ABSTRACT
A specific monoclonal antibody (mAb) against trimethoprim (TMP) was produced and a mAb-based indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) was established to detect TMP residues in milk, honey, and fish samples. The working range of ic-ELISA and IC50 was 1.83–9.36 and 4.14 µg L−1. The average recoveries of TMP from TMP-spiked milk, honey, and fish sample were 103.5–118.0%, 100.3–107.1%, and 91.5–108.1%, respectively. The results revealed that our developed mAb-based ic-ELISA can be effectively applied to detect TMP residues in these food products.
Introduction
Trimethoprim (TMP, 5-[(3′, 4′, 5′-trimethoxyphenyl) methyl]-2, 4-pyrimidinediamine) is a member of the 2, 4-diamino pyrimidine class of dihydrofolate reductase inhibitors and an antibiotic synergists (Luo et al., Citation2013). In animal husbandry, the single administration of TMP causes microbial resistance; therefore, TMP is widely administered in combination with sulfonamides or other antibiotics for the therapy of animal diseases, including fowl cholera, respiratory tract secondary bacterial infection, and pullorum disease (Liu, Wu, Sun, & Wan, Citation2012). The potency of TMP is enhanced 10× when administered with sulfonamides. However, the excessive use of TMP has contributed to the presence of drug residue in foods, which result in antibiotic-resistance, nausea, emesis, headache, pruritus, and rush in consumers (Croubels, Wassink, & De Backer, Citation2002). Additionally, the long term and excessive use of TMP reduces serum leukocyte and thrombocyte and induces teratogenesis.
Several countries have established the maximum residue limits (MRLs) of TMP in foods. In the European Union and China, the MRL of TMP residues is 50 µg kg−1 in pork, chicken, and fish (Märtlbauer, Meier, Usleber, & Terplan, Citation1992) (Andrade, de Moraes, Rocha-Filho, Fatibello-Filho, & Cass, Citation2009). In light of its adverse effects on consumer health, it is important to develop an effective and rapid detection method of TMP residues that can be used to screen a large number of samples.
Currently, there are several methods developed to analyze TMP residues in food, which are mainly dependent on instruments including high-performance liquid chromatography (HPLC)-ultraviolet (HPLC-UV) (Batzias, Botsoglou, Kotsaki-Kovatsi, & Kounenis, Citation2002; Batzias, Delis, & Koutsoviti-Papadopoulou, Citation2005), HPLC-diode array detector (Bedor et al., Citation2008), and liquid chromatography-mass spectrometry (Economou, Petraki, Tsipi, & Botitsi, Citation2012; Fernandez-Torres, Lopez, Consentino, Mochon, & Payan, Citation2011), and hydrophilic interaction chromatography (Fontanals, Marcé, & Borrull, Citation2011; Uchiyama et al., Citation2011). These methods, which have adequate sensitivity and specificity, are expensive and require laborious and time-consuming sample pre-treatments steps and highly skilled personnel.
The enzyme-linked immunosorbent assay (ELISA) is a sensitive, simple, and cost-effective method, which has been widely used in the analyses of large compounds such as microbes (Feng et al., Citation2013), proteins (Peng et al., Citation2014), and allergens (Peng et al., Citation2013) and small compounds such as antibiotics (Chen et al., Citation2016; Suryoprabowo, Liu, Peng, Kuang, & Xu, Citation2014), pesticides (Chen, Liu, Kuang, Song, & Xu, Citation2013; Chen, Xu, et al., Citation2013), hormones (Kong et al., Citation2015; Liu, Peng, Jin, & Xu, Citation2007), and toxins (Zhang et al., Citation2013). There are also some papers which have reported the detection of TMP in food-based antibodies. The polyclonal antibody against TMP was produced with limit of detection (LOD) of 6 ng mL−1 (Märtlbauer, Usleber, Schneider, & Dietrich, Citation1994). An ELISA based on antibodies produced from rabbit was developed with LOD of 12.5 µg kg−1 in raw milk (Albrecht, Hammer, & Heeschen, Citation1996). However, there is still no report about the monoclonal antibody (mAb). The objectives of this study were to produce a sensitive and specific mAb and develop a mAb-based indirect competitive ELISA (ic-ELISA) for TMP residue detection in food samples.
Materials and methods
Chemicals and reagents
TMP, sulfadiazine, sulfamethazine, sulfamethoxazole, sulfamethizole, and 25% glutaraldehyde (GA) solution were obtained from J&K Scientific Ltd. (Beijing, China). Diaveridine, sulfadimethoxine, sulfathiazole, bovine serum albumin (BSA), ovalbumin (OVA), Freund’s complete and incomplete adjuvant, 3,3′,5,5′-tetramethylbenzidine (TMB), and polyethylene glycol 1500 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Enzyme immunoassay-grade horseradish peroxidase labelled goat anti-mouse immunoglobulin was supplied by Hua Mei Co. (Shanghai, China). RPMI-1640 cell culture medium, 50× HAT supplement (containing hypoxanthine aminopterin thymidine), 100× HT supplement (containing hypoxanthine thymidine), and fetal bovine serum were obtained from Thermo Fisher Scientific Inc. (Waltham, MA, USA). All other chemicals and solvents were of analytical grade.
Instruments
Absorbance measurements were performed with a spectrophotometric microtiter plate reader (Thermo), and UV spectra were determined with an UV–VIS spectrophotometer (Agilent, LA, USA). Pure water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA).
Buffer solutions
The buffer solutions used in this study referred to our previous work with some modifications (Xu, Xu, Ma, Kuang, & Xu, Citation2015). (1) coating buffer (0.05 M carbonate buffer, CB, pH 9.6); (2) blocking buffer (0.05 M CB with 0.2% gelatin, w/v); (3) washing buffer (0.01 M phosphate buffered solution (PBS) with 0.05% Tween-20 v/v, PBS-T, pH 7.2); (4) assay buffer (0.01 M PBS, pH 7.2); (5) antibody diluent (0.01 M PBS-T with 0.1% gelatin, w/v); (6) citrate buffer (100 mL of 0.1 M citrate phosphate buffer containing 18 µL of 30% H2O2, pH 5.0); (7) TMB (60 mg TMB dissolved in 100 mL ethylene glycol); (8) substrate solution (5:1 v/v mixture of citrate buffer and TMB solution); and (9) Stop buffer (2 M H2SO4).
Synthesis of immunogens and coating antigens
Immunogens and coating antigens were synthesized via GA method, in which amino groups of TMP are covalently attached to carrier proteins (e.g. BSA or OVA) (Chen et al., Citation2015). Briefly, 13 mg of TMP previously dissolved in 3 mL of acetonitrile was mixed with 50 µL of 25% GA solution for reacting for 5 min. Then, the mixture solution was added dropwise into 100 mg of BSA or OVA dissolved in 10 mL of 0.01 M PBS, allowed to react for 2 h in room temperature, and dialyzed against PBS for 3 d. The formation of conjugates was confirmed by UV–VIS.
Immunization schedule
The immunization schedule was performed based on standard protocol (Yin, Liu, Song, Kuang, & Xu, Citation2015). Briefly, five BALB/c female mice were subcutaneously injected at multiple points with 100 µg of immunogen emulsified with Freund’s complete adjuvant. Four weeks after the initial injection, booster immunizations were performed with 50 µg of immunogen emulsified with Freund’s incomplete adjuvant. After the third immunization, titer and IC50 of sera collected from mice were evaluated by ic-ELISA. The mouse with the highest titer and lowest IC50 was screened for spleen donation for cell fusion. Twenty days prior to cell fusion, a final booster intra-peritoneal injection (25 µg of immunogen directly dissolved in 100 µL of physiological saline) was administered.
Cell fusion and hybridoma screening
Cell fusion and hybridoma screening were performed as previously reported (Kuang, Xu, Cui, Ma, & Xu, Citation2010). Firstly, spleen was removed from mouse with the highest titer and lowest IC50 and ground to yield splenocytes. In the presence of PEG 1500, splenocytes were fused with mouse Sp 2/0 myeloma cells. The hybridoma cells distributed in 96-well plates were cultured in HAT medium supplemented with 20% bovine fetal serum for 7 d at 37°C and 5% CO2. The optimum cell line with the highest titer and lowest IC50 was screened by evaluating the supernatants via ic-ELISA. Sub-clones from the cell lines were intraperitoneally administered to mice primed with paraffin to obtain ascites. Monoclonal antibodies were obtained following the purification of ascites by the saturated ammonium sulfate method. The concentration of mAb was measured by UV–VIS spectroscopy at 278 nm. The mAb was labeled and stored at −20°C.
ic-ELISA
The most appropriate mAb concentration and suitable coating concentration for ic-ELISA were determined by bi-dimensional titration assays (Jiang et al., Citation2011). Briefly, 100 µL of TMP-OVA serially diluted in coating buffer was coated on microtiter plates for 2 h at 37°C. Subsequently, the plates were blocked washed and blocked for 2 h at 37°C. Blank assay buffer (50 µL) and diluted mAb (50 µL) were added into every well and incubated for the antigen-antibody reaction for 30 min at 37°C. The reaction was terminated by washing steps. Peroxidase-labeled goat anti-mouse IgG (100 µL, diluted 3000 times with antibody diluent) was added and incubated for 30 min at 37°C. Following the 30-min incubation, the plates were washed to remove excess peroxidase-labeled goat anti-mouse IgG. TMB substrate solution (100 µL) was added, and the plates were incubated for 15 min at 37°C. Finally, the reaction was stopped with 50 µL of 2 M H2SO4. Optical density (OD) was measured at 450 nm in a microplate reader. The procedure of bi-dimensional titration assay was similar to ic-ELISA, except that the blank assay buffer was replaced with diluted analyte. ODs were plotted against the logarithm of analyte concentration to obtain the standard inhibition curve.
Sensitivity and specificity
IC50, which represents the concentration of competing compound that produced a 50% inhibition of antibody binding to the coating antigen, is an important indicator of mAb sensitivity (Jiang et al., Citation2011). The concentration that resulted in 20–80% inhibition and 15% inhibition were defined as detection range and LOD of ic-ELISA, respectively (Wang, Zhang, Gao, Duan, & Wang, Citation2010).
Specificity was defined as the ability of structurally related compounds to combine with antibody. Generally, cross-reactivity (CR) was measured to assess specificity. CR was calculated according to the following equation (Liu, Kuang, Peng, Wang, & Xu, Citation2014):
Seven analogues (diaveridine, sulfadiazine, sulfamethazine, sulfadimethoxine, sulfathiazole, sulfamethoxazole, and sulfamethizole) were used to evaluate the specificity of mAb. These compounds, previously dissolved in dimethyl formamide at 1 mg mL−1, were diluted to 5, 10, 20, 50, 100, and 200 µg L−1 with PBS.
Sample pretreatment
Three different food matrices were used in this study: milk, honey, and fish tissue (crucian carps). TMP-negative samples confirmed by HPLC were provided by Jiangsu Entry-Exit Inspection and Quarantine Bureau. Milk (5 mL) and honey (5 g) samples were spiked with TMP resulting in three different TMP concentrations (20, 40, and 80 µg L−1 in milk and 20, 40, and 80 µg kg−1 in honey). The TMP-spiked milk and honey samples were subsequently diluted 10× with 0.01 M PBS to reduce matrix effects, resulting in final TMP concentrations of 2, 4, and 8 µ L−1 in milk and 2, 4, and 8 µg kg−1 in honey.
Edible muscle from crucian carps was used. The fish samples were eviscerated, peeled, minced, and homogenized. Fish tissue (5 g) in a 50-mL centrifuge tube was spiked with TMP resulting in three different TMP concentrations (8, 16, and 32 µg kg−1). The spiked fish tissues were placed in an ultrasonic bath for 5 min to thoroughly disperse TMP into the matrix (Cháfer-Pericás, Maquieira, Puchades, Miralles, & Moreno, Citation2010). Twenty-five milliliter of acetonitrile was added into the sample and mixed on a votex for 10 min. The mixture was centrifuged at 875 g for 5 min. Organic solvent in the supernatant was evaporated under a nitrogen stream. The remainder of samples were re-suspended in 4 mL of PBS-T containing 10% acetonitrile and filtered through 0.22 µm membrane (Cháfer-Pericás et al., Citation2010). This procedure diluted the fish tissue 4× to final TMP concentrations of 2, 4, and 8 µg kg−1.
Results and discussions
Synthesis of immunogen
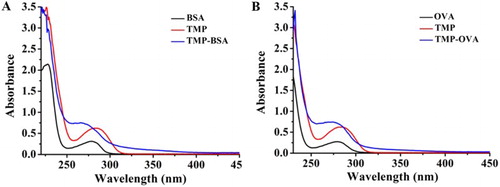
Small molecules such as TMP are incapable of triggering immune responses and eliciting antibody synthesis in animals. To be immunogenic, TMP has to be coupled to a carrier protein such as BSA or keyhole limpet hemocyanin; OVA is often used as a coating antigen. The three methoxy groups of TMP on benzene ring are unsuitable for conjugation. In contrast, the amino groups on pyrimidine ring are active and easily conjugate with the amino groups of carrier proteins. GA is a common homobifunctional crossing-linking agent that has been widely applied in conjugation reactions. The maximum absorbance peak of BSA/OVA is at 278 nm, and the characteristic absorbance peak of TMP is at 285 nm. As shown in , the maximum absorbance of TMP-BSA and TMP-OVA obviously blueshifted to 266 nm, which confirms that the conjugates were successfully synthesized.
Optimization of ic-Elisa
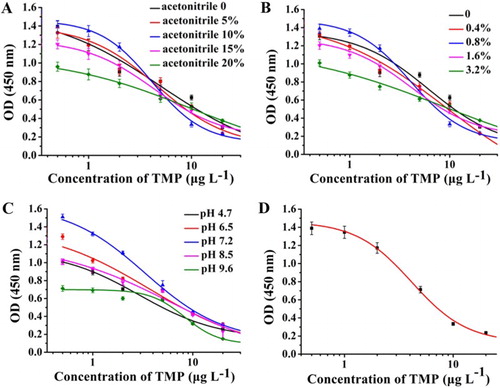
Certain parameters of the assay buffer, for example, organic solvent content, ionic strength, and pH, affect protein interactions and antigen-antibody reactions. Organic solvent contributes to the solubility of hydrophobic compounds. Excessive volumes of organic solvent negatively affect the antibody, leading to low OD values. As shown in (a), the maximum OD varied with acetonitrile concentration. Under condition of 20% acetonitrile (v/v) in 0.01 M PBS, the sensitivity of antibody was poor. At 10% acetonitrile (v/v) in 0.01 M PBS, OD values and sensitivity were optimum. As shown in (b), IC50 was the lowest and OD was low at 3.2%. Therefore, 0.8% ionic strength was optimal. As shown in (c), the maximum OD was <1 at pH 9.6. At pH 7.2, OD was the highest and IC50 was the lowest. (d) shows the standard sigmoidal inhibition curve of TMP under optimized conditions. The results revealed that the IC50 value and quantitative detection range (IC20–IC80) were 4.14 and 1.83–9.36 µg L−1, respectively.
Figure 2. Optimization of assay buffer for ic-ELISA: (a) Effect of acetonitrile content in PBS on ic-ELISA performance; (b) Effect of ionic strength of assay buffer on ic-ELISA performance; (c) Effect of pH value of assay buffer on ic-ELISA performance; (d) Standard curve of inhibition under the optimized conditions. Coating antigen: 0.2 µg mL−1; mAb: 1: 32000; standards: 0.5, 1, 2, 5, 10, 20, and 50 µg L−1. Each point presents the mean of ± SD of three replicates.
Cross-reactivity
There was no CR between the analogues and TMP, which illustrated that the produced mAb was specific to TMP (). The difference in chemical structure between TMP and diaveridine was an extra methoxy group on the benzene ring. However, diaveridine has no CR with TMP, which proved that the methoxy groups were the antigenic determinant.
Table 1. CR of mAb.
Recovery tests
Dilution is commonly used to reduce the matrix interferences; however, this procedure reduce the sensitivity. In this study, the sensitivity of ic-ELISA still satisfied the recovery tests after dilution. The fish tissues were treated with organic buffers to extract TMP residues. Acetonitrile, which is poorly tolerated in immunoassays, was completely removed. The four-fold dilution can obviously reduce the matrix effects. As shown in , the average recoveries of TMP spiked in milk, honey, and fish tissue were 103.5–118.0%, 100.3–107.1%, and 91.5–108.1%, respectively, which revealed that mAb-based ic-ELISA can be used for TMP residue analysis.
Table 2. Recoveries of TMP from spiked samples by ic-ELISA.a
Conclusions
In this paper, a sensitive and specific mAb against TMP was produced for the first time, and a rapid and simple ic-ELISA was developed for the detection of TMP in milk, honey, and fish samples. The simple sample pretreatment steps would allow the rapid screening of a large number of samples. Based on the results, mAb had excellent working range (1.83–9.36 µg L−1), which can be employed for commercial ELISA kits and gold immunochromatographic strips.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Yanni Chen got her bachelor’s degree from Liaoning Medical University, Jinzhou, China, in 2012 and then she began to study in Jiangnan University (Wuxi, China) as a Ph.D. student in Food Science. Her research interest includes immunoassay applications in food.
Liqiang Liu got his Ph.D. in Food Science in 2014 from Jiangnan University, Wuxi, China, and then became a faculty in College of Food Science and Technology of Jiangnan University. His research interests are immunochromatographic strip design and application.
Shanshan Song got her master’s degree in Food Science in 2012 from Jiangnan University, Wuxi, China, and then became a research assistant in College of Food Science and Technology of Jiangnan University. Her research interest includes monoclonal antibody development.
Hua Kuang got her Ph.D. from China Agricultural University in 2009 and then began to work as a faculty in College of Food Science and Technology of Jiangnan University. She is currently a full professor in food safety. Her research interest includes biosensor development.
Chuanlai Xu is a full professor of Food Science and Technology of Jiangnan University. He got his Ph.D. in Food Science in 2002. His research interests are fast detection technology and food safety evaluation.
Additional information
Funding
References
- Albrecht, U., Hammer, P., & Heeschen, W. (1996). Enzyme immunoassay for the detection of trimethoprim in raw milk. Milchwissenschaft-Milk Science International, 51(9), 515–516.
- Andrade, L. S., de Moraes, M. C., Rocha-Filho, R. C., Fatibello-Filho, O., & Cass, Q. B. (2009). A multidimensional high performance liquid chromatography method coupled with amperometric detection using a boron-doped diamond electrode for the simultaneous determination of sulfamethoxazole and trimethoprim in bovine milk. Analytica Chimica Acta, 654(2), 127–132. doi: 10.1016/j.aca.2009.09.035
- Batzias, G., Botsoglou, N., Kotsaki-Kovatsi, V.-P., & Kounenis, G. (2002). New simple liquid chromatographic method for the determination of trimethoprim, sulfadiazine and N 4-acetylsulfadiazine in plasma of broilers. Journal of Chromatography B, 769(2), 253–259. doi: 10.1016/S1570-0232(01)00625-0
- Batzias, G., Delis, G., & Koutsoviti-Papadopoulou, M. (2005). Bioavailability and pharmacokinetics of sulphadiazine, N4-acetylsulphadiazine and trimethoprim following intravenous and intramuscular administration of a sulphadiazine/trimethoprim combination in sheep. Veterinary Research Communications, 29(8), 699–712. doi: 10.1007/s11259-005-3868-6
- Bedor, D., Gonçalves, T., Ferreira, M., De Sousa, C., Menezes, A., Oliveira, E., & De Santana, D. (2008). Simultaneous determination of sulfamethoxazole and trimethoprim in biological fluids for high-throughput analysis: Comparison of HPLC with ultraviolet and tandem mass spectrometric detection. Journal of Chromatography B, 863(1), 46–54. doi: 10.1016/j.jchromb.2007.12.027
- Cháfer-Pericás, C., Maquieira, Á, Puchades, R., Miralles, J., & Moreno, A. (2010). Fast screening immunoassay of sulfonamides in commercial fish samples. Analytical and Bioanalytical Chemistry, 396(2), 911–921. doi: 10.1007/s00216-009-3229-3
- Chen, X., Liu, L., Kuang, H., Song, S., & Xu, C. (2013). A strip-based immunoassay for rapid determination of fenpropathrin. Analytical Methods, 5(21), 6234–6239. doi: 10.1039/c3ay41030g
- Chen, X., Xu, L., Ma, W., Liu, L., Kuang, H., Peng, C., … Xu, C. (2013). Development of an enzyme-linked immunosorbent assay for cyhalothrin. Immunological Investigations, 42(6), 493–503. doi: 10.3109/08820139.2013.797909
- Chen, Y., Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of an enzyme-linked immunosorbent assay (ELISA) for natamycin residues in foods based on a specific monoclonal antibody. Analytical Methods, 7(8), 3559–3565. doi: 10.1039/C5AY00404G
- Chen, Y., Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Analytical Methods, 9(4), 905–915. doi: 10.1007/s12161-015-0262-z
- Croubels, S., Wassink, P., & De Backer, P. (2002). Simultaneous determination of sulfadiazine and trimethoprim in animal feed by liquid chromatography with UV and tandem mass spectrometric detection. Analytica Chimica Acta, 473(1), 183–194. doi: 10.1016/S0003-2670(02)00976-5
- Economou, A., Petraki, O., Tsipi, D., & Botitsi, E. (2012). Determination of a liquid chromatography–tandem mass spectrometry method for the determination of sulfonamides, trimethoprim and dapsone in honey and validation according to Commission Decision 2002/657/EC for banned compounds. Talanta, 97, 32–41. doi: 10.1016/j.talanta.2012.03.058
- Feng, M., Yong, Q., Wang, W., Kuang, H., Wang, L., & Xu, C. (2013). Development of a monoclonal antibody-based ELISA to detect Escherichia coli O157: H7. Food and Agricultural Immunology, 24(4), 481–487. doi: 10.1080/09540105.2012.716026
- Fernandez-Torres, R., Lopez, M. B., Consentino, M. O., Mochon, M. C., & Payan, M. R. (2011). Enzymatic-microwave assisted extraction and high-performance liquid chromatography–mass spectrometry for the determination of selected veterinary antibiotics in fish and mussel samples. Journal of Pharmaceutical and Biomedical Analysis, 54(5), 1146–1156. doi: 10.1016/j.jpba.2010.12.002
- Fontanals, N., Marcé, R. M., & Borrull, F. (2011). On-line solid-phase extraction coupled to hydrophilic interaction chromatography–mass spectrometry for the determination of polar drugs. Journal of Chromatography A, 1218(35), 5975–5980. doi: 10.1016/j.chroma.2010.12.028
- Jiang, J., Wang, Z., Zhang, H., Zhang, X., Liu, X., & Wang, S. (2011). Monoclonal antibody-based ELISA and colloidal gold immunoassay for detecting 19-nortestosterone residue in animal tissues. Journal of Agricultural and Food Chemistry, 59(18), 9763–9769. doi: 10.1021/jf2012437
- Kong, N., Guo, L., Guan, D., Liu, L., Kuang, H., & Xu, C. (2015). An ultrasensitive ELISA for medroxyprogesterone residues in fish tissues based on a structure-specific hapten. Food Analytical Methods, 8(6), 1382–1389. doi: 10.1007/s12161-014-0023-4
- Kuang, H., Xu, L., Cui, G., Ma, W., & Xu, C. (2010). Development of determination of di-n-octyl phthalate (DOP) residue by an indirect enzyme-linked immunosorbent assay. Food and Agricultural Immunology, 21(3), 265–277. doi: 10.1080/09540101003758962
- Liu, L., Kuang, H., Peng, C., Wang, L., & Xu, C. (2014). Fragment-based hapten design and screening of a highly sensitive and specific monoclonal antibody for ractopamine. Analytical Methods, 6(1), 229–234. doi: 10.1039/C3AY41827H
- Liu, L., Peng, C., Jin, Z., & Xu, C. (2007). Development and evaluation of a rapid lateral flow immunochromatographic strip assay for screening 19-nortestosterone. Biomedical Chromatography, 21(8), 861–866. doi: 10.1002/bmc.832
- Liu, Z. Y., Wu, Y., Sun, Z. L., & Wan, L. (2012). Characterization of in vitro metabolites of trimethoprim and diaveridine in pig liver microsomes by liquid chromatography combined with hybrid ion trap/time-of-flight mass spectrometry. Biomedical Chromatography: BMC, 26(9), 1101–1108. doi: 10.1002/bmc.1754
- Luo, H., Zhang, L., Xue, F., Li, Y., Wang, X., Fei, C., & Zhang, C. (2013). Simultaneous determination of trimethoprim and diaveridine in tissues of chicken, porcine, and fish by hydrophilic interaction liquid chromatography–tandem mass spectrometry. Food Analytical Methods, 7(2), 308–317. doi: 10.1007/s12161-013-9628-2
- Märtlbauer, E., Meier, R., Usleber, E., & Terplan, G. (1992). Enzyme immunoassays for the detection of sulfamethazine, sulfadiazine, sulfamethoxypyridazine and trimethoprim in milk. Food and Agricultural Immunology, 4(4), 219–228. doi: 10.1080/09540109209354771
- Märtlbauer, E., Usleber, E., Schneider, E., & Dietrich, R. (1994). Immunochemical detection of antibiotics and sulfonamides. The Analyst, 119(12), 2543–2548. doi: 10.1039/AN9941902543
- Peng, J., Meng, X., Deng, X., Zhu, J., Kuang, H., & Xu, C. (2014). Development of a monoclonal antibody-based sandwich ELISA for the detection of ovalbumin in foods. Food and Agricultural Immunology, 25(1), 1–8. doi: 10.1080/09540105.2012.716398
- Peng, J., Song, S., Xu, L., Ma, W., Liu, L., Kuang, H., & Xu, C. (2013). Development of a monoclonal antibody-based sandwich ELISA for peanut allergen Ara h 1 in food. International Journal of Environmental Research and Public Health, 10(7), 2897–2905. doi: 10.3390/ijerph10072897
- Suryoprabowo, S., Liu, L., Peng, J., Kuang, H., & Xu, C. (2014). Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Analytical Methods, 7(10), 2163–2168. doi: 10.1007/s12161-014-9863-1
- Uchiyama, K., Kondo, M., Yokochi, R., Takeuchi, Y., Yamamoto, A., & Inoue, Y. (2011). Derivative spectrum chromatographic method for the determination of trimethoprim in honey samples using an on-line solid-phase extraction technique. Journal of Separation Science, 34(13), 1525–1530. doi: 10.1002/jssc.201100112
- Wang, L., Zhang, Y., Gao, X., Duan, Z., & Wang, S. (2010). Determination of chloramphenicol residues in milk by enzyme-linked immunosorbent assay: Improvement by biotin-streptavidin-amplified system. Journal of Agricultural and Food Chemistry, 58(6), 3265–3270. doi: 10.1021/jf903940h
- Xu, N., Xu, L., Ma, W., Kuang, H., & Xu, C. (2015). Development and characterisation of an ultrasensitive monoclonal antibody for chloramphenicol. Food and Agricultural Immunology, 26(3), 440–450. doi: 10.1080/09540105.2014.950201
- Yin, Y., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of a highly sensitive icELISA to detect semicarbazide based on a monoclonal antibody. Food and Agricultural Immunology, 26(3), 356–365. doi: 10.1080/09540105.2014.914891
- Zhang, X., Feng, M., Liu, L., Xing, C., Kuang, H., Peng, C., … Xu, C. (2013). Detection of aflatoxins in tea samples based on a class-specific monoclonal antibody. International Journal of Food Science & Technology, 48(6), 1269–1274. doi: 10.1111/ijfs.12086