Purified mAb was slowly mixed with gold solution (20 ml), resulting in solution A. Solution A (pH 8.2) was mixed with 0.1 M K2CO3 at room temperature for 2 h. Bovine serum albumin (BSA, 10%, 100 ml) was added, thereby resulting in solution B. Solution B was stirred for 2 h and centrifuged three times at 8000 rpm for approximately 20 min each time. Gold-labeled mAb was re-suspended in borate solution and stored at 4°C (Guo, Liu, et al., Citation2015).
Immunochromatographic strip preparation
The immunochromatographic strip consists of four parts: a conjugate pad, a nitrocellulose (NC) membrane, a sample pad, and an absorbent pad. The control and test line were loaded onto the goat anti-mouse IgG, and the coating antigen was added to the NC membrane using a membrane dispenser. There are two lines (control and test) with a 0.5 cm separation in-between. The NC membrane was dried at 37°C for 30 min and attached to the back of the plate. Therefore, the NC membrane, a conjugate pad, and sample pad were loaded to the back of the plate. The absorbent pad was attached to the upper section of the plate. Finally, the assembled plate was cut into 3-mm wide strips (Guo, Song, et al., Citation2015).
Immunochromatographic strip assay
Swine urine sample (∼80 μl) was added to the sample pad and, by capillarity, it migrated to the absorption pad. Gold-labeled mAb became soluble and migrated to the coating antigen of the NC membrane. In RCT-negative swine urine, the gold-labeled mAb was captured by the coating antigen resulting in a visible red test line. A control line may also be generated because of redundant mAb captured by goat anti-mouse IgG. In RCT-positive swine urine, the free RCT would compete with the immobilized RCT for the gold-labeled mAb; however, mAb would not complex with the immobilized RCT, leading to the disappearance of the test line. At high RCT concentrations in the sample, the test line would have a faint color.
Analysis of spiked swine urine samples
RCT-negative swine urine was confirmed by a commercial immunochromatographic strip assay (Beijing wei de wei kang Biotech Co, Ltd). Swine urine was purified by centrifugation (8000 rpm, 10 min), spiked with different RCT concentrations, and analyzed by ic-ELISA.
Results and discussion
Conjugated antigen preparation
KLH was selected as the carrier protein for immunization, while OVA was selected as the coating antigen. The production of immunogen and coating antigen was based on the EDC method. In the EDC method, the proportion of organic phase and water phase is crucial, because EDC activity in water is higher than in organic solutions. In this method, DMF was approximately 30% (v/v). Additionally, the pH value should be approximately 4.7 in the active system, and kept between 9 and 10 when the active solution was added into the protein solution.
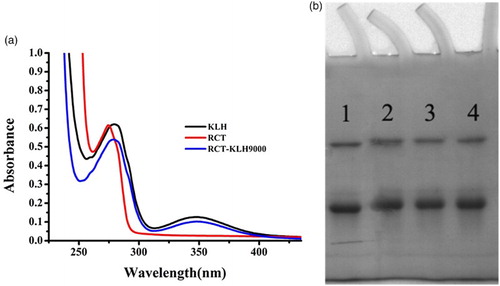
The immunogen RCT hapten-KLH was confirmed by UV–VIS spectroscopy ((a)), and the coating antigen was confirmed by polyacrylamide gel electrophoresis ((b)). The main UV peak of the RCT hapten was 27 nm, and KLH carrier protein were at 280, 350 nm, respectively. And the main UV peak of the RCT hapten-KLH was between 270 and 280 nm, it had the offset. The conjugated antigen also had the carrier protein KLH UV peak 350 nm. The findings revealed that the production of immunogen and coating antigen was successful. Peak 1 corresponded to OVA, the carrier protein, while peaks 2, 3, and 4 corresponded to conjugated proteins (the coupling ratio in RCT-EDC-OVA was 60, 80, and 100 M (RCT)/M (OVA), respectively). The molecular weight of OVA is 45,000 Daltons. If the RCT hapten was successfully conjugated with the carrier protein KLH, the molecular weight of RCT hapten-KLH was higher than OVA, and it resulted that the stripe of RCT hapten-KLH was behind of the OVA. The findings proved that RCT hapten was successfully linked with carrier protein KLH.
Preparation of mAb
MAb was obtained following ascite purification and confirmed by ic-ELISA. The cell lines 1B4, 2B11, 3E7, 4B3, 5B4, and 6D1 had similar IC50 values (0.05 ng/ml) in negative swine urine. The cell line 1B4 was selected for the immunochromatographic strip assay.
Optimization of ic-ELISA
RCT-EDC-OVA was selected as the coating antigen at a concentration of 0.1 µg/ml, with an antibody concentration of 0.06 µg/ml. The optimization experiments were based on the monoclonal antibody 1B4.
The effect of pH (5.0–9.6) was evaluated. As shown in (a), the Absorbance valuemax (Absmax)/IC50 value at pH 7.4 was the highest among the tested pH values. Furthermore, IC50 (0.05 ng/ml) was the lowest and Absmax was the highest at pH 7.4. Therefore, pH 7.4 was selected for subsequent experiments.
Figure 3. (a) The influence of pH; (b) The influence of ionic strength; (c) The influence of methanol content; (d) Standard inhibition curve for RCT by the optimized conditions.
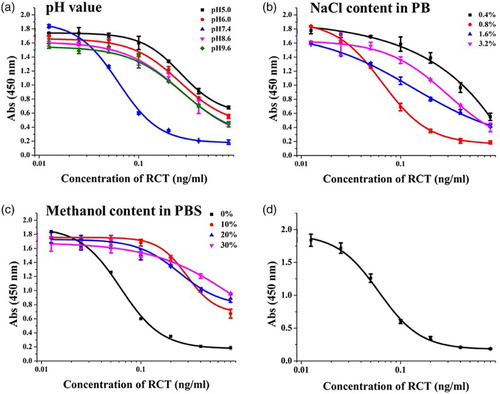
The effect of ionic strength was evaluated using four different NaCl concentrations (0.4–3.2%) in PB ((b)). Absmax was similar between 0.4% and 0.8% NaCl; however, IC50 was lower at 0.8% than at 0.4% NaCl. At > 0.8% NaCl, Absmax decreased. Therefore, 0.8% NaCl was selected for subsequent experiments.
Finally, the effect of different methanol concentrations (0–30%, v/v) was evaluated. With increasing methanol concentration, Absmax decreased and IC50 increased ((c)). IC50 was lower at 0% methanol than at other methanol concentrations. Therefore, 0% methanol was selected for subsequent experiments.
Therefore, the optimal conditions were pH 7.4, 0.8% NaCl in PB buffer, and 0% methanol concentration. At these conditions, IC50 was 0.05 ng/ml and IC20 (20% inhibition concentration) was 0.0286 ng/ml ((d)).
Cross-reactivity
The specificity of the synthesized mAb was assessed by determining CR with other beta-adrenergic agonists such as salbutamol, clenbuterol, phenylethanolamine A, arformoterol, and formoterol, The developed RCT antibody had high specificity (). The CR values with the beta-adrenergic agonists were < 0.1%.
Table 1. CR of related compounds
RCT recovery by ic-ELISA
Swine urine was spiked with different RCT concentrations (0.025–0.1 ng/ml) and analyzed by ELISA. RCT recovery was 110–120% (), and the effect of the urine matrix led to the recovery was very high. The intra-day coefficient variation (CV) was 0.34–3.87%, and the inter-day CV was 0.14–1.46%.
Table 2. Recoveries of RCT in swine urine samples.
RCT detection by immunochromatographic strip assay
The immunochromatographic strip was assayed by the dry method. Swine urine does not require complex pretreatment steps. The results revealed that swine urine was RCT-negative based on commercial immunochromatographic strip assays. In this study, 3 ng/ml RCT was added to swine urine. Firstly, we used the wet method to assess the optimal antibody and coating antigen concentrations. Using coating antigen (RCT-BSA-60) and different antibody concentrations, we optimized the assay. The results revealed that the optimum conditions were 1 ml of gold nanoparticle with 4 μl K2CO3, 8 μg/ml antibody, and 1 mg/ml coating antigen. However, these conditions were not suitable for subsequent experiments. Therefore, we selected RCT-EDC-OVA 60 as the coating antigen at a concentration of 0.5 ng/ml (). At 1 ml of gold nanoparticle with 6 μl K2CO3, the RCT cell line 1B4 was selected for subsequent experiments.
Figure 4. The sensitivity of the immunochromatographic assay in urine for RCT different antibodies (0.3 ng/ml from left to right).
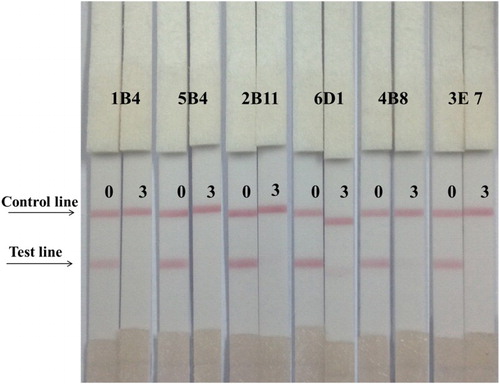
Different RCT standard concentrations (0–3 ng/ml) were added to swine urine. An aliquot (80 µl) of the spiked urine sample was added to the sample pad of the strips. The results were visible in 5 min, and as showed in and , the strip performed well when the concentrations (0, 1, 2, and 3 ng/ml) were analyzed, the RCT strip cutoff value was 2 ng/ml.
Table 3. Accuracy and reproductivity evaluation of the RCT strip.
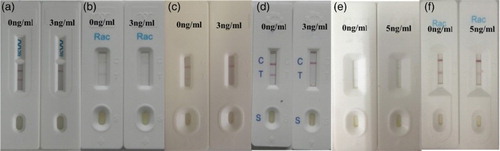
There are several RCT-detecting immunochromatographic strips commercially available () with RCT cutoff values of 3 ng/ml ((a), the company A; (b), the company B; (c), the company C; (d), the company D) and 5 ng/ml ((e), the company E; (f), the company F). In general, the optimum RCT-detecting strips have an RCT cutoff value of 3 ng/ml. In this study, the RCT cutoff value was 2 ng/ml in swine urine. Therefore, our developed immunochromatographic strip assay is practical for the detection of RCT residues in urine samples.
Figure 5. Comparison with other market products. (a) The company A for RCT, it cuts off at 3 ng/ml; (b) The company B for RCT, it cuts off at 3 ng/ml; (c) The company C for RCT, it cuts off at 3 ng/ml; (d) The company D for RCT, it cuts off at 3 ng/ml; (e) The company E for RCT, it cuts off at 5 ng/ml.; (f) The company F for RCT, it cuts off at 5 ng/ml.
Conclusions
In this study, we developed an ultrasensitive mAb for RCT detection, with an IC50 of 0.05 ng/ml. CR was very low, indicative that the developed mAb was highly specific. The developed mAb can be applied for RCT detection in swine urine samples. By optimizing the coating antigen and gold nanoparticle, a rapid and portable immunochromatographic strip assay was also successfully developed with an RCT cutoff value of 2 ng/ml in swine urine.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Huaiying Gu obtained her bachelor’s degree from Southwest University for Nationalities, Chengdu, China in 2013 and then her master’s degree in food science from Jiangnan University (Wuxi, China). Her research interest includes immunoassay development for veterinary drugs.
Liqiang Liu earned his Ph.D. in Food science in 2014 from Jiangnan University, Wuxi, China and then became a faculty in college of Food science and technology of Jiangnan University. His research interests include immunochromatographic strip design and application.
Shanshan Song received her Master’s degree in Food science in 2012 from Jiangnan University, Wuxi, China and then became a research assistant in college of Food science and technology of Jiangnan University. Her research interest includes monoclonal antibody development.
Hua Kuang obtained her Ph.D. from China Agricultural University in 2009 and then began to work as a faculty in college of Food science and technology of Jiangnan University. She is currently a full professor in nanoscience and food safety. Her research interests are biosensor development.
Chuanlai Xu is a full professor of Food science and technology of Jiangnan University. He earned his Ph.D. in 2002. His research interests are fast detection technology and food safety evaluation.
Additional information
Funding
References
- Amelin, V., Korolev, D., & Tret'yakov, A. (2015). QuEChERS sample preparation in the simultaneous determination of diethylstilbestrol and ractopamine in food by gas-liquid chromatography. Journal of Analytical Chemistry, 70(4), 419–423. doi: 10.1134/S1061934815040024
- Anurukvorakun, O., Buchberger, W., Himmelsbach, M., Klampel, C. W., & Suntornsuk, L. (2010). A sensitive non-aqueous capillary electrophoresis-mass spectrometric method for multiresidue analyses of β-agonists in pork. Biomedical Chromatography, 24(6), 588–599.
- Cao, B., He, G., Yang, H., Chang, H., Li, S., & Deng, A. (2013). Development of a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) for the detection of phenylethanolamine A in tissue and feed samples and confirmed by liquid chromatography tandem mass spectrometry (LC–MS/MS). Talanta, 115(17), 624–630. doi: 10.1016/j.talanta.2013.06.026
- Deng, X., Liu, L., Ma, W., Xu, C., Wang, L., & Kuang, H. (2012). Development and validation of a sandwich ELISA for quantification of peanut agglutinin (PNA) in foods. Food & Agricultural Immunology, 23(3), 265–272. doi: 10.1080/09540105.2011.617358
- Dong, J. X. (2012). Development of a single-chain variable fragment-alkaline phosphatase fusion protein and a sensitive direct competitive chemiluminescent enzyme immunoassay for detection of ractopamine in pork. Analytica chimica Acta, 736(14), 85–91. doi: 10.1016/j.aca.2012.05.033
- Dong, Y., Xi, X., Xia, W., Ding, S., Li, X., Zhang, S., … Feng, Z. (2011). Validation of an ultra-performance liquid chromatography-tandem mass spectrometry method for determination of ractopamine: Application to residue depletion study in swine. Food Chemistry, 127(1), 327–332. doi: 10.1016/j.foodchem.2010.12.138
- Fang, G., Lu, J., Pan, M., Li, W., Ren, L., & Wang, S. (2011). Substitution of antibody with molecularly imprinted film in enzyme-linked immunosorbent assay for determination of trace ractopamine in urine and pork Samples. Food Analytical Methods, 4(4), 590–597. doi: 10.1007/s12161-011-9206-4
- Gao, H., Jing, H., Yang, S., Wang, Z., Lin, W., & Fu, Z. (2014). Highly sensitive multianalyte immunochromatographic test strip for rapid chemiluminescent detection of ractopamine and salbutamol. Analytica chimica acta, 839, 91–96. doi: 10.1016/j.aca.2014.05.024
- Guan, D., Guo, L., Liu, L., & Kong, N. (2015). Development of an ELISA for nitrazepam based on a monoclonal antibody. Food & Agricultural Immunology, 26, 611–621. doi: 10.1080/09540105.2014.998637
- Guo, J., Liu, L., Xue, F., Xing, C., Song, S., Kuang, H., & Xu, C. (2015). Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food & Agricultural Immunology, 26, 282–292. doi: 10.1080/09540105.2014.907242
- Guo, L., Song, S., Liu, L., Peng, J., Kuang, H., & Xu, C. (2015). Comparsion of an immunochromatographic strip with ELISA for simultaneous detection of thiamphenicol, florfenicol and chloramphenicol in food samples. Biomedical Chromatography, 29, 1432–1439. doi: 10.1002/bmc.3442
- Hu, Y., Liu, R., Li, Y., & Li, G. (2010). Investigation of ractopamine-imprinted polymer for dispersive solid-phase extraction of trace β-agonists in pig tissues. Journal of Separation Science, 33(13), 2017–2025. doi: 10.1002/jssc.201000063
- Jiang, X. F., Zhu, Y. H., & Liu, X. Y. (2014). Identification of ractopamine glucuronides and determination of bioactive ractopamine residues and its metabolites in food animal urine by ELISA, LC-MS/MS and GC-MS. Food Additives and Contaminants – Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 31(1), 29–38.
- Jing, Z., Xintian, S., Jingli, Y., Donghui, L., & Zhenhua, C. (2014). Preparation of ractopamine-tetraphenylborate complexed nanoparticles used as sensors to rapidly determine ractopamine residues in pork. Nanoscale Research Letters, 9(1), 639. doi: 10.1186/1556-276X-9-588
- Jing-Kai, H., Teh-Ia, H., Lie-Chwen, L., & Tung-Hu, T. (2014). Pharmacokinetics of ractopamine and its organ distribution in rats. Journal of Agricultural & Food Chemistry, 62(38), 9273–9278. doi: 10.1021/jf5026168
- Li, X., Zhang, G., Deng, R., Yang, Y., Liu, Q., Xiao, Z., … Cai, S. (2010). Development of rapid immunoassays for the detection of ractopamine in swine urine. Food Additives & Contaminants Part A Chemistry Analysis Control Exposure & Risk Assessment, 27(8), 1096–1103.
- Li, Y., Ji, B., Chen, W., Liu, L., Xu, C., Peng, C., & Wang, L. (2008). Production of new class-specific polyclonal antibody for determination of fluoroquinolones antibiotics by indirect competitive ELISA. Food & Agricultural Immunology, 19(4), 251–264. doi: 10.1080/09540100802471538
- Liu, G., Chen, H., Peng, H., Song, S., Gao, J., Lu, J., … Zou, Z. (2011). A carbon nanotube-based high-sensitivity electrochemical immunosensor for rapid and portable detection of clenbuterol. Biosensors & Bioelectronics, 28(1), 308–313. doi: 10.1016/j.bios.2011.07.037
- Liu, L., Kuang, H., Peng, C., Wang, L., & Xu, C. (2013). Fragment-based hapten design and screening of a highly sensitive and specific monoclonal antibody for ractopamine. Analytical Methods, 6(1), 229–234. doi: 10.1039/C3AY41827H
- Liu, L., Yan, H., & Zhang, X. (2014). Development of an anti-chlorothalonil monoclonal antibody based on a novel designed hapten. Food & Agricultural Immunology, 26, 410–419. doi: 10.1080/09540105.2014.938319
- Liu, M., Ning, B., Qu, L., Peng, Y., Dong, J., Gao, N., … Gao, Z. (2012). Development of indirect competitive immunoassay for highly sensitive determination of ractopamine in pork liver samples based on surface plasmon resonance sensor. Sensors & Actuators B Chemical, 161(1), 124–130. doi: 10.1016/j.snb.2011.09.078
- Liu, S., Lin, Q., Zhang, X., He, X., Xing, X., Lian, W., & Huang, J. (2011). Electrochemical immunosensor for salbutamol detection based on CS-Fe 3O 4-PAMAM-GNPs nanocomposites and HRP-MWCNTs-Ab bioconjugates for signal amplification. Sensors & Actuators B Chemical, 156(1), 71–78. doi: 10.1016/j.snb.2011.03.074
- Mei, L. R., Xue, L. C., Chao, H. L., Bo, X. U., Wen, J. L., Heng Yi, X. U., & Yong, H. X. (2014). Lateral flow immunoassay for quantitative detection of ractopamine in swine urine. Biomedical & Environmental Sciences, 27(2), 134–137.
- Radu, D., Aciu, F., Constantin, C., Leauta, I., Dermengiu, D., Hostiuc, S., … Curca, G. C. (2012). Study regarding drugs in blood with ELISA and chemiluminiscence versus ELISA with spectrophotometric detection. Romanian Journal of Legal Medicine, 20, 61–64. doi: 10.4323/rjlm.2012.61
- Rajkumar, M., Li, Y. S., & Chen, S. M. (2013). Electrochemical detection of toxic ractopamine and salbutamol in pig meat and human urine samples by using poly taurine/zirconia nanoparticles modified electrodes. Colloids & Surfaces B Biointerfaces, 110c(10), 242–247. doi: 10.1016/j.colsurfb.2013.03.038
- Sairi, M., & Arrigan, D. W. M. (2015). Electrochemical detection of ractopamine at arrays of micro-liquid | liquid interfaces. Talanta, 132(132), 205–214. doi: 10.1016/j.talanta.2014.08.060
- Sun, F., Liu, L., Ma, W., Xu, C., Wang, L., & Kuang, H. (2012). Rapid on-site determination of melamine in raw milk by an immunochromatographic strip. International Journal of Food Science & Technology, 47(7), 1505–1510. doi: 10.1111/j.1365-2621.2012.02998.x
- Suryoprabowo, S., Liu, L., Peng, J., Kuang, H., & Xu, C. (2014). Antibody for the development of specific immunoassays to detect nadifloxacin in chicken muscles. Food & Agricultural Immunology, 26(3), 317–324. doi: 10.1080/09540105.2014.914469
- Wang, H., Yong, Z., He, L., Du, B., Ma, H., Dan, W., & Qin, W. (2013). A silver-palladium alloy nanoparticle-based electrochemical biosensor for simultaneous detection of ractopamine, clenbuterol and salbutamol. Biosensors & Bioelectronics, 49(10), 14–19. doi: 10.1016/j.bios.2013.04.041
- Wang, Z., Liu, M., Shi, W., Li, C., Zhang, S., & Shen, J. (2015). New haptens and antibodies for ractopamine. Food Chemistry, 183, 111–114. doi: 10.1016/j.foodchem.2015.03.043
- Wei, D., Gang, Z., Qiang, F., Min, S., Zhou, H., & Chang, C. (2014). Combined microextraction by packed sorbent and high-performance liquid chromatography-ultraviolet detection for rapid analysis of ractopamine in porcine muscle and urine samples. Food Chemistry, 145(7), 789–795.
- Xing, C. & Kuang, H. (2013). A highly sensitive enzyme-linked immunosorbent assay for copper(II) determination in drinking water. Food & Agricultural Immunology, 25(3), 432–442. doi: 10.1080/09540105.2013.821600
- Yue, N., Wu, L., Li, L., & Xu, C. (2009). Multi-residue detection of benzodiazepines by ELISA based on class selective antibodies. Food and Agricultural Immunology, 20(4), 281–293. doi: 10.1080/09540100903199475
- Zhang, L., Gong, Y., Zhang, M., Xi, X., Li, M., Chen, Z., Yu, X., & Zhou, Y. (2014). Development of a monoclonal antibody-based direct competitive enzyme-linked immunosorbent assay for a new β-adrenergic agonist phenylethanolamine A. Analytical Methods, 6, 5942–5950. doi: 10.1039/C4AY00682H