ABSTRACT
During the period from September to November 2014, occurrences of Aflatoxin B1, B2, G1, and G2 (AFB1, AFB2, AFG1, and AFG2) were determined in 76 pepper samples consisting black pepper (n = 40) and red pepper (n = 36) obtained from local markets of Isfahan province, Iran. Aflatoxins' (AFs') analyses were carried out by using the high-performance liquid chromatography (HPLC) method. AFB1 levels in 32 (88.9%) of 36 red pepper samples were higher than the Iranian and European maximum permitted level (>5 µg/kg). Total AFs were detected in 41 out of 76 samples (53.9%) while 25 pepper samples (32.9%) had levels of the toxin above the Iranian and European permitted level, that is, 10 μg/kg. This study shows that incidence of AFs' contamination in red pepper in Iran was significantly higher than black pepper (p < .05) and indicates a serious hazard for human health.
KEYWORDS:
Introduction
Mycotoxins are fungal secondary metabolites mostly produced by toxigenic molds such as Aspergillus, Penicillium, and Fusarium. Aflatoxins (AFs) are a major group of mycotoxins, mainly produced by the species of Aspergillus, specifically Aspergillus flavus and Aspergillus parasiticus (Fallah, Barani, & Nasiri, Citation2015; Zhao, Schaffner, & Yue, Citation2013). Aflatoxin B1, B2, G1, and G2 (AFB1, AFB2, AFG1, and AFG2) are the most important mycotoxins that appear in foodstuffs as a result of fungal contamination during harvest and post-harvest practices (Fallah, Citation2010; Kamkar, Fallah, & Mozaffari Nejad, Citation2014).
AFs are known to be mutagenic and teratogenic natural compounds and have also been classified by the International Agency for Research on Cancer as a Group 1 carcinogen component due to its carcinogenic and cytotoxic effects in humans (International Agency for Research on Cancer [IARC], Citation2002). Because of high toxic effects of AFs, many countries have established a maximum limit for AFs in different types of foods. The European Commission has set an acceptable level of 5 µg/kg for AFB1 and 10 µg/kg for total AFs in foodstuff (European Commission [EC], Citation2006b). Also in Iran the maximum limit of AFs in foodstuff is 5 µg/kg and 10 µg/kg for AFB1 and total AFs, respectively (Institute of Standard and Industrial Research of Iran [ISIRI], Citation2002).
Spices are widely used for flavoring and seasoning of foods. They also have antimicrobial activities that help in food preservation (Mozaffari Nejad, Sabouri Ghannad, & Kamkar, Citation2014; Prelle, Spadaro, Garibaldi, & Gullino, Citation2014). Pepper is one of the most popular seasoning spices in the world and, depending on humidity and temperature conditions, is often contaminated with AFs during storage and drying processing stages (Hammami, et al., Citation2014; Kong, et al., Citation2014; Ozbey & Kabak, Citation2012). Contamination of spices with AFs can cause many diseases in humans even when consumed in small amounts.
Natural occurrence of AFs in different types of pepper has been studied in few surveys worldwide (Adzahan, Jalili, & Jinap, Citation2009; Martins, Martins, & Bernardo, Citation2001; Móricz, Fatér, Otta, Tyihák, & Mincsovics, Citation2007). Enzyme-linked immunosorbent assay, thin-layer chromatography, high-performance liquid chromatography (HPLC) coupled to fluorescence detector and mass spectrometry are the methods that are used for determination of AFs (Colak, Bingol, Hampikyan, & Nazli, Citation2006; Erdogan, Citation2004; Yao, Hruska, & Di Mavungu, Citation2015). However, immunoaffinity column clean-up, followed by HPLC with fluorescence detection is the most popular method for detection of AFs in different food types (Ali, Hashim, & Shuib, Citation2015; Papp, Klara, Záray, & Mincsovics, Citation2002).
In Iran, different kinds of spices are commonly used in food preparation. Referring to scientific literature, very few data have been published on the occurrence of AFs in spices in Iran. Therefore, this study aimed to determine the occurrence and levels of AFB1, AFB2, AFG1, and AFG2 in black and red pepper samples in the Isfahan province of Iran.
Materials and methods
Sample collection
During the period from September to November 2014, a total of 76 spice samples including black pepper (n = 40) and red pepper (n = 36) were purchased randomly from local markets in the Isfahan province of Iran. The samples were taken and analyzed according to European Union sampling and analyses methods for the official control of the levels of mycotoxins in foodstuffs (EC, Citation2006a). The samples were transported to the laboratory and stored in the dark at 4°C until analyses.
Chemicals and reagents
Acetonitrile, methanol, and distilled water were HPLC grade and purchased from Merck (Darmstadt, Germany), and other chemicals and reagents were of analytical grade. Aflatoxin B1, B2, G1, and G2 standards were obtained from Sigma–Aldrich (St. Louis, MO, USA) and immunoaffinity columns for sample clean-up were obtained from Neogen (Neogen Europe, Ltd., Scotland, UK).
Sample extraction
The samples were extracted and cleaned up with the reference official HPLC-fluorescence detector method described by the Institute of Standards and Industrial Research of Iran (ISIRI, Citation2010). A portion of sample (50 g) was blended with 5 g of sodium chloride and 200 ml of methanol 80% at high speed for 3 min. The mixture was filtered with filter paper (Whatman No. 1) and 20 mL of aqueous phase was mixed with 130 mL of phosphate buffer saline (PBS). This diluted solution was filtered through a glass microfiber filter (Whatman, Inc., Clifton, NJ, USA). Then 70 mL of solution was passed through an immunoaffinity column at a flow rate of about 3 ml/min; the column was previously conditioned with 10 ml of PBS. Afterwards, the column was washed with 10 ml distilled water and flushed with air to remove any remaining water. Finally, AFs were eluted from the column with 1500 µl of methanol followed by 1500 µl of distilled water and collected in a glass tube. The mixture was filtered through a 0.45 µm PTFE syringe filter and the volume of 20 µl of this mixture was injected into the HPLC device.
High-performance liquid chromatography analysis
The HPLC analyses were carried out in an Agilent 1260 Infinity HPLC system (Agilent Corporation, USA), with a quaternary pump, an auto sampler, a vacuum degasser, and a fluorescence detector. The chromatographic separation was performed on a Discovery® C18 HPLC column (5 µm particle size, 250 mm × 4.6 mm i.d.; Supelco, Bellefonte, USA) protected with a Discovery® C18 Supelguard column (5 µm particle size, 20 mm × 4.6 mm i.d.; Supelco, Bellefonte, USA). The post-column derivatization of AFs was carried out by using a Kobra cell electrochemical bromine system (Libios, Chemin de plagne 69210 Bully, France). The mixture solution of water, acetonitrile, and methanol (6:2:3, v/v/v) was used as mobile phase with a flow rate of 1.0 ml/min and the column temperature was set at 40°C. The fluorescence detector was set at wavelengths of 360 and 430 nm for excitation and emission, respectively.
Methods' validation
Validation of analytical procedure was carried out on the basis of recovery, limit of detection (LOD), limit of quantification (LOQ), linearity, repeatability, and within-laboratory reproducibility. The recoveries were determined by spiking the blank samples at three different levels (2.5, 5, and 10 µg/kg) in six replicates with the working standard solution (100 µg/kg). The LOD and the LOQ were calculated from signal to noise ratios of 3:1 and 10:1, respectively. The repeatability and within-laboratory reproducibility of the method was expressed as the relative standard deviation (RSD) of the recovery. A five-point calibration curve was prepared at concentrations of 0.5, 1, 2, 5, and 10 µg/l to check the linearity and quantify the AFs in the samples.
Statistical analyses
The statistical analyses were performed using t-test and chi-square test of the SPSS software version 20 for windows (SPSS Inc., Chicago, IL, USA) to compare AFs' concentrations.
Results and discussion
The HPLC Chromatograms of Aflatoxin B1, B2, G1, and G2 standards (5 µg/l), blank and positive sample are shown in . According to the validation procedure the average recovery was in the range of 79.8–90.4%. The obtained recovery values are in compliance with Commission Regulation (EC) No. 401/2006, which stated the recovery values of 70–120% for spiking levels of 1–10 μg/kg (EC, Citation2006a).
Figure 1. Chromatographic response for AFs: (a) a standard solution at 5ng/ml; (b) a blank pepper sample; (c) a positive pepper sample.
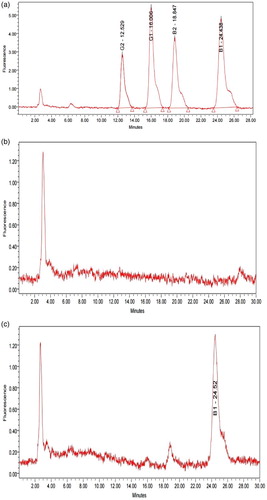
The RSD values for spiked samples were ranging from 5.57 to 7.28, indicating the acceptable precision for analysis of AFs in pepper samples. The LODs were 0.05 µg/kg for AFB1 and AFG1, 0.04 µg/kg for AFB2, and 0.1 µg/kg for AFG2. The LOQs were 0.2 µg/kg for AFB1 and AFG1, 0.11 µg/kg for AFB2, and 0.25 µg/kg for AFG2 ().
Table 1. Validation of AFs' determination by HPLC analysis.
The occurrence and concentrations of AFs in black and red pepper samples obtained from the local market of the Isfahan province of Iran are shown in . According to the results, AFB1 was detected in 5 out of 40 black pepper samples (12.5%) ranging from 0.88 to 1.45 μg/kg and all of the red pepper samples (100%) ranging from 4.22 to 28.6 μg/kg. AFB1 levels in 32 (88.9%) of 36 red pepper samples were higher than the Iranian and European regulations' permitted limit (>5 µg/kg). All together total AFs were detected in 41 out of 76 samples (53.9%) while 25 samples (32.9%) had levels of the toxin above the ISIRI and EC limit, that is, 10 μg/kg. As shown in , AFG1 and AFG2 were not detected in samples.
Table 2. The occurrence of AFs in pepper samples in the Isfahan province, Iran.
In Iran, few studies have been performed on AFs' level in pepper. In previous surveys conducted in the Mazandaran province of Iran, AFB1 was found in all of the red and black pepper samples ranging from 0.063 to 0.627 µg/kg (Mozaffarinejad & Giri, Citation2015). In another study performed in the Razavi Khorasan province of Iran (Habibi Najafi & Mortazavi, Citation2012), a high incidence of AFs contamination (70%) was found in red peppers, which is comparable to the results of this study.
Besides Iran, occurrence of AFs in black and red pepper was investigated by several studies in different countries (Cho, et al., Citation2008; Fazekas, Tar, & Kovacs, Citation2005; Jalili, Jinap, & Radu, Citation2010; O'Riordan & Wilkinson, Citation2008). In Turkey, Karaaslan and Arslanğray (Citation2015) reported that 90.5% (38/42) of powdered red pepper samples contain AFs ranging from 0.38 to 86.01 μg/kg and 13 samples (34%) had AFs higher than the EC limit (10 μg/kg). In another study, Hazir and Coksoyler (Citation1998) showed that 46 out of 141 red pepper samples (32.6%) contained AFs. In Italy, Romagnoli, Menna, Gruppioni, and Bergamini (Citation2007) did not detect any AFs in black pepper samples. In Malaysia, Adzahan et al. (Citation2009) found AFs in 27 out of 39 (69.2%) black pepper powder samples.
Conclusions
This study shows that the incidence of AFs' contamination in red pepper in Iran was significantly higher than black pepper (p < .05). Routine monitoring of AFs' contamination in various spices should be performed regularly and the spices with the toxin levels higher than the legal regulations must be prohibited for human consumption to control the health risks associated from these toxins. Good processing, handling, transportation, and storage system can reduce the production of AFs.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Afshin Barani http://orcid.org/0000-0002-3772-7305
Notes on contributors
Afshin Barani is PhD candidate in food hygiene and his main interest is detecting drug residue and mycotoxins in food and he expert in analytical method development.
Zeinab Nasiri is graduate in analytical chemistry, her main interest is chromatography and she expert in methods development.
Nafiseh Jarrah is graduate in analytical chemistry and her main interest is laboratory work such as chromatography and analytical methods.
References
- Adzahan, N., Jalili, M., & Jinap, S. (2009). Survey of aflatoxins in retail samples of whole and ground black and white peppercorns. Food Additives and Contaminants: Part B, 2, 178–182. doi: 10.1080/19440040903384190
- Ali, N., Hashim, N. H., & Shuib, N. S. (2015). Natural occurrence of aflatoxins and ochratoxin A in processed spices marketed in Malaysia. Food Additives & Contaminants: Part A, 32, 518–532. doi: 10.1080/19440049.2015.1011712
- Cho, S. H., Lee, C. H., Jang, M. R., Son, Y. W., Lee, S. M., Choi, I. S., … Kim, D. B. (2008). Aflatoxins contamination in spices and processed spice products commercialized in Korea. Food Chemistry, 107, 1283–1288. doi: 10.1016/j.foodchem.2007.08.049
- Colak, H., Bingol, E. B., Hampikyan, H., & Nazli, B. (2006). Determination of aflatoxin contamination in red-scaled, red and black pepper by ELISA and HPLC. Journal of Food and Drug Analysis, 14, 292–296.
- Erdogan, A. (2004). The aflatoxin contamination of some pepper types sold in Turkey. Chemosphere, 56, 321–325. doi: 10.1016/j.chemosphere.2004.02.020
- European Commission (EC). (2006a). Commission Regulation 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Official Journal of European Communities, L 70, 12–34.
- European Commission (EC). (2006b). Commission Regulation. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of European Communities, L 364, 5–24.
- Fallah, A. (2010). Aflatoxin M 1 contamination in dairy products marketed in Iran during winter and summer. Food Control, 21, 1478–1481. doi: 10.1016/j.foodcont.2010.04.017
- Fallah, A., Barani, A., & Nasiri, Z. (2015). Aflatoxin M1 in raw milk in Qazvin Province, Iran: A seasonal study. Food Additives & Contaminants: Part B, 8, 195–198. doi: 10.1080/19393210.2015.1046193
- Fazekas, B., Tar, A., & Kovacs, M. (2005). Aflatoxin and ochratoxin A content of spices in Hungary. Food Additives and Contaminants, 22, 856–863. doi: 10.1080/02652030500198027
- Habibi Najafi, M. B., & Mortazavi, S. A. (2012). Assessment of the microbiological quality and mycotoxin contamination of Iranian red pepper spice. Journal of Agriculture Science and Technology, 14, 1511–1521.
- Hammami, W., Fiori, S., Al Thani, R., Kali, N. A., Balmas, V., Migheli, Q., & Jaoua, S. (2014). Fungal and aflatoxin contamination of marketed spices. Food Control, 37, 177–181. doi: 10.1016/j.foodcont.2013.09.027
- Hazir, Z., & Coksoyler, N. (1998). Aflatoxin levels of red peppers which are produced with different methods in different regions. In Food Engineering Congress (16–18 September 1998), Gaziantep, Turkey (pp. 479–483).
- Institute of Standard and Industrial Research of Iran (ISIRI). (2002). Maximum tolerated limits of mycotoxins in foods and feeds. Standard No. 5925. Tehran, Iran.
- Institute of Standard and Industrial Research of Iran (ISIRI). (2010). Foods and feeds: Determination of aflatoxins B and G by HPLC method and immunoaffinity column clean up, test method. Standard No. 6872. Tehran, Iran.
- International Agency for Research on Cancer (IARC). (2002). Aflatoxins. IARC monograph on the evaluation of carcinogenic risk to humans (Vol. 82, p. 171). Lyon: World Health Organization.
- Jalili, M., Jinap, S., & Radu, S. (2010). Natural occurrence of ochratoxin A contamination in commercial black and white pepper products. Mycopathologia, 170, 251–258. doi: 10.1007/s11046-010-9320-7
- Kamkar, A., Fallah, A., & Mozaffari Nejad, A. S. (2014). The review of aflatoxin M1 contamination in milk and dairy products produced in Iran. Toxin Reviews, 33, 160–168. doi: 10.3109/15569543.2014.922580
- Karaaslan, M., & Arslanğray, Y. (2015). Aflatoxins B1, B2, G1, and G2 contamination in ground red peppers commercialized in Sanliurfa, Turkey. Environmental Monitoring and Assessment, 187, 1–9. doi: 10.1007/s10661-015-4402-0
- Kong, W., Wei, R., Logrieco, A. F., Wei, J., Wen, J., Xiao, X., & Yang, M. (2014). Occurrence of toxigenic fungi and determination of mycotoxins by HPLC-FLD in functional foods and spices in China markets. Food Chemistry, 146, 320–326. doi: 10.1016/j.foodchem.2013.09.005
- Martins, M. L., Martins, H. M., & Bernardo, F. (2001). Aflatoxins in spices marketed in Portugal. Food Additives & Contaminants, 18, 315–319. doi: 10.1080/02652030120041
- Móricz, Á. M., Fatér, Z., Otta, K. H., Tyihák, E., & Mincsovics, E. (2007). Overpressured layer chromatographic determination of aflatoxin B1, B2, G1 and G2 in red paprika. Microchemical Journal, 85, 140–144. doi: 10.1016/j.microc.2006.03.007
- Mozaffarinejad, A. S., & Giri, A. (2015). The measurement of Aflatoxin B1 in chilli and black peppers of qaemshahr, Iran. Journal of Kerman University of Medical Sciences, 22, 185–193.
- Mozaffari Nejad, A. S., Sabouri Ghannad, M., & Kamkar, A. (2014). Determination of aflatoxin B1 levels in Iranian and Indian spices by ELISA method. Toxin Reviews, 33, 151–154. doi: 10.3109/15569543.2014.942319
- O'Riordan, M. J., & Wilkinson, M. G. (2008). A survey of the incidence and level of aflatoxin contamination in a range of imported spice preparations on the Irish retail market. Food Chemistry, 107, 1429–1435. doi: 10.1016/j.foodchem.2007.09.073
- Ozbey, F., & Kabak, B. (2012). Natural co-occurrence of aflatoxins and ochratoxin A in spices. Food Control, 28, 354–361. doi: 10.1016/j.foodcont.2012.05.039
- Papp, E., Klara, H., Záray, G., & Mincsovics, E. (2002). Liquid chromatographic determination of aflatoxins. Microchemical Journal, 73, 39–46. doi: 10.1016/S0026-265X(02)00048-6
- Prelle, A., Spadaro, D., Garibaldi, A., & Gullino, M. L. (2014). Co-occurrence of aflatoxins and ochratoxin A in spices commercialized in Italy. Food Control, 39, 192–197. doi: 10.1016/j.foodcont.2013.11.013
- Romagnoli, B., Menna, V., Gruppioni, N., & Bergamini, C. (2007). Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. Food Control, 18, 697–701. doi: 10.1016/j.foodcont.2006.02.020
- Yao, H., Hruska, Z., & Di Mavungu, J. D. (2015). Developments in detection and determination of aflatoxins. World Mycotoxin Journal, 8, 181–191. doi: 10.3920/WMJ2014.1797
- Zhao, X., Schaffner, D. W., & Yue, T. (2013). Quantification of aflatoxin risk associated with Chinese spices: Point and probability risk assessments for aflatoxin B 1. Food Control, 33, 366–377. doi: 10.1016/j.foodcont.2013.03.012
