ABSTRACT
The present study evaluated the immune function of extract obtained from yellow pond turtle (YPT). Two YPT extract doses (250 mg/kg and 500 mg/kg·bw/d) were administered orally to normal and cyclophosphamide (CY)-induced immune-suppressed male Kunming mice, respectively. The immune function were evaluated by testing the body weight growth curve, thymus index and spleen index in CY-induced mice as well as mononuclear-macrophage phagocytic function by the carbonic particle clearances index and the macrophage phagocytose index. After a 10-day administration, both the low-dose group (250 mg/kg·bw/d) and high-dose group (500 mg/kg·bw/d) showed a marked increase in immune organs indices and the carbonic particle clearances index and macrophage phagocytose index. Moreover, immune function showed significant dose–effect relationship. These results demonstrated the potential use of YPT extract as a functional medicine for enhancing human immunity and immunomodulatory for adjuvant treatment in cancer.
1. Introduction
Modulation of immune response to alleviate disease has been of interest for a long time (Bani et al., Citation2005; Mehrotra et al., Citation2002). Extracts of a diverse range of plants have been shown to possess immunomodulatory properties (Nicholl et al., Citation2001), such as cordyceps (Wang et al., Citation2012) and makino (Ren et al., Citation2014), but there are few reports about animal materials. The immune system plays an important role in the pathophysiology of diseases such as cancer (Kobra et al., Citation2015). An etiological analysis reveals an intriguing fact of breast cancer: critical role of cellular immunity in breast cancer progression. During initial stages of cancer, acute inflammatory reactions can favour tumour rejection (Pawar et al., Citation2014). Some of the factors such as stress, chemotherapy and other drug therapies may lead to immune suppression (Kobra et al., Citation2015). Also, some studies have proved that a long-term low immune person is more vulnerable to suffer from cancer. So the immunomodulatory plays a further role in the treatment of cancer.
The Asian yellow pond turtle (YPT), Mauremys mutica (Cantor), is a member of the family Bataguridae (Joyce, Parham, & Gauthier, Citation2004). It is of rich nutrition with high ornamental and medicinal value. They are mainly distributed in Zhejiang, Anhui, Guangdong, Hainan, Fujian, Taiwan and Vietnam. The higher values for food and remedy than other species of turtle has made it one of the most commonly and highly traded turtles in Asia (Zhao et al., Citation2014). The report in Shen Nong's herbal stressed that turtleback can subdue inflammation. Besides, it has been proved that YPT, as a folk medicine, can enhance human immunity and treat various diseases in Shen Nong's herbal, Shan Hai Ching and Compendium of Materia Medica.
Cyclophosphamide (CY) is the most widely used alkylating agent in chemotherapy with a high therapeutic index and is one of the major immunosuppressive drugs used in clinical studies with its broad spectrum of activity against a variety of cancers since the late 1950s (Mei, Chen, Zhang, Zhang, & Liang, Citation2013; Pass et al., Citation2005). However, CY acts on the tumour cell as well as the normal cell with its non-selective cytotoxicity and immunosuppression effect. High morbidity and mortality in CY-induced leukopenia, myelosuppression and immunosuppression are the major limiting factors in clinical chemotherapy without efficacious remedies (Rood, Macdonald, & Packer, Citation2004; Rossi, Caracciolo, Russo, Reiss, & Giordano, Citation2008). Immunosuppression is a major side effect caused by the long-term CY therapy in cancer patients. With high doses of CY, body weight, the weights of spleen and thymus and NK (Natural Killer) cell activity were decreased (Kobra et al., Citation2015). To enhance the efficacy and reduce the toxicity, it has been demonstrated recently that CY can be combined with various detoxifying and protective agents in clinical adjuvant chemotherapy, such as many traditional Chinese medicinal herbs, especially Cassia occidentalis (Bin, Ahmad, Haque, & Raisuddin, Citation2001), Phyllanthus amarus (Kumar & Kuttan, Citation2005), Andrographis paniculata (Sheeja & Kuttan, Citation2006) and Ganoderma atrum (Yu et al., Citation2014). The research in traditional folk medicine is of great value for CY's low toxicity and curative effects.
This study investigated the immunomodulatory effects of YPT extract on the enhancement of the innate immune response by employing a number of immune response assays for the first time. By establishing an immune suppression animal model, the immune function evaluations of YPT extract were carried out in two different parts: (1) to evaluate the immune organs indices in normal and CY-induced immune-suppressed mice, (2) to evaluate the carbonic particle clearances and mononuclear-macrophage phagocytic function through ink experiment in CY-induced mice.
2. Materials and methods
2.1. Reagents
CY was purchased from Jiangsu Engrain Co., Ltd. NaCl was bought from Sinopharm Chemical Reagent Co., Ltd. Yidege ink was purchased from Beijing Yidege Ink Industry Co., Ltd. And the ink was diluted fourfold with sterile physiological saline when used. The solution of Na2CO3 was purchased from Sinopharm Chemical Reagent Co., Ltd. The heparin sodium was bought from Shanghai Kehua Bio-engineering Co., Ltd. All other reagents were of analytical grade.
2.2. The preparation of YPT extract
The seven-year Asian YPTs were donated by the Tortoises and Turtles Association of Dongguan City, Guangdong Province, China in June 2012. The Asian YPTs were authenticated by Yangyan Jiang, vice-chairman of the Tortoises and Turtles Association of Dongguan City. A voucher specimen (No.120612) has been deposited in College of Food Science and Technology, Huazhong Agricultural University.
The YPT was killed in hot water, and its turtle shell and flesh were collected. After the bloodstains were washed away, the flesh was cut up into small pieces and frozen in the refrigerator, while the shell was cut up into pieces and immersed in 5% acetic acid. Then the solvent was replaced with fresh acetic acid in a day. And the shell would be soaked for another day.
The extract of turtle meat: The frozen turtle flesh was thawed naturally at room temperature, then boiled with water in the cooking pot for 2 h to obtain the white thick soup. Two hours later, the hot soup was filtrated into a 500 ml round-bottom flask for spin steaming with the rotary evaporator at the temperature of 55°C (RE-52AA, Shanghai Yarong Biochemistry Instrument Factory). When there was basically no more condensed fluid coming out, the condensed fluid was collected into a 250 ml of plastic cup sealed by a plastic wrap, and then stored at 4°C. After being added with distilled water, the residues were boiled for another 2 h to be thickened and then filtered again. The spin steaming would be repeated once again to get the second condensed fluid. For the last time, the remaining residues would be boiled for 1.5 h to get the third condensed fluid. The collected condensed fluid from the above process would undergo the process of spin steaming enrichment. The total extracts were obtained and preserved in plastic beaker to be frozen to get bulk gel. Then the bulk gel was weighed by an electronic balance (BSA124S, Sartorius).
The extract of turtle shell: The broken tortoise shells were cleaned and placed in a 1000 ml round-bottom flask. Then the shells were boiled with 500 ml 50% ethanol for 2.5 h. The hot liquid was filtrated into a 500 ml round-bottom flask for spin steaming for 2 h to get the condensed fluid. After being added with 500 ml 80% ethanol, the residues were boiled for 2 h. The hot liquid would undergo the process of spin steaming once again to get the second condensed fluid. The collected condensed fluid from the above process would undergo the process of spin steaming enrichment. The total extracts were obtained and preserved in a plastic beaker to be frozen to get bulk gel. Then the bulk gel was weighed by an electronic balance (BSA124S, Sartorius).
The freeze-dry of samples: The frozen samples of the extracts were packaged into small plastic beakers. The thickness of the frozen samples was controlled at about 3 cm. The plastic beakers were sealed with plastic wraps and fastened with elastics. A small spear was used to punch several holes on the plastic wraps. Then the plastic beakers were put into the Freeze Drying Equipment to be freeze-dried for 48 h. Finally, they were transferred into the fridge for cryopreservation.
In this study, the flesh of YPT was extracted with water, and the shell of YPT was extracted with 80% ethanol aqueous in reflux conditions. Two kinds of solutions were concentrated under reduced pressure and lyophilized in vacuum, respectively. The YPT extract was the mixture of the turtle flesh extract and turtle shell extract, with the ratio of 2:1.
2.3. Main compositions of the YPT extract
Moisture content was determined by a moisture analyser (MJ33, Switzerland, Mettler Toledo). The cholesterol content was determined by the direct saponification and colorimetric method (Kovacs, Anderson, & Ackman, Citation1979), the extractive conditions were as follows: extraction solvent was 50% potassium hydrate and absolute alcohol, ratio of YPT extract, 50% potassium hydrate and absolute alcohol was 2:5:45(W:V:V), extracted 30 min at 80°C and volatilized temperature was 65°C (He, Citation2011). Then the amino acids content was determined by ninhydrin reaction, to present colour with ninhydrin and measure the absorbance at 570 nm. The crude protein content was determined by the Kjeldahl method (Chen, Zhang, & Sundar, Citation2007), using K9860 automatic Kjeldahl analyzer (China, Jinan Hanon Instruments Co., Ltd). The Kjeldahl method consists of three main steps: sample digestion, distillation and ammonia determination, and a factor of 6.25 was used to calculate crude protein from the total N (Noblet, Shi, & Dubois, Citation1993). Then, the crude oil content was determined by the method of Soxhlet extraction using petroleum ether and a Soxhlet extractor (China, Jinan Dongyi Instruments Co., Ltd) (Hoonsoo et al., Citation2013).
2.4. Animals and drug administration
Kunming mice (KM, males, SPF, 20 ± 2 g) used in this study were procured from the Animal Center for Disease Prevention and Control in Hubei province (Certificate No. SCXK 2008-0005, Wuhan, China). All mice were housed at a constant temperature of 24 ± 2°C and constant humidity of 50–70% under a 12 h light-dark cycle, with free access to food and water. All animal studies were performed strictly according to the international rules considering animal experiments and the internationally accepted ethical principles for laboratory animal use and care.
Low-dose YPT extract (250 mg/kg body weight): The ratio of turtle shell and turtle flesh was 1:2, that is, 0.25 g turtle shell and 0.5 g turtle flesh were mixed together and diluted with sterile physiological saline to 30 ml to get the reagent.
High-dose YPT extract (500 mg/kg body weight): The ratio of turtle shell and turtle flesh was 1:2, that is, 0.5 g turtle shell and 1.0 g turtle meat were mixed together and diluted with sterile physiological saline to 30 ml to get the reagent.
2.5. The effect of YPT extract on normal mice without CY inducement
The normal mice were assigned into 3 groups (10 mice per group) at random. Group 1 (normal group) was given 0.1 ml/10 g·bw/d of sterile physiological saline (vehicle). Groups 2 and 3 were administered YPT extract at a dose of 250 mg/kg·bw/d (low-dose group) and 500 mg/kg·bw/d (high-dose group), respectively. All the groups were administered the common diets orally with the same kind of cannula throughout the study. The mice were intragastrically administered saline and YPT extract, respectively, once daily for 10 days. The body weight was measured every day.
After an overnight fast, the animals were weighed and then killed by decapitation. Spleen and thymus were exercised from the animal and weighed immediately. The thymus and spleen indices were calculated according to the following formula:
2.6. The effect of YPT extract on CY-induced mice
All the mice used for the experiment were randomly divided into 4 groups. Each group consisted of 10 normal mice. Group I (normal group) was given 0.1 ml/10 g·bw/d of sterile physiological saline (vehicle). Group II (model group) was given 0.1 ml/10 g·bw/d of sterile physiological saline (vehicle). Groups III and IV were administered YPT extract at a dose of 250 mg/kg·bw/d (low-dose group) and 500 mg/kg·bw/d (high-dose group), respectively. The mice of Groups II–IV were injected with CY (i.p. 80 mg/kg) on days 4–6 to establish the immune suppression model, while the mice of Group I were injected with the same volume of sterile physiological saline. All the groups were administered the common diets orally with the same kind of cannula throughout the study. The mice were intragastrically administered once daily for 10 days. The body weight was measured every day.
After an overnight fast, the animals were weighed and then killed by decapitation. Spleen and thymus were exercised from the animal and weighed immediately. The thymus and spleen indices were calculated according to the following formula:
2.7. The effect of YPT extract on mononuclear-macrophage phagocytic function in hypo-immunologic model mice
In the mononuclear-macrophage phagocytic function study, KM were divided into 4 groups (10 mice for each group). The normal group was given 0.1 ml/10 g·bw/d of sterile physiological saline (vehicle). The model group was given 0.1 ml/10 g·bw/d of sterile physiological saline (vehicle). Low-dose group and high-dose group were administered YPT extract at a dose of 250 mg/kg·bw/d and 500 mg/kg·bw/d, respectively. The CY-induced hypo-immunologic model was established in accordance with the above method (2.5). All the groups were administered the common diets orally with the same kind of cannula throughout the study. The mice were intragastrically administered once daily for 10 days. The body weight was measured every day.
One hour after the last drug administration, the animals were weighed. The ink diluents was injected from the tail vein and timed immediately. After the ink injection, at the time of 1 min (t1) and 6 min (t2), heparin sodium was used to get 25 μl blood from orbital venous plexus and the blood was immediately added with 3 ml 0.1% Na2CO3 solution and the absorbance of the mixture was measured at 675 nm. The absorbances were A1 (at the time of t1) and A2 (at the time of t2), respectively, using the absorbance of Na2CO3 solution as a blank control. Then mice were killed by decapitation. Liver and spleen were exercised from the animal and were weighed immediately. The carbonic particle clearances index (K) and macrophage phagocytose index (α) were measured to evaluate the phagocytic function of mononuclear-macrophage.
2.8. Statistical analysis
The data were expressed as the mean ± standard deviation and analysed using SPSS version 17.0 for windows. Significant differences among groups were evaluated using a one-way analysis of variance (one-way ANOVA). A level of P < .05 was considered as statistically significant.
3. Results
3.1. The main compositions of the YPT extract
The extract of YPT is rich in high-quality protein. As shown in , the major contents in the extract of YPT are protein and fat. The protein was 55.96–57.98 g/100 g, and the content of fat was 24.42–27.32 g/100 g. While the moisture was 0.629%, cholesterol and amino acid of the YPT extract averaged 312.91 and 53.60 mg/100 g, respectively.
Table 1. The main compositions of YPT extract ( ) (n = 3).
) (n = 3).
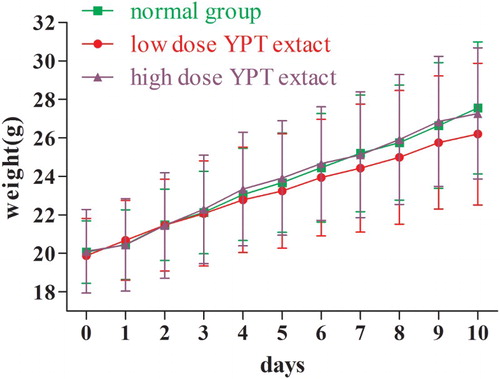
3.2. Effect of YPT extract on body weight of normal mice without CY inducement
The body weights of normal mice in Groups 1–3 are shown in . In the normal mice experiment, the body weight of all mice increased, no deaths occurred during the experimental period and there were no significant difference between different groups, indicating that the YPT extract had not much influence on the normal mice. After 10 days of administration, low- or high- dose YPT extract showed no significant effect on the body weight of normal mice (P > .05).
3.3. Effect of YPT extract on immune organs indices of normal mice without CY inducement
As shown in , there was no difference in the thymus index and spleen index of normal group and YPT extract group. It indicated that the YPT extract had no effect on the growth of immune organs in the normal mice without CY inducement.
Table 2. The influence of YPT extract on immune organs indices in normal mice without CY inducement ( ) (n = 10).
) (n = 10).
3.4. Effect of YPT extract on body weight of CY treatment mice
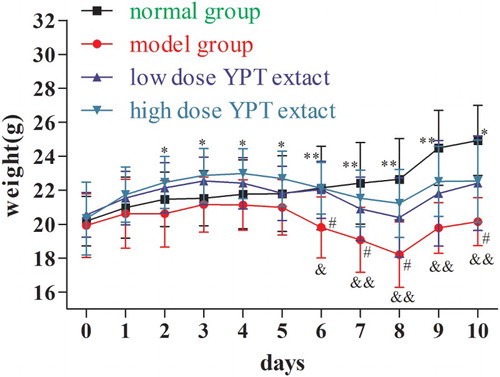
As shown in , the body weight of all mice increased before the intraperitoneal injection of CY. The weight of Groups II–IV decreased from the fourth day to the eighth day. On the eighth day, the mice weight of model group decreased most significantly (P < .01, compared to the normal group). Low-dose YPT extract produced an increase in the weight compared to the model group (P < .05). High-dose YPT extract produced a significant increase in the weight compared to the model group (P < .01). The results suggested that the YPT extract could help recover the suppressed effect on weight of CY-induced hypo-immunologic model mice.
Figure 2. The effect of YPT extract on body weight of CY treatment mice. Note: &, P < .05 as model group vs. normal group; &&, P < .01 as model group vs. normal group; #, P < .05 as low-dose YPT extract group vs. model group; *, P < .05 as high-dose YPT extract vs. model group; **, P < .01 as high-dose YPT extract vs. model group.
3.5. Effect of YPT extract on immune organs indices of CY-induced mice
As shown in , the thymus index and spleen index of model group were markedly reduced compared to the normal group (P < .01), indicating that the modelling was successful. Compared to the model group, there was a significant difference in the spleen index in the group of treatment with low- and high-dose YPT extract (P < .01), suggesting that the spleen shrinking induced by CY could be recovered to normal size. In addition, the thymus index significantly increased in the group of low-dose YPT extract, compared to the model group (P < .05), while the group of the high-dose YPT extract showed particularly significant difference, compared to the model group (P < .01), suggesting that the YPT extract of low-and high-dose could also recover the thymus damage.
Table 3. The influence of YPT extract on immune organs indices in CY-induced mice ( ) (n = 10).
) (n = 10).
3.6. Effect of YPT extract on mononuclear-macrophage phagocytic function
As shown in , compared to the model group, 250 mg/kg YPT extract can increase the carbonic particle clearances index (K) and macrophage phagocytose index (α) (P < .05), while 500 mg/kg YPT extract can markedly increase the carbonic particle clearances index (K) and macrophage phagocytose index (α) (P < .01). This result suggested that the YPT extract can protect the CY-induced immune-suppressed mice and improve the phagocytosis of mononuclear-macrophage in immunocompromised mice.
Table 4. The influence of YPT extract on monocyte-macrophages in CY-induced mice ( ) (n = 10).
) (n = 10).
4. Discussion
Long-term CY chemotherapy usually leads to immunosuppressive and cytotoxic effects, particularly of humoral immunity, regardless of their great curative effects (Bin, Ahmad, Haque, & Raisuddin, Citation2001; Yu et al., Citation2014). CY is known to cause leucopenia for its cytotoxic not only to cancer cells but also to leukocytes. Despite the fact that CY is the main cancer chemotherapy agent, its immunocompromised activity and the side effects such as neutropenia, anorexia and proteinemia are major limiting factors for sustained clinical application (Rafiul Haque, Ansari, & Rashikh, Citation2013; Wang et al., Citation2012; Zhu, Chen, & Lin, Citation2007). Therefore, it is necessary to seek for an agent with low toxicity and protective effect of normal tissues in chemotherapy (Wang et al., Citation2012).
Macrophages are an important part of the innate immune system and play an important role in the defense mechanism against host infection and the killing of tumour cells (Leonora & Elena, Citation2011). There is a close correlation between the modulation of antitumour properties of macrophages by various biological response modifiers and immunomodulating activity (Manosroi, Saraphanchotiwitthaya, & Manosroi, Citation2003). Macrophages are also involved in the initiation of adaptive immune responses. To fulfill these functions, macrophages produce and release cytokines and superoxide anions, such as TNF-γ and ILs, to kill the tumour cell. In this study, the increase in the carbonic particle clearances index (K) and macrophage phagocytose index (α) (P < .01) indicated that the YPT extract can improve the phagocytosis of mononuclear-macrophage in immunocompromised mice, suggesting that the YPT extract could enhance non-specific immune function.
Thymus is the organ in which T lymphocytes develop, differentiate and mature. The immune organs of thymus and spleen play an important role in body's immune function. The weight of the thymus and spleen could be enhanced by the immune system activators. In this study, the effects of YPT extract on the thymus and spleen indices were measured. The increase in the spleen index of mice treated with YPT extract indicated that the extract was able to act against the immunosuppression induced by CY in vivo. In this study, a significant increase was observed in the thymus and spleen indices. These data were consistent with that of previously published studies (Cui, Yuan, & Zhang, Citation2010; Zong et al., Citation2015).
Over these years, different kinds of side effects in many Western medicines have been reported, and people have paid more attention to the traditional folk medicine exacted from natural plants and animals, resulting in significant developments in functional foods, pharmaceuticals and nutraceuticals. There is a growing interest among producers and the public in those areas, which may provide health benefits beyond basic nutrition. This has resulted in greater interest for new functional ingredients, which can contribute to develop new opportunities in the relevant applications (Lee & Jeon, Citation2013). The development of natural health products that could potentially modulate the immune system provides an alternative source of bioactive agents with medical significance (Leonora & Elena, Citation2011).
Judging from the design of the experiment, this study is of important practical significance. When the mice were treated with CY, the thymus and spleen indices remarkably reduced. But with the YPT extract administration at different doses to the CY-induced mice, a significant increase was observed in the thymus and spleen indices. And the in vivo treatments with high-dose YPT extract (500 mg/kg body weight) ameliorated the immunosuppression in CY-treated mice through enhancement of phagocytic activity.
This study assessed the immunomodulatory potential of YPT that it can elevate immune indices of normal mice and immune-suppressed mice. Results revealed the immunopotentiating activities of YPT extract on the innate immunity of KM in vivo. The protective effects of YPT extract to the immune system were confirmed in this study. The results of this study on YPT are very encouraging, which could be used as standard when studies on the different mechanisms of its immunomodulatory properties are undertaken. This research has laid a good foundation for the future studies on medicinal mechanisms.
5. Conclusion
In conclusion, as our study demonstrated, the nutrients of YPT extract were as follows: crude protein (56.97 ± 1.01 g/100 g), crude fat (25.87 ± 1.45 g/100 g), amino acid (53.60 ± 2.39 mg/100 g), cholesterol (312.91 ± 5.79 mg/100 g) and moisture (0.629 ± 0.043 g/100 g). For the first time, the YPT extract can result in accelerating recovery of immunosuppression in CY-treated mice in this study. However, the questions about the specific immunological mechanism, the main effective components and the interaction with other medicines are not clear and require more studies. There is a huge potential for applying YPT as an efficacious adjuvant therapy for immunomodulation. Our findings may provide experimental evidences for further research and clinical application of the Asian YPT.
Acknowledgement
The authors would like to thank the Tortoises and Turtles Association of Dongguan City (Guangdong Province, China) for providing the seven-year-old Asian yellow pond turtles.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Jiao-jiao Yin obtained her bachelor's degree from HuaZhong Agricultural University, Wuhan, China, in 2015 and her master's degree in hydrobiology in Fisheries College from HuaZhong Agricultural University (Wuhan, China). Her research includes the quality and safety of aquatic products.
Qing Zhou obtained her bachelor's degree from Tongji School of Pharmacy, Huazhong University of Science and Technology, Wuhan, China, in 2004 and her master's degree in medicinal chemistry in the same school in 2008. Her research interest includes the natural products which could enhance human immunity.
Lin Wang obtained his bachelor's degree from HuaZhong Agricultural University, Wuhan, China, in 2015 and his master's degree in food science in the same school. His research includes chemicals in natural product.
Wei Xu obtained her bachelor's degree from HuaZhong Agricultural University, Wuhan, China, in 2015 and her master's degree in food science and engineering from Shanghai Jiao Tong University (Shanghai, China). Her research includes the relationship between natural products and immunity.
Jiu-liang Zhang obtained his bachelor's degree from Tongji School of Pharmacy, Huazhong University of Science and Technology, Wuhan, China, in 2004 and his doctor's degree in pharmacology in the same school in 2009. He is interested in natural product research and development.
Additional information
Funding
References
- Bani, S., Kaul, A., Khan, B., Ahmad, S. F., Suri, K. A., Satti, N. K., … Qazi, G. N. (2005). Immunosuppressive properties of an ethyl acetate fraction from Euphorbia royleana. Journal of Ethnopharmacology, 99, 185–192. doi: 10.1016/j.jep.2004.12.017
- Bin, H. B., Ahmad, I., Haque, R., & Raisuddin, S. (2001). Protective effect of Cassia occidentalis L. on cyclophosphamide-induced suppression of humoral immunity in mice. Journal of Ethnopharmacology, 75, 13–18. doi:10.1016/S0378-8741(00)00382-2
- Chen, D. W., Zhang, M., & Sundar, S. (2007). Compositional characteristics and nutritional quality of Chinese mitten crab (Eriocheir sinensis). Food Chemistry, 103(4), 1343–1349. doi:10.1016/j.foodchem.2006.10.047
- Cui, J. J., Yuan, J. F., & Zhang, Z. Q. (2010). Anti-oxidation activity of the crude polysaccharides isolated from Polygonum cillinerve (Nakai) Ohwi in immunosuppressed mice. Journal of Ethnopharmacology, 132, 512–517. doi:10.1016/j.jep.2010.08.052
- He, C. M. (2011). Analysis of cholesterol in egg yolk by direct saponification-colorimetric method. Guangdong Agricultural Sciences, 38(9), 110–113. Retrieved from http://lib.cqvip.com/read/detail.aspx?ID=38486167
- Hoonsoo, L., Byoung, K. C., Moon, S. K., Wang, H. L., Jagdish, T., Hanhong, B., … Hee, Y. C. (2013). Prediction of crude protein and oil content of soybeans using Raman spectroscopy. Sensors and Actuators B: Chemical, 185, 694–700. doi: 10.1016/j.snb.2013.04.103
- Joyce, W. G., Parham, J. F., & Gauthier, J. (2004). Developing a protocol for the conversion of rank-based taxon names to phylogenetically defined clade names, as exemplified by turtles. Journal of Paleontology, 78, 989–1013. doi:10.1666/0022-3360(2004)078<0989:DAPFTC>2.0.CO;2
- Kobra S., Faezeh, V. H., Kamal, R. A., Somayeh, H., Bamdad R. Z., & Gholamreza, K. (2015). Phytotrapy of cyclophosphamide-induced immunosuppression. Environmental Toxicology and Pharmacology, 39, 1262–1275. doi:10.1016/j.etap.2015.04.012
- Kovacs, M. I. P., Anderson, W. E., & Ackman, R. G. (1979). A simple method for the determination of cholesterol and some plant sterols in fishery-based food products. Journal of Food Science, 44, 1299–1305. doi:10.1111/j.1365-2621.1979.tb06423.x
- Kumar, K. B. H., & Kuttan, R. (2005). Chemoprotective activity of an extract of Phyllanthus amarus against cyclophosphamide induced toxicity in mice. Phytomedicine, 12, 494–500. doi:10.1016/j.phymed.2004.03.009
- Lee, S. H., & Jeon Y. J. (2013). Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia, 86, 129–136. doi:10.1016/j.fitote.2013.02.013
- Leonora, P. N., & Elena, S. C. (2011). Immunostimulatory effects of Uncaria perrottetii (A. Rich.) Merr. (Rubiaceae) vinebark aqueous extract in Balb/C mice. Journal of Ethnopharmacology, 133, 613–620. doi:10.1016/j.jep.2010.10.044
- Manosroi, A., Saraphanchotiwitthaya, A., & Manosroi, J. (2003). Immunomodulatory activities of Clausenia excavata Burm. F. wood extracts. Journal of Ethnopharmacology, 89, 155–160. doi:10.1016/S0378-8741(03)00278-2
- Mehrotra, S., Mishra, K.P., Maurya, R., Srimal, R.C., & Singh, V.K. (2002). Immunomodulation by ethanolic extract of Boerhaavia diffusa roots. Journal of Ethnopharmacology, 2, 987–996. doi:10.1016/S1567-5769(02)00031-0
- Mei, Y. X., Chen, H. X., Zhang, J., Zhang, X. D., & Liang, Y. X. (2013). Protective effect of chitooligosaccharides against cyclophosphamide-induced immunosuppression in mice. International Journal of Biological Macromolecules, 62, 330–335. doi: 10.1016/j.ijbiomac.2013.09.038
- Nicholl, D. S., Daniels, H. M., Thabrew, M., Grayer, R. J., Simmonds, M. S., & Hughes, R. D. (2001). In vitro studies on the immunomodulatory effects of extracts of Osbeckia aspera. Journal of Ethnopharmacology, 78, 39–44. doi:10.1016/S0378-8741(01)00319-1
- Noblet, B. Y. J., Shi, X. S., & Dubois, S. (1993). Metabolic utilization of dietary energy and nutrients for maintenance energy requirements in sows: Basis for a net energy system. Brirish Journal of Nutrition, 70, 407–419. doi:10.1079/BJN19930135
- Pass, G. J., Carrie, D., Boylan, N., Lorimore, S., Wright, E., Houston, B., … Wolf, C. R. (2005). Role of hepatic cytochrome P450s in the pharmacokinetics and toxicity of cyclophosphamide: Studies with the hepatic cytochrome P450 reductase null mouse. Cancer Research, 65, 4211–4217. doi: 10.1158/0008-5472.CAN-04-4103
- Pawar, V. K., Panchal, S. B., Singh, Y., Meher, J. G., Sharma, K., Singh, P., … Chourasia, M. K. (2014). Immunotherapeutic vitamin E nanoemulsion synergies the antiproliferative activity of paclitaxel in breast cancer cells via modulating Th1 and Th2 immune response. Journal of Controlled Release, 196, 295–306. doi: 10.1016/j.jconrel.2014.10.010
- Rafiul Haque, M., Ansari, S. H., & Rashikh, A. (2013). Coffea arabica seed extract stimulate the cellular immune function and cyclophosphamide-induced immunosuppression in mice. Iranian Journal of Pharmaceutical Research, 12, 101–108. Retrieved from http://www.ijpr.ir
- Ren, Z., He, C. H., Fan, Y. H., Guo, L. W., Si, H. M., Wang, Y. W., … Zhang, H. B. (2014). Immuno-enhancement effects of ethanol extract from Cyrtomium macrophyllum (Makino) Tagawaon cyclophosphamide-induced immunosuppression in BALB/c mice. Journal of Ethnopharmacology, 155, 769–777. doi: 10.1016/j.jep.2014.06.021
- Rood, B. R., Macdonald, T. J., & Packer, R. J. (2004). Current treatment of medulloblas-toma: Recent advances and future challenges. Seminars in Oncology, 31, 666–675. doi:10.1053/j.seminoncol.2004.07.009
- Rossi, A., Caracciolo, V., Russo, G., Reiss, K., & Giordano, A. (2008). Medulloblastoma: From molecular pathology to therapy. Clinical Cancer Research, 14, 971–976. doi:10.1158/1078-0432.CCR-07-2072
- Sheeja, K., & Kuttan, G. (2006). Protective effect of Andrographis paniculata and andrographolide on cyclophosphamide-induced urothelial toxicity. Integrative Cancer Therapies, 5, 244–251. doi:10.1177/1534735406291984
- Wang, J. X., Tong, X., Li, P. B., Cao, H., & Su, W. W. (2012). Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. Journal of Ethnopharmacology, 139, 788–795. doi:10.1016/j.jep.2011.12.019
- Yu, Q., Nie, S. P., Wang, J. Q., Liu, X. Z., Yin, P. F., Huang, D. F., … Xie, M. Y. (2014). Chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. International Journal of Biological Macromolecules, 64, 395–394. doi: 10.1016/j.ijbiomac.2013.12.029
- Zhao, M., Shi, Y., Zhao, J., Zhu, X. P., Chen, K. C., Pan, D. B., & Wei, C. Q. (2014). Molecular characterization and expression analysis of matrix metalloproteinase 3 in the Asian yellow pond turtle Mauremys mutica. Asian Herpetological Research, 5(1): 38–48. doi:10.3724/SP.J.1245.2014.00038
- Zhu, X. L., Chen, A. F., & Lin, B. Z. (2007). Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. Journal of Ethnopharmacology, 111, 219–226. doi:10.1016/j.jep.2006.11.013
- Zong, A. Z., Liu, Y. H., Zhang, Y., Song, X. L., Shi, Y. K., Cao, H. Z., … Wang, F. S. (2015). Anti-tumor activity and the mechanism of SIP-S: A sulfatedpolysaccharide with anti-metastatic effect. Carbohydrate Polymers, 129, 50–54. doi: 10.1016/j.carbpol.2015.04.017