ABSTRACT
To supply reference for further antitumor mechanism studies, the antitumor effect of acylated blueberry anthocyanin (ABA) was studied on H22 murine-transplanted tumor experiment. Mice treated with ABA in combination with cyclophosphamide (CTX) had 53.74% life extension rate. CTX also has significant antineoplastic effect, which could inhibit tumor growth by 63.12%. The spleen and thymus indices decreased by CTX also ameliorated considerably. The activities of alanine aminotransferase and aspartate aminotransferase were significantly lower compared with model group. Malondialdehyde, the product of lipid peroxidation was also decreased. Meanwhile, the activities of antioxidant enzymes, such as catalase, superoxide dismutase, and glutathione peroxidase, were enhanced in a dose-dependent manner (P < .05). Electron microscopy images showed that tumor cells in the mix group had a large sheet of necrosis than the other groups. ABA had strong antitumor effect on H22, as well as synergetic and attenuated toxic effect when combined with CTX.
Introduction
Numerous kinds of drugs are used to treat liver cancer at present, but clinical potential side effects also increased prominently. Thus, attention was focused on obtaining safe active ingredients from plants and using them for the treatment of diseases such as cancer (Sun & Liu, Citation2008). Anthocyanin, a type of water-soluble natural pigment generally existing in plants, has a variety of biological functions, including antioxidation, prevention of cardiovascular disease, immunity enhancement, and anticancer properties. However, anthocyanins are easily oxidized and unstable, thus limiting its application in food processing (Awika, Rooney, & Waniska, Citation2004; Matsumoto, Nakamura, Tachibanaki, Kawamura, & Hirayama, Citation2003; Wu, Cao, & Prior, Citation2002).
Aromatic acids or fatty acids were added to modify the structure of anthocyanins, which can improve its stability though acylating. Donald, David, Elena, Marc, and Neil (Citation1998) added styrene acid and other aromatic acids in carrot cell cultures to obtain 14 new acylated anthocyanins, which can maintain their original colors under the near-neutral condition. Jungsook et al. (Citation2003) studied the stability of purple corn and purple potato anthocyanin extracts under different pH, temperature, and light conditions. The results showed that the stability of purple potato, which is rich in acylated anthocyanins, was better than purple corn.
At present, transplanted tumor experiment on animals is still the most common method used in antitumor medicine research because of its practicality compared with spontaneous and induced tumor. The inoculation success rate of transplanted tumor experiments on animals reached 100%, and it can provide chances to observe changes in the animal, as well as the growth of tumor (Cheuk et al., Citation2008; NakaZato, Ito, Ikeda, & Kizaki, Citation2005; Nguyen et al., Citation2004). The results can benefit the clinical efficacy of antitumor drugs.
H22 is an ascites hepatoma cell strain that grows as ascites if inoculated intraperitoneally or as tumor block if inoculated in physical organization. H22 can grow in Kunming mouse and other strains and is suitable for antitumor experiments (Graziani et al., Citation2005; Horvathova, Chalupa, Sebova, Tóthová, & Vachálková, Citation2005; Liu, Liu, & Chen, Citation2005; Sen, Chakraborty, & Raha, Citation2006). The current experiment applied mouse H22 hepatoma cell strain and EAC (transplanted mice ascites tumor) as transplanting tumor model. We observed the effect of acylated blueberry anthocyanin (ABA) in different concentrations on antitumor and life extension in animal models to provide basic data for further antitumor mechanism research.
Materials and methods
Materials and reagents
Blueberry was provided by Dandong organic food Co., Ltd (Liaoning, China), Lauric acid (Sinopharm Chemical Reagent, China), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimi-de, N-hydroxybenzotriazole (HOBt; Aladdin, China), dimethylformamide (DMF; Aladdin, China), trifluoroacetic acid (Aladdin, China), methyl alcohol, acetonitrile (Fisher, USA), and citric acid were of analytical grade, XAD-7 resin were all provided by Beijing chemical reagent Co., China.
Cyclophosphamide (CTX) was purchased from Hualian pharmaceutical Co., Ltd. in Shanghai province. Iscove's modified dubecco's medium (IMDM) culture medium, physiological saline, and ConA were purchased from Sigma Corporation of America. Sodium chloride solution (analytical grade) was provided by reagent Co., Ltd (Beijing, China). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST), commercial antioxidant assay kits for malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) were purchased from Nanjing Jiancheng bioengineering institute (Nanjing, China).
Clean ICR mice (50% male and 50% female), aged 6–8 weeks old and weighing 18–22 g, were purchased from laboratory animal center of the China academy of military medical sciences. Mice hepatoma H22 cell strains were provided by Beijing union medical college hospital (Beijing, China).
Chemical acylation procedure
According to our laboratory research methods, Blueberry anthocyanin was purified using XAD-7 resin column to remove proteins and polysaccharides. Purified blueberry anthocyanin (2 g) was combined with two molar equivalents of acyl donor substrates by using DMF as solvent. The samples were placed in a round bottom flask by using argon as protective gas. The reaction was continuously stirred from 0 to 48 h at 4°C. Samples (10 μL) were obtained every 12 h, diluted with methanol, and analyzed by high-performance liquid chromatography mass spectra to monitor the extent of reaction. Acyl donor substrates included one molar equivalent of lauric acid compared with cyanidin-3-glucoside, one and a half molar equivalents of 1-(3-dimethylaminopropy)-3-ethycarbodiimide, and one molar equivalent of HOBt. The hybrid reaction was performed at 20°C and proceeded for 30 min. When the reaction was completed, methanol was used to stop the reaction (Zhao et al., Citation2015)
Recovery of cell strains
A frozen tube of H22 cell strain was taken out from liquid nitrogen tank, rapidly placed into 37°C water bath case, and shaken repetitively until no solid substance was left in the tube. The mouth of the frozen tube was cleaned with 75% ethanol on super clean bench. Approximately 1000 µL of H22 cell strain fluid was drawn with a micropipette and transferred to 50 mL centrifuge tube. In addition, 10 mL of IMDM culture medium was added and centrifuged at 1000 rpm for 5 min and the supernatant was discarded. Approximately 10 mL of 0.9% physiological saline (20 times dilution) was added to the mixture and centrifuged at 1000 rpm for 5 min and the supernatant was discarded. Moreover, 2 mL of 0.9% physiological saline was added, and 0.2 mL of the final solution was rapidly injected to the abdominal cavity of each ICR mouse (Bendini et al., Citation2006; Ding et al., Citation2003; Liang, Sun, Wu, & Pan, Citation2002).
Establishment of tumor model
The condition of ascites of mice was observed for 5 days after cell strain recovery. When the ascites quality met the experimental requirement for 7–10 days, the mice were executed by cutting off heads and taking ascites from them sterilely. The ascites were placed into 50 mL centrifuge tube, mixed with physiological saline (20 times dilution), and centrifuged at 1000 rpm for 5 min. The supernatant was then discarded. The solution was once again mixed with physiological saline (20 times dilution), diluted by physiological saline to 5 × 106 live cells/mL after counting, and preserved in ice water. The right armpit of each mouse was disinfected and inoculated (0.2 mL inoculation on subcutaneous) rapidly, which prepared H22 liver ascites models. Axillary subcutaneous inoculation in mice was conducted with the same method, which prepared H22 liver cancer model. The experiment was repeated three times (Aprikian et al., Citation2003; Romain et al., Citation2005).
Animal grouping and medication
Mice were randomly divided into seven groups the next day (five male and five female mice in each group). The groups are the control group, model group (0.9% sodium chloride solution), CTX group (CTX was dissolved with 0.9% physiological saline to formulate 20 mg/kg solution), combined treatment group (20 mg/kg CTX + 20 mg/kg sample), and low, medium, and high-dose groups (5, 10, and 20 mg/kg sample, respectively). Each group was treated with 0.2 ml/10 g drugs successively for 30 days. All other groups were established tumor models except for the control group, and the live days of each group were recorded. The experiment was repeated three times.
Influence of ABA on the immune organs
The spleen and thymus were weighed after blocking the tumor in mice from different groups. The spleen and thymus indices were calculated using the following formula (Sembries, Dongowski, Mehrländer, Will, & Dietrich, Citation2004; Weng, Cai, & Liu, Citation2001):
Observation of tumor cell morphology
The tumor tissue was cut into the size of 3 × 3 × 3 mm3 and fixed in 4% neutral formaldehyde after 12–24 h. The slice (4 μm) was embedded by paraffin and HE staining, and morphological changes were observed in tumor cells.
Determination of ALT, AST, SOD, CAT, GSH-Px, and MDA level in serum
ALT, AST, SOD, CAT, GSH-Px, and MDA levels in serum were determined according to the kit method by Nanjing Institute of Biological Engineering. Its level refers to the standard curve.
Calculation of tumor inhibition rate
At the next day of last medication, the mouse was weighed and executed by cutting off head, and dissected. The tumor was taken off and weighed. The average weight of tumor in each group was calculated, and the tumor inhibition rate and mice life extension rate were calculated, respectively (Sembries et al., Citation2004; Weng et al., Citation2001).
where T is the average tumor weight of treated group and C is average tumor weight of control group.
where T is the average survival days of treated group and C is average survival days of control group.
Statistical analysis
All results were analyzed by using analysis of variance by using SPSS 21.0 statistical software. The significance of difference was tested, and the results were shown in mean ± standard deviation ( ± sd). ANOVA was employed to assess the differences among groups. Significant differences were denoted by P < .05.
Results and discussion
Antitumor effect of ABA on mice with H22 murine tumors
According to , the inhibitory rate of low-, medium-, and high-dose ABA mice with H22 murine tumors were 13.45%, 25.32%, and 34.18%, respectively. Compared with the model group, the tumor weight of medium- and high-dose mice was significantly lower (P < .05, P < .01). The inhibitory rate of the CTX group was 57.24%, whereas the inhibitory rate of ABA and CTX mix group reached 63.12%, which was significantly higher than that of the CTX group (P < .05). The high dose of ABA group has obvious inhibitory effect on H22 murine tumors growing in mice.
Table 1. Effects of ABA on tumor inhibitory rates in H22 liver cancer solid bearing mice (x¯ ± sd, n = 10).
Effects of ABA on survival period of ICR mice with H22 murine tumors
Six days after inoculation, ascites began to appear in the model group. The ascites tumor grew rapidly after 8 days, and the mice experienced shortness of breath, as well as disorderly and lackluster hair that fall of easily. Ascites in low-dose group of ABA began to appear after 8 days, and the state of life of the mice was slightly better than the model group. Ascites in the medium- and high-dose groups of ABA began to appear 1 day later than the low-dose group, with the ascites tumor growing slowly in a relatively stable condition. High-dose of ABA alone or in combination with CTX both prolonged the survival time of the mice with H22 murine tumors, with the extension rate of 20.30% and 53.74%, respectively. The survival extension rate of CTX group was 46.78% ().
Table 2. Effects of ABA on the life extension rates of H22 liver cancer ascites-bearing mice (x¯ ± sd, n = 10).
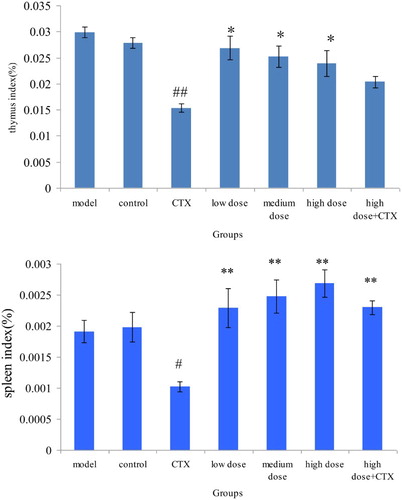
Effects of ABA on immune organs of H22 murine tumor mice
Immune function is closely linked with the occurrence and development of tumor, which inhibits our body's immune function. Application of chemotherapy drug further intensifies organic immune suppression. Therefore, improving our body's immune function is vital to prevent serious affliction from chemotherapy. The spleen and thymus indices are one of the important indices in evaluating the human body's immune status. None of the dose groups had obvious toxicity for immune organs of the mice with H22 murine tumors.
The spleen and thymus indices in CTX group were significantly lower in those of the model group (P < .05), showing that CTX had obvious toxicity. The spleen and thymus indices in the mix group were significantly higher than those of the CTX group (P < .05), indicating that ABA can effectively improve immune organ atrophy caused by CTX ().
Effects of ABA on oxidative stress levels in serum of mice
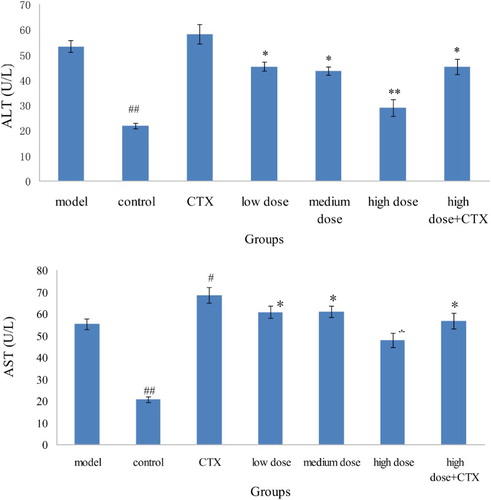
Given that AST was in the mitochondria of liver cells and ALT mainly existed outside the mitochondria, mitochondrial membrane will only be significantly damaged when the liver cell was severely damaged. Therefore, ALT primarily reflects the liver damage index, and a significantly high AST value indicates apparent liver damage. According to , after H22 murine tumor injured the liver tissue, the tumor promoted the release of enzymes in liver cells, resulting in the high ALT and AST levels of the model group compared with the control group. ALT and AST levels in the CTX group were also elevated possibly because of the side effects of CTX. Compared with the model group, ALT and AST levels in the sample group were lower, had obvious differences (P < .05), and presented a certain effect. An AST/ALT ratio greater than 1 suggests serious liver damage. Aside from the control group, the AST/ALT ratio of each group was greater than 1 (), indicating that the mice had substantial liver damage when hepatocellular carcinoma model was set up.
Figure 2. Effect of ABA on ALT and AST in serum in H22 liver tumor mice ( ± sd, n = 10). Note: acylated blueberry anthocyanin (ABA), alanine aminotransferase (ALT), aspartate aminotransferase (AST) compared with model group, #P < .05, ## P < .01; compared with CTX group, *P < .05, ** P < .01.
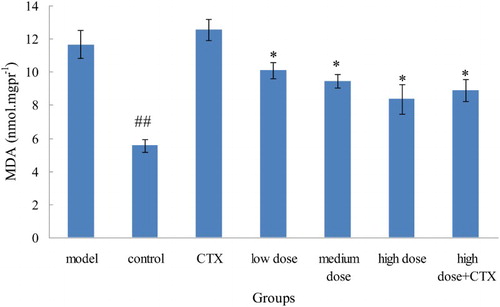
Similar to protein and nucleic acid macromolecular crosslinking polymerization, MDA is a radical lipid peroxidation product. Thus, MDA production can often reflect the degree of lipid peroxidation in the body and indirectly reflect the degree of cell damage. The MDA level of the model group was significantly higher than that of control and other groups, indicating that liver damage can cause a significant increase in MDA level. The control group and acylation anthocyanins dose groups had MDA level lower than that of model group. MDA level decreased significantly as the dose increased, indicating that acylation anthocyanins can significantly reduce MDA level in mice with liver injury ().
Effects of ABA on oxidative stress levels in liver of mice
GSH-Px, an important peroxide decomposition enzyme, widely exists in our body. GSH-Px is mainly used for catalytic GSH in peroxidation, is eliminated in the process of cellular respiration and metabolism of superoxide and hydroxyl radicals, and thus reduces the cell membrane peroxidation of polyunsaturated fatty acids.
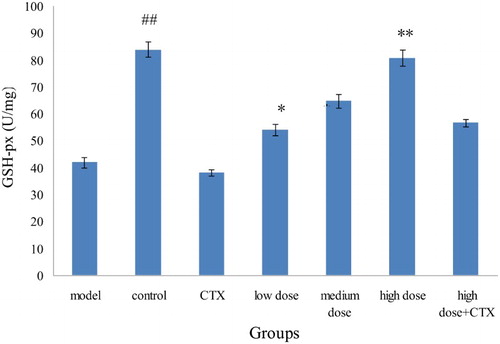
H22 murine tumor cells were injected to form acute liver cancer in mice, and GSH-Px level in model group showed significant difference compared with the control group (P < .01), indicating that a successful liver cancer model was established. The GSH-Px level of sample groups increased compared with the model group and showed significant difference (P < .05). The GSH-Px level in high-dose group maintained at a high level, presenting a certain concentration–response relationship. Compared with the model group, the GSH-Px level in the CTX group was slightly lower, indicating that CTX could reduce the body's antioxidant ability. The GSH-Px level in the mix group was higher than that of the CTX group, and high doses of acylation of anthocyanins have lower damage to cyclophosphamide on antioxidant capacity ().
Figure 4. Effect of ABA on GSH-Px in the liver tissue of H22 liver tumor mice ( ± sd, n = 10). Note: acylated blueberry anthocyanin (ABA), glutathione peroxidase (GSH-Px), compared with model group, #P < .05, ## P < .01; compared with CTX group, *P < .05, ** P < .01.
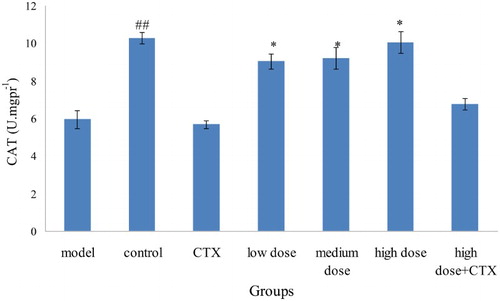
Compared with the model group, the CAT level in the CTX group was slightly lower because CTX could weaken the body's decomposition and ability to scavenge for free radicals. The CAT level in the three dose groups increased in comparison with the model group, with the CAT in high-dose group at a high level, which is only slightly lower than the control group ().
Figure 5. Effect of ABA on CAT in liver tissue in liver tumor in mice ( ± sd, n = 10). Note: acylated blueberry anthocyanin (ABA), catalase (CAT), compared with model group, #P < .05, ## P < .01; compared with CTX group, *P < .05, **P < .01.
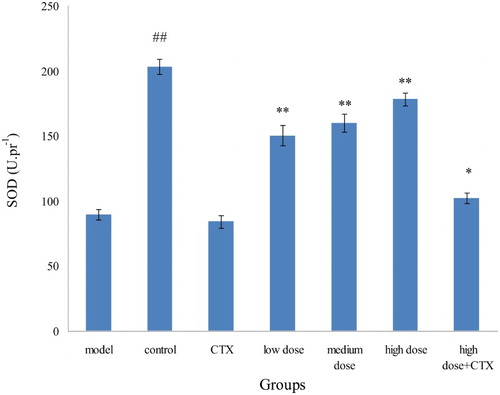
Compared with the control group, the model group was significantly reduced. H22 murine tumors can destroy the body's redox balance and reduce the content of SOD in the body. SOD vitality in the CTX group was slightly lower than that of the model group, indicating that CTX can also destroy the body's redox equilibrium as a side effect. SOD vitality in the ABA groups with three doses were higher than that of model group, presenting the certain proportion relationship and illustrating that acylation of anthocyanins can improve the body's antioxidant levels ().
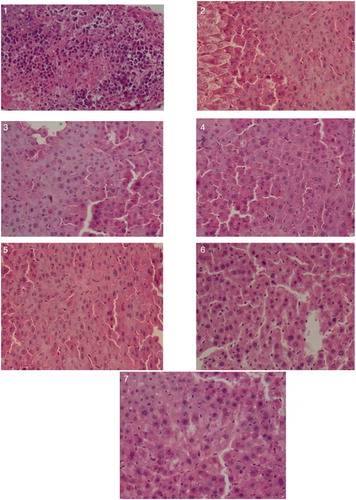
Morphological changes of ABA on mice liver tissue
The results of HE dyeing experiment showed that more nuclear hyperchromatic cells were visible in liver tissue of the model group mice, which had obviously abnormal nuclear/cytoplasmic ratios, nuclear karyolysis and pyknosis phenomenon were gradually increased. Liver cells in CTX group were increased obviously, cytoplasm was transparent, lightly stained, loose, some cells appeared obviously ballooning degeneration. Cells arranged in ABA groups were relatively uniform, the nuclear were slightly out of shape, with occasional nuclear pyknosis phenomenon, but the liver damage was in a lesser degree overall. The mixed group remained visible ballooning degeneration, indicated that CTX had larger damage on liver, but high dose ABA could partly reduce liver damage ().
Conclusions
The high-dose group of ABA in combination with CTX (anticancer drugs) can increase the enzyme activity of T-SOD, CAT, and GSH-Px of liver tissue in mice with H22 murine tumors. Moreover, the MDA, ALT, and AST levels were effectively reduced, whereas tumor inhibitory rate and life extension rate were increased. According to the tumor tissue pathological slices, the tumor cells in the mix group were a larger sheet of necrosis than in the other group. ABA can effectively reduce the side effects of anticancer drugs on the body and has recognizable protection for the liver tissue.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Wenlong Liu is a Master in food quality and safety. Presently, he is working as a researcher in P & G company of Beijing province, China. His research is about design and evaluation of functional food.
Jian Chen is a Ph.D. in microbiology. Presently, he is a teacher in Hainan University of Hainan province, China. His research is about design and evaluation of functional food.
Qiang Li is a Master in food quality and safety. His research is about design and evaluation of functional food.
Aidong Sun is a Ph.D. in Processing and storage of Agricultural products. Presently, she is working as Doctoral Supervisor, Professor at the college of food science in Beijing Forestry University of Beijing province, China. Her research is about design and evaluation of functional food.
Additional information
Funding
References
- Aprikian, O., Duclos, V., Guyot, S., Besson, C., Manach, C., Bernalier, A. … Demigné, A. (2003). Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. Journal of Nutrition, 133, 1860–1865.
- Awika, J., Rooney, L., & Waniska, R. (2004). Anthocyanins from black sorghum and their antioxidant properties. Food Chemistry, 90, 293–301. doi: 10.1016/j.foodchem.2004.03.058
- Bendini, A., Cerretani, L., Pizzolante, L., Toschi, T. G., Guzzo, F., Ceoldo, S., …, Levi, M. (2006). Phenol content related to antioxidant and antimicrobial activities of Passiflora spp. extracts. European Food Research and Technology, 223, 102–109. doi: 10.1007/s00217-005-0150-7
- Cheuk, K. L., Zhang, Z., Yu, H., Tsang, S. Y., Huang, Y., & Chen, Z. Y. (2008). Apple polyphenols inhibit plasma CETP activity and reduce the ratio of non-HDL to HDL cholesterol. Molecular Nutrition & Food Research, 52, 950–958. doi: 10.1002/mnfr.200700319
- Ding, M., Lu, Y., Bowman, L., Huang, C., Leonard, S., Wang, L., Castranova, V., & Shi, X. (2003). Inhibition of AP-1 and neoplastic transformation by fresh apple peel extract. Journal of Biological Chemistry, 279(11), 10670–10676. doi: 10.1074/jbc.M311465200
- Donald, K. D., David, C. B., Elena, G. G., Marc, A. R., & Neil, A. W. (1998). Anthocyanins from wild carrot suspension cultures acylated with supplied carboxylic acid. Carbohydate Research, 310, 177–189. doi: 10.1016/S0008-6215(98)00124-4
- Graziani, G., D'Argenio, G., Tuccillo, C., Loguercio, C., Ritieni, A., Morisco, F., … Romano, M. (2005). Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut, 54, 193–200. doi: 10.1136/gut.2004.046292
- Horvathova, K., Chalupa, I., Sebova, L., Tóthová, D., & Vachálková, A. (2005). Protective effect of quercetin and luteolin in human melanoma HMB-2 cells. Mutation Research, 565(2), 105–112. doi: 10.1016/j.mrgentox.2004.08.013
- Jungsook, C., Seong, K. J., Hoai, L. P., Jhang, J., Yiho, B., & Kyeong-Soo, C. (2003). Antioxidant and memory enhancing effects of purple sweet potato anthocyanin and cordyceps mushroom extract. Archives of Pharmacal Research, 26(10), 821–825. doi: 10.1007/BF02980027
- Liang, Y., Sun, Z. J., Wu, S. L., & Pan, C. G. (2002). Effect of resveratrol on cell cycle protein in mice transplanted hepatoma tissue. Journal of the Fourth Military Medical University, 23(23), 2172–2174.
- Liu, R. H., Liu, J., & Chen, B. (2005). Apples prevent mammary tumors in rats. Journal of Agricultural and Food Chemistry, 53, 2341–2343. doi: 10.1021/jf058010c
- Matsumoto, H., Nakamura, Y., Tachibanaki, S., Kawamura, S., & Hirayama, M. (2003). Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. Journal of Agricultural and Food Chemistry, 51, 3560–3563. doi: 10.1021/jf034132y
- NakaZato, T., Ito, K., Ikeda, Y., & Kizaki, M. (2005). Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clinical Cancer Research, 11(16), 6040–6049. doi: 10.1158/1078-0432.CCR-04-2273
- Nguyen, T. T. T., Tran, E., Nguyen, T. H., Do, P. T., Huynh, T. H., & Huynh, H. (2004). The role of activated MEKERK pathway in quercetin induced grown inhibition and apoptosis in A549 lung cancer cell. Carcinogenesis, 25(5), 647–659. doi: 10.1093/carcin/bgh052
- Romain, V., Sandra, H. V., Thomas, P., Odile, T., Monique, R., Jean, C., … Jean-Marc, L. (2005). Apple procyanidins decrease cholesteroles terification and lipoproteins secretionin Caco-2/TC7 enterocytes. Journal of Lipid Research, 46, 258–268.
- Sembries, S., Dongowski, G., Mehrländer, K., Will, F., & Dietrich, H. (2004). Dietary fiber-rich colloids from apple pomace extraction juices do not affect food intake and blood serum lipid levels, but enhance fecal excretion of steroids in rats. The Journal of Nutritional Biochemistry, 15(5), 296–302. doi: 10.1016/j.jnutbio.2003.12.005
- Sen, P., Chakraborty, P. K., & Raha, S. (2006). Tea polyphenol epigallcoatcehin 3-gallate impedes the anti-apoptotic effects of low-grade repetitive stress through inhibition of Akt and Nfkappaβ survival pathways. FEBS Letters, 580(1), 278–284. doi: 10.1016/j.febslet.2005.12.013
- Sun, J., & Liu, R. H. (2008). Apple phytochemical extracts inhibit proliferation of estrogen-dependent and estrogen-independent human breast cancer cells through cell cycle modulation. Journal of Agricultural and Food Chemistry, 56(24), 11661–11667. doi: 10.1021/jf8021223
- Weng, H. L., Cai, W. M., & Liu, R. H. (2001). Animal experiment and clinical study of effect of gamma-interferon on hepatic fibrosis. World Journal of Gastroenterology, 7(1), 42–48. doi: 10.3748/wjg.v7.i1.42
- Wu, X., Cao, G., & Prior, R. L. (2002). Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. Journal of Nutrition, 132, 1865–1871.
- Zhao, L. Y., Chen, J., Wang, Z. Q., Shen, R. M., Cui, N., & Sun, A. D. (2015). Direct acylation of cyanidin-3-glucoside with lauric acid in blueberry and its stability analysis. International Journal of Food Properties. doi:10.1080/10942912.2015.1016577