ABSTRACT
Hexestrol (HES) and Diethylstilbestrol (DES) are synthetic hormones, which have been found abuse use in animal-origin food production. We developed an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) and immune-chromatographic strip to detect these compounds based on monoclonal antibody. Under optimized conditions, the half-inhibition concentration (IC50) values of ic-ELISA were 0.15 µgL−1 and 0.23 µgL−1 for HES and DES, respectively. Spiked milk samples were analyzed. The recovery of both synthetic hormones in the milk samples was 60.48–102.19% (HES) and 89.34–100.16% (DES). In immune-chromatographic assay, the visual cutoff values at 0.5 and 1.0 µgL−1 respectively for HES and DES, which allow us to detect milk samples of a concentration low to 10 µgL−1 for HES and 15 µgL−1 for DES.
Introduction
Estrogens are group of hormones that refer to any substance either natural or synthetic that mimics the effects of the natural hormones. Hexestrol (HES) and Diethylstilbestrol (DES) are included in synthetic estrogen-like substances, which are widely used in agriculture industries as hormonal growth promoters (Dickson, MacNeil, Reid, & Fesser, Citation2003). Implantation or administration of hormonal growth promoters into cattle reportedly increased their feed efficiency and formation of lean muscle mass as well as milk production (Socas-Rodríguez, Asensio-Ramos, Hernández-Borges, Herrera-Herrera, & Rodríguez-Delgado, Citation2013). However, the use of HES and DES as growth promoters in animals’ production is confronted with many setbacks in the light of food safety. Many researches have reported a great potential for carcinogenic, mutagenic, teratogenic effects associated with their use (Dickson et al., Citation2003; Tang et al., Citation2014).
Consequently, HES, DES and other stilbenes are banned from the list of safe veterinary drugs that must be used for animal production. For instance, the European Union (EU) has forbidden the use of all hormonal growth promoters in cattle production and have developed hormone-free cattle programs for exporting countries where animals are certified to have been grown to market weight free from hormonal growth promoters (Dickson et al., Citation2003). The cow’s milk naturally contains a number of steroid and peptide hormones in minute quantities. However, its estrogen levels vary according to the level of fat and the physiological status of the cow; as a result, there is more likelihood of finding traces of synthetic nonsteroidal estrogens in cow’s milk when the lactating animal is under treatment with synthetic hormonal growth promoters. HES and DES are among the group of growth promoters employed in the treatment of estrogen-deficiency disorders in veterinary medicine (Socas-Rodríguez et al., Citation2013; Wang, Fang Peng, Chen, & Xu, Citation2008).
In view of the potential threat to human health associated with the use of growth promoters in animal breeding, measures must be put in place to ensure safe animal food products on the markets. The common techniques used to detect defective foods are liquid chromatography–mass spectrometry (LC–MS) and gas chromatography–mass spectrometry. Nevertheless, these methods are not suitable for on-site analysis (Wang et al., Citation2016; Xu et al., Citation2006); moreover, they are expensive and require more skilled personnel to operate them. Therefore, rapid, sensitive, inexpensive and accurate simple and time-saving methods such as Lateral immunochromatographic strips and indirect competitive enzyme-linked immunosorbent assay (ic-ELISA), which require less knowledge for operation, must be developed complementary to conventional instrumental techniques (Song et al., Citation2015; Tochi et al., Citation2015; Xu et al., Citation2015). In this work, we reported the production of a high-affinity monoclonal antibody against HES, the optimization of an Ic-ELISA and immunochromatographic assay to detect HES and DES residues in cow’s milk.
Materials and methods
Chemicals and materials
HES and DES were obtained from J&K Scientific Ltd. (Beijing, China). Bovine serum albumin (BSA), Ovalbumin (OVA), Keyhole limpet hemocyanin (KLH), Freund’s complete and incomplete adjuvant, 3,3′,5,5′-tetramethylbenzidine (TMB), and polyethylene glycol 1500 (PEG 1500) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG was obtained from Hua Mei Co. (Shanghai, China). RPMI-1640 cell culture medium, 50 × Culture medium with Hypoxanthine, Aminopterin and Thymidine (HAT) supplement (containing hypoxanthine aminopterin thymidine), 100 × HT supplement (containing hypoxanthine thymidine) and fetal bovine serum were obtained from Gibco BRL (Paisley, Scotland). Nitrocellulose membrane and absorbent pad were purchased from Millipore (Bedford, MA). All other chemicals and solvents used were obtained from the National Pharmaceutical Group Chemicals Reagent Co. Ltd. (Beijing, China).
Instruments
BioDot TSR3000 membrane strip reader was obtained from Gene Company, whereas, Biostrip dispenser and cutter were from XinqidianGene-Technology Co. Ltd (Beijing, China). Agilent Cary 60 UV-Vis Spectrophotometer purchased from Thermo, USA was used to confirm successful antigen-carrier protein coupling. BioTek Eon Microplate Spectrophotometer also from Thermo Fisher operating at 450 nm was used to read Elisa plates. A Milli-Q water purification system was purchased from Millipore (Bedford, MA, USA).
Buffers and solutions
Buffers and solutions used were: (1) 0.01 M phosphate buffer saline (PBS) 8.0 g of NaCl, 3.62 g of Na2HPO4·12H2O, 0.2 g of KH2PO4 and 0.2 g of KCl dissolved in 1 l of distilled water and adjusted to (pH 7.4); (2) 0.05 M carbonate buffer (CB, pH 9.6) without and with 0.2% (w/v, g/l) gelatin, respectively, for coating and blocking solutions. (3) 0.01 M PBS containing 0.05% Tween-20 (v/v) (PBST) was used as washing buffer; (4) 0.01 M PBS containing 0.1% (w/v, g/L) gelatin and 0.05% (v/v) Tween 20 was used as antibody dilution buffer; (5) substrate stock solution buffer; 100 ml of 0.1 M citrate-phosphate buffer (pH 5.0) containing 180 µl of 30% H2O2. (6) TMB solution (60 mg of TMB dissolved in 100 ml glycol); (7) TMB-Substrate solution [5:1 (v/v) mixture of substrate buffer and TMB solution]; and (8) 2 M H2SO4 solution for stopping color development.
Hapten synthesis and immunogen preparation
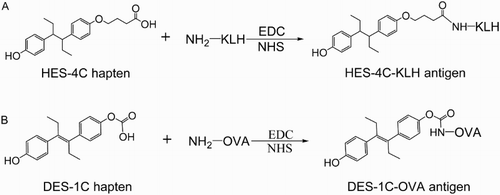
The synthesis of 4-(4-(4-(4-hydroxyphenyl)hexan-3-yl)phenoxy)butanoic acid (HES-4C) was accomplished by linking ethyl 4 bromobutanoate to one of HES′ –OH group using the method as described by Li et al. (Citation2011) and Wang et al. (Citation2008) with slight modification (). Briefly, 200 µl of ethyl 4 bromobutanoate dissolved into 1 ml of DMSO was slowly introduced into a solution of 15 ml of DMSO containing 0.5 mg of HES and 0.25 g of K2CO3; then, the mixture was heated to 60°C for 6 h. After cooling to room temperature, the mixture was extracted with Diethyl ether and the ether extract was dried in fume hood; the product obtained was saponified to produce HES-4C acid. By using the same procedures, DES-1C hapten was synthesized using DES and ethyl carbonobromidate as materials.
Figure 1. Schematic diagram illustrating the route of [4-(4-(4-(4-hydroxyphenyl) hexan-3-yl) phenoxy) butanoic acid] hapten synthesis.
![Figure 1. Schematic diagram illustrating the route of [4-(4-(4-(4-hydroxyphenyl) hexan-3-yl) phenoxy) butanoic acid] hapten synthesis.](/cms/asset/72866efa-efe8-47f3-b458-01036d9b578f/cfai_a_1183601_f0001_b.gif)
The conjugation of HES-4C to carrier protein KLH involved 8.0 mg of the hapten, 13.0 mg of EDC (1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide) and 8.0 mg of N-hydroxysuccinimide (NHS) dissolved in 0.6 ml of DMSO and 0.4 ml of MES then activated for 5 h at room temperature, yielding solution I. Meanwhile, 2 ml of KLH (5 mg/ml) was diluted by 1 ml of carbonate buffer (CB, pH 9.6) to prepare solution II. Having prepared the two solutions, the solution I was slowly added to solution II with a constant stirring; this reaction lasted for a period of 3 h at room temperature. The conjugate obtained was then purified by dialysis in 0.01 M PBS for 3 days with intermittent changing of buffer solution three times daily. Successful conjugations were confirmed by comparing the UV spectrum of conjugates against UV spectrum of hapten and carrier protein. The coating antigen was prepared in the same way but using (DES-1C) hapten and OVA as a carrier protein.
Immunization
Ten BALB/c female mice aged eight to ten weeks were subcutaneously immunized with HES-4C-KLH immunogen. The primary immunization was accomplished by administering each mouse with 100 µg of immunogen prepared in physiological saline and emulsified with complete Freund’s adjuvant (2:1:1; w/v/v/). Four subsequent boosters were applied at an interval of three weeks per booster and each mouse received 50 µg of immunogen emulsified with incomplete Freund’s adjuvant (1:1:1; w/v/v/). One week after the fourth booster, the antisera were collected and their titer was analyzed by ic-ELISA with HES as inhibitor. The mouse whose antiserum exhibited a highest affinity with HES was intraperitoneally injected with 30 µg of immunogen prepared in 0.2 ml saline and after three days, it was sacrificed to give spleen for cell fusion.
Production of monoclonal antibodies
The production of monoclonal antibody was done according to the method as described by Chen et al. (Citation2015). Murine myeloma cells (SP20-Ag14) and mouse spleen cells were fused at a ratio of 1:1 in the presence of PEG-1500 resulting in hybridoma cells. The hybridoma cells were dispersed into 96-well plates supplemented withCulture medium with Hypoxanthine, Aminopterin and Thymidine (HAT) medium containing 20% bovine fetal serum and incubated at 37°C in the presence of 5% CO2 for seven days. The supernatants of the hybridoma colonies were evaluated by ic-ELISA. The cell lines with highest titer were used to generate sub-clones, which later were injected intraperitoneally into mice primed with 0.5 ml of paraffin oil for antibody production. The ascites were collected from mice after seven days and purified according to caprylic acid-ammonium sulfate precipitation method as previously described (Kuang et al., Citation2013; Tochi et al., Citation2016). The collected monoclonal antibodies were evaluated and quantified; the quantification was done by using the following equation: [X1/Ab1 = X2/Ab2 (where X1 and Ab1 are, respectively, the concentration and absorbance at 278 nm of immunoglobulin G; and X2 is the unknown concentration and Ab2 the absorbance at 278 nm of the mAb)]. The antibodies were labeled and stored at −20°C.
Development of indirect competitive ELISA
Optimization of coating antigen and antibody dilution
The checker board titration procedure was used to optimize the coating antigen DES-1C-OVA (1.0, 0.5, 0.25 and 0.125 ng/ml) and the primary antibody (1:1000, 1:2000, 1:4000, 1:8000, 1:16,000 and 1:64,000) concentrations. Other parameters were evaluated based on the optimum condition of coating antigen and primary antibody working point. As previously described (Sun, Liu, Song, Kuang, & Xu, Citation2016), the ic-ELISA procedure was adapted to adequate anti-HES mAb’s assays. Briefly, each well of a Microtiter plate was coated with 100 µl of DES-1C-OVA solution prepared in bicarbonate buffer (0.05 M, pH 9.6). The plates were incubated at 37°C for 2 h, washed three times with PBST and blocked with 200 µl/well. After incubation at 37°C for 2 h, plates were washed twice with PBST. Standards of HES, prepared in 0.01 M PBS containing 20% of methanol, were used as positive control against a blank buffer control of 0.01 M PBS also containing 20% of methanol; 50 µl of blank buffer and 50 µl of standards were added to each well followed by the addition of 50 µl of the primary antibody. The reaction was improved by incubation at 37°C for 30 min and terminated by washing the plates three times with PBST. Goat anti-mouse IgG HRP (1:3000) dissolved in antibody diluents was added to each well 100 µl/well and incubated for 30 min at 37°C. The reaction also was completed by washing the microtiter plates four times using PBST as washing buffer. Lastly, 100 µl/well of TMB-substrate solution was added to each well, incubated for 15 min and then 50 µl/well of 2 M H2SO4 was added to stop color development. The absorbance values were determined at 450 nm by a microtiter plate reader.
Effect of physical–chemical parameters on ELISA performance
Immunoassay performance can be improved by altering some physical–chemical parameters such as organic solvent content, ionic strength, pH of the buffers and other substances in the assay buffer (Chen & Jiang, Citation2013; Kong et al., Citation2015). In this work, the effects of methanol, NaCl and pH were evaluated by running standard curves using a modified PBS buffer (the buffer which was used to prepare standards of HES) . The maximum absorbance (Amax), half-inhibition concentration (IC50), maximal Amax/IC50 ratio and R2 were used as conclusive criteria. To evaluate the effect of methanol content, 0.01 M PBS, pH 7.4 buffer was prepared with different concentrations of methanol (0%, 10%, 20%, 30% and 40%). For ionic strength, different concentrations of NaCl (0.10, 0.15, 0.20, 0.40 and 0.80 M) were prepared using phosphate buffer containing methanol previously optimized. Lastly, pH effects were studied by measuring (5.0, 6.0, 7, 8.0 and 9.0 pH values).
Table 1. Effects of methanol, ionic strength and pH contents on the performance of ic-ELISA.
Specificity and sensitivity of monoclonal antibodies
The specificity of the monoclonal antibody produced was evaluated by determination of its capability of detecting other related structural analogues. The cross-reactivity (CR) values were calculated according to the following equation (Chen et al., Citation2015):
Development of immunochromatographic assay test kit
Synthesis of gold nanoparticles
The colloidal gold nano particles (GNPs) were prepared according to the method described in (Chen et al., Citation2015; Khaemba et al., Citation2015). A volume of 2 ml of freshly prepared 1% (w/v) trisodium citrate was added to 100 ml of HAuCl4·4H2O solution (0.01%, w/v) (previously heated up to its boiling point under continuous stirring). The mixture obtained was heated for about 6 min until a red-wine like color was formed; the heat was removed and after cooling to room temperature, it was kept at 4°C. The diameter profile of GNPs was characterized by transmission electron microscope.
Preparation of colloidal gold-labeled monoclonal antibodies
The anti-HES mAb was labeled with GNPs according to the methods previously described by Gu, Liu, Song, Kuang, and Xu (Citation2015), Khaemba et al. (Citation2015) and Liu et al. (Citation2014) with minimal modifications. The procedure was as follows: 40 μl of 0.1 M K2CO3 was mixed together with 10 ml GNPs and the ion strength of the mixture was adjusted to pH 8.2. The antibody (0.1 mg) was added dropwise into the GNP solution and kept at room temperature for 50 min with constant stirring, BSA (50 mg dissolved in 1 ml of dd water) was slowly added to the mixture and stirred for 30 min. The mixture was then centrifuged at 8000 rpm for 25 min to remove free blocking agent and excess monoclonal antibodies. The precipitates were collected and washed with gold-labeled re-suspension buffer (0.02 M PBS, containing 1% BSA, 5% sucrose and 0.5% PEG 6000, pH 7.4); at the end of washing, the conjugate was dissolved in 4 ml PBS containing 0.2% NaN3 and stored at 4oC until use.
Immunochromatographic strip preparation
Essentially, an ideal immunochromatographic strip comprises four main sections; as illustrated in , there are an absorbent pad, a nitrocellulose (NC) membrane (, conjugate pad and a sample pad, assembled in layers. The test and control lines located on NC membrane were respectively sprayed with coating antigen and goat anti-mouse IgG by using a BJQ 3000 Membrane dispenser machine, and the sample pad, together with the conjugate pad, was attached to one side of the polystyrene backing card close to the test line, while the absorbent pad was attached on the other side of the card close to the control line and overlapping with the NC membrane (Feng et al., Citation2015; Liu, Xing, Yan, Kuang, & Xu, Citation2014).
Recovery test in milk
The recovery test in negative milk samples was done by ic-ELISA. Milk samples were spiked with HES (0.5, 1.0 and 5.0 ng/ml) and DES (1.5, 3.0 and 15.0 ng/ml). Before conducting ic-ELISA analysis, spiked milk samples were diluted with an equal volume of 0.01 M PBS containing 20% Methanol.
Samples for immunochromatographic assay were prepared as follows: negative milk samples were spiked with HES (0, 0.25, 0.5, 1.0, 2.5, 5.0 and 10.0 ng/ml) and DES (0, 0.5, 1.0, 2.5, 5.0, 10.0 and 15.0 ng/ml). Samples were directly analyzed without any further pretreatments.
Results and discussion
Hapten, immunogen and coating antigen synthesis
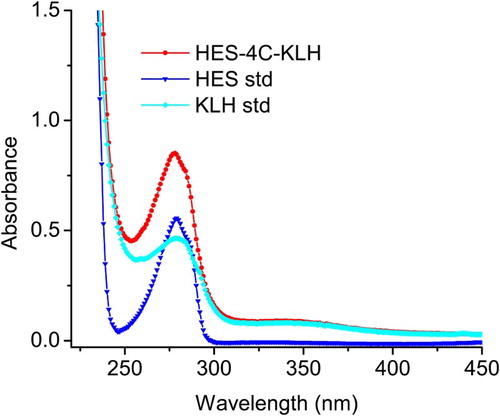
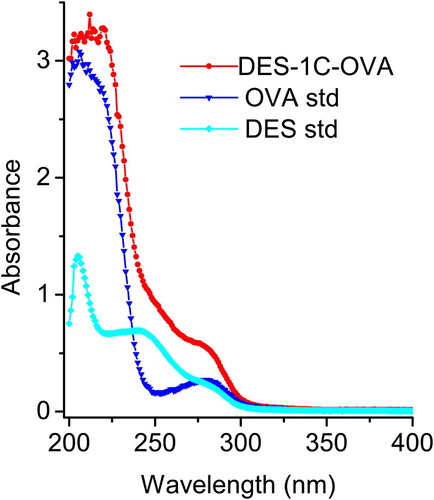
Haptens are small molecules that can induce an immune response only when they are linked to a larger antigenic molecule such as KLH, BSA and OVA. It is important to note that some haptens require marginal modification so that they can bind to their carrier proteins. The hapten synthesis route is summarized in . HES molecule was linked to ethyl 4 bromobutanoate to generate an intermediate compound following which its ester group was converted into carboxyl group by saponification to produce 4-(4-(4-(4-hydroxyphenyl)hexan-3-yl)phenoxy)butanoic acid (the name was generated by ChemBioDraw ultra 14.0 and it was labeled HES-4C). The mass spectrum of HES-4C compound was analyzed by LC–MS and it was compared with that of parental molecule; HES m/z: 270.16 and HES-4C m/z: 356.20. The same procedures were applied to synthesize a heterologous coating antigen DES-1C and the LC mass spectrum that resulted was as follows: DES m/z: 268.15 and DES-1C m/z: 354.18. The introduction of two different spacer arms of four carbon lengths and one carbon length, respectively, for HES and DES, was not only to create a carboxyl group of both haptens but also to increase the affinity, sensitivity and specificity of the antibody. Tochi et al. (Citation2016) also reported that an immunogen with a longer spacer arm, between hapten and carrier protein, could produce an antibody with higher affinity. shows the synthesis of immunogen and coating antigen, the carboxyl group of HES-4C was covalently linked to KLH free amino group to yield HES-4C-KLH, while DES-1C was linked to OVA to prepare coating antigen DES-1C-OVA. Successful conjugates were confirmed by UV-Vis spectroscopy. HES-4C-KLH showed a characteristic absorption peaks at 350 and 280 nm (), whereby the peak at 350 nm was merely generated by KLH, whereas the major peak detected at 280 mm was generated via both KLH and HES-4C ensuring that the conjugation was successful. Based on the Beer-Lambert Law, the conjugation ratio was determined using multi-wavelength linear regression method and it was estimated to be 17. The same Law was applied to determine the conjugation ratio of DES-1C-OVA, which was 13 ().
Production of monoclonal antibodies
During development of monoclonal antibodies, harvested cell culture samples must be screened for IgG titer and the antibodies of choice must be characterized and quantified. In this study, after getting pure colonies from the hybridoma cells, we screened out most prominent three cells (2E6, 2H2 and 2H6). These cells were used to produce mAbs whereby 2 ml of each cell sample was injected intraperitoneally into a healthy mature female BLB/c mouse primed with 0.5 ml of paraffin oil for seven to ten days. Purified mAbs were evaluated against HES standards (0, 0.03, 0.1, 0.3, 0.9, 2.7 and 8.1 ng/ml) and their IC50 values (0.31 , 0.35 and 0.32 ng/ml respectively for 2E6, 2H2 and 2H6) were compared to select the best antibody. Monoclonal antibody 2E6 carried further experiments as a result of exhibiting high titer and being specific toward HES drug.
Optimization of ic-ELISA
Optimization of coating antigen and antibody dilution
The optimization of monoclonal antibody and coating antigen was done by assessing the interaction between antibody and the coating antigen. The optimum coating antigen concentration was 0.25 ng/ml in contrast to 2E6 mAb titer of 1:5000 with an average optical density of 1.6 ± 0.1 read at 450 nm by microtiter plate reader. Further experiments were conducted depending on the above optimal working point.
Effect of methanol concentration in assay buffer
HES and other Stilbenes are practically insoluble in water but soluble in organic solvents; therefore, it is very crucial to fully dissolve them before making subsequent serial dilutions. This explains why it is important to study the effects triggered by methanol concentration during the development of ic-ELISA of anti-HES mAb. High concentration of methanol was found to increase the Amax values and resulted to poor IC50 whereas low concentration of methanol enhanced the performance of ELISA (). At a concentration of 10% Methanol, the result exhibited a maximum Amax/IC50 value of 10.54, indicating that the mAb produced had a strong affinity with HES drug. From , it is clearly shown that the increment of methanol concentration gradually increased the Amax and decreased the sensitivity of the antibody. The same trend was observed by previous authors (Chen & Jiang, Citation2013; Kong et al., Citation2015).
Effect of ionic strength in assay buffer
The addition of salt into the PBS buffer stabilizes and improves the interaction of various molecules of analytes in assay buffer. As depicted from , it was obviously shown that the salt concentration above 0.2 M suppressed the Amax values, though the IC50 values remained almost constant. This phenomenon might be due to the effect of salting out on protein, which would denature the antibodies’ structure and hinder their activities as a result of high salt concentration in assay buffer.
Effect of pH in assay buffer
To study the influence of pH on the ELISA performance, five pH values ranging from 5 to 9 were considered as indicated in . The result showed a progressive decrease in IC50 values while the Amax values remained almost constant. The isoelectric point (pI) of the majority antibodies tends to be in the pH 6–10 region; thus, this could explain the reason why the Amax values stayed almost the same within the pH 5–9 region while the antibody activity was declining. The decrease in IC50 values meant that there was an increase in sensitivity of HES as long as high alkaline solution was a good solvent for HES dissociation. The pH value of 8 corresponded with highest Amax/IC50 ratio, indicating that there was a desirable IC50 value which was our target objective. From these findings, it could be concluded that HES drug was better detected in assay buffer with 10% Methanol PBS, upgraded with 0.2 M of Salt at a pH value of 8. Further experiments were carried out based on these optimized ic-ELISA system.
Sensitivity and specificity of anti-HES monoclonal antibody on ic-ELISA assay
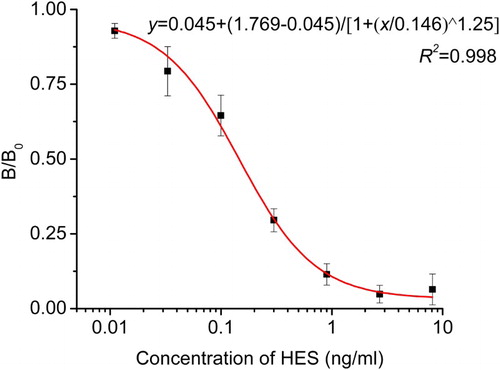
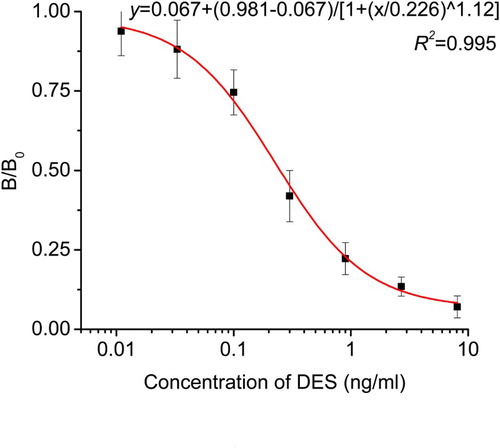
The mAb developed showed a higher specificity against HES compound and more sensitivity to other stilbene compounds; it is a common phenomenon that an antibody would recognize compounds structurally similar to that of antigen used for immunization. Among stilbenes tested, DES compound had the highest CR (65.21%). shows the CR of anti-HES mAb with different compounds. It was obviously noticed that there was no significant CR with other estrogen used in this study; this finding was in agreement with other research reported on stilbene antibodies (Li et al., Citation2011; Wang et al., Citation2008). Therefore, the results revealed that the produced 2E6 mAb had a high specificity against HES and more affinity against DES. In addition, it assured that the developed ic-ELISA method could be used to screen out animal food samples with HES and DES residues. The standard curve for HES and DES is presented in and ; the curves were fitted with four-parameter logistic model, where B/B0 was plotted against Log(x) [B is the absorbance of analyte at a given concentration, B0 is the absorbance of the analyte at zero concentration and Log(x) is the transformed x-values]. For HES, the standard curve generated a regression equation y = 0.045 + (1.769–0.045)/ [1 + (x/0.146)^1.25], with (R2 = 0.998, n = 3), IC50 = 0.146 ng/ml and IC10 = 0.019 ng/ml. For DES standard curve, the regression equation was y = 0.067 + (0.981–0.067)/ [1 + (x/0.226) ^1.12], with (R2 = 0.995, n = 3), IC50 = 0.226 ng/ml and IC10 = 0.028 ng/ml ().
Figure 7. The principle of immunochromatographic strip inhibition assay format. A test is positive when a sample with target analyte is loaded on the sample application pad and flows through the strip to produce a colored line only in the control zone. On the other hand, a negative test occurs when a sample with or without target analyte is loaded on the sample application pad and flows through the strip to produce colored lines in both control zone and testing zone.
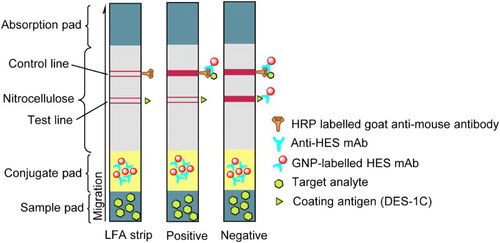
Table 2. CR of anti-HES mAb with various compounds.
Sensitivity of immunochromatographic assay
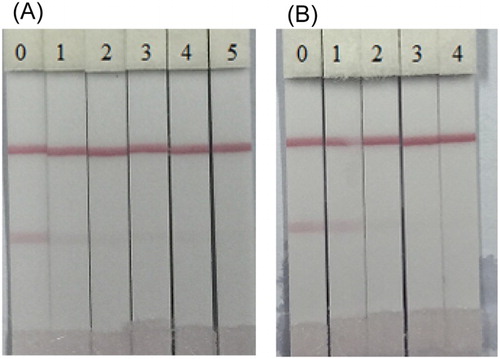
Immunological assays are commonly used to detect veterinary drug residues present in animal foods and the testing method does not require much knowledge as long as you have already obtained the strip kit on your side. The developed strip was found to be effective when 1 ml of gold nanoparticle was used in conjugation with 8 ng/ml of 2E6 antibody and it was found that the colloidal gold solution was stable at pH 8. The optimum coating concentration was 1 mg/ml in 5 min and the assay sensitivity of developed strips was evaluated with serial dilutions of HES and DES standards as shown in . The negative test criteria were characterized as follows: the sample of DES or HES which could produce clear visible lines both at control line and detection line, while a positive test would produce only one visible line at the control line. shows that the test lines gradually decreased the color intensity until it completely disappeared at 0.5 ng/ml concentration for HES and at 1 ng/ml for DES.
Figure 8. Images of immunochromatographic strip analysis of HES (A) and DES (B) in 0.01 M PBS. The concentrations of HES: (0 = Blank sample, 1 = 0.025 ng/ml, 2 = 0.05 ng/ml, 3 = 0.1 ng/ml, 4 = 0.25 ng/ml and 5 = 0.5 ng/ml); the concentrations of DES: (0 = Blank sample, 1 = 0.1 ng/ml, 2 = 0.25 ng/ml, 3 = 0.5 ng/ml and 4 = 1.0 ng/ml).
Performance of ic-ELISA and LFA in Milk
Milk samples were defatted and then spiked with different amounts of HES (0.0, 0.5, 1.0 and 5.0 ng/ml) and DES without any other further treatment. The results obtained by ic-ELISA method are shown in . The milk sample spiked with 0.5, 1.0 and 5.0 ng/ml of HES showed a recovery rate of 60.48–102.19% and 65.94–99.39%, respectively, for intra and inter-assays; For DES, samples spiked with 1.5, 3.0 and 15 ng/ml of DES showed a recovery rate of 89.34–100.16% and 88.49–97.53%, respectively, for intra and inter-assays.
Table 3. Recovery, intra and inter-assay analysis of the ic-ELISA for HES and DES spiked milk samples.
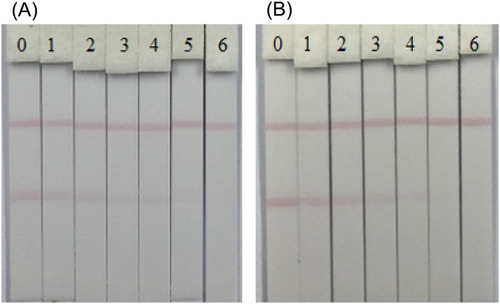
The application of the LFA in the determination of HES and DES residues in milk was also tested. As depicted in , the test lines showed the visual cut-off values at 10 ng/ml for HES and 15 ng/ml for DES. In contrast with the results obtained during LFA sensitivity evaluation and that of milk samples, it was perceived that milk samples had a matrix effects on the immunochromatographic strip assay provoking the visual cut-off values to shift from 0.5 ng/ml (assay in PBS) to 10 ng/ml (assay in milk samples) for HES.
Figure 9. Images of immunochromatographic strip analysis of HES (A) and DES (B) in milk samples. The concentrations of HES spiked in milk samples were: (0 = Blank sample, 1 = 0.25 ng/ml, 2 = 0.5 ng/ml, 3 = 1.0 ng/ml, 4 = 2.5 ng/ml, 5 = 5.0 ng/ml and 6 = 10 ng/ml); the concentrations of DES spiked in milk samples were: (0 = Blank sample, 1 = 0.5 ng/ml, 2 = 1.0 ng/ml, 3 = 2.5 ng/ml, 4 = 5.0 ng/ml, 5 = 10.0 ng/ml and 6 = 15.0 ng/ml).
Conclusion
In this research, a sensitive gold-based lateral-flow immunochromatography strip was developed for the control of HES and DES residues in bovine milk samples. A high-quality monoclonal antibody for HES was produced through female BALB/c mice immunized with HES-4C-KLH conjugates. The ic-ELISA based on this antibody showed high sensitivities toward stilbene than other estrogen hormones. Altogether, with lateral-flow immunochromatography strip, the results proved that they could be an alternative method for the routine screening of HES and DES residues in bovine milk samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Daniel Mukunzi was born in Kenya and began to study as a PhD student in college of Food science and technology of Jiangnan University in 2012. His research interests are immunoassays development for agonists.
Bitange Nipa Tochi was born in Kenya and began to study as a PhD student in college of Food science and technology of Jiangnan University in 2012. His research interests are monoclonal antibody development and applications.
Joel Isanga was born in Uganda and began to study as a PhD student in college of Food science and technology of Jiangnan University in 2013. His research interests are immunoassays development for veterinary drugs.
Liqiang Liu earned his PhD in Food science in 2014 from Jiangnan University, Wuxi, China and then became a faculty in college of Food science and technology of Jiangnan University. His research interests include immunochromatographic strip design and application.
Hua Kuang obtained her PhD. from China Agricultural University in 2009 and then began to work as a faculty in college of Food science and technology of Jiangnan University. She is currently a full professor in food safety. Her research interests are biosensor development.
Chuanlai Xu is a full professor of Food science and technology of Jiangnan University. He earned his PhD. in food science in 2002. His research interests are fast detection technology and food safety evaluation.
Additional information
Funding
References
- Chen, J. J., & Jiang, J. Q. (2013). Monoclonal antibody-based solvent tolerable indirect competitive ELISA for monitoring ciprofloxacin residue in poultry samples. Food and Agricultural Immunology, 24, 331–344. doi: 10.1080/09540105.2012.689817
- Chen, Y., Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Analytical Methods, 9, 905–914. doi: 10.1007/s12161-015-0262-z
- Dickson, L., MacNeil, J. D., Reid, J., & Fesser, A. C. E. (2003). Validation of screening method for residues of diethylstilbestrol, dienestrol, hexestrol, and zeranol in bovine urine using immunoaffinity chromatography and gas chromatography/mass spectrometry. Journal of AOAC International, 86, 631–639.
- Feng, M., Kong, D., Wang, W., Liu, L., Song, S., & Xu, C. (2015). Development of an immunochromatographic strip for rapid detection of Pantoea stewartii subsp. stewartii. Sensors (Basel), 15, 4291–4301. doi: 10.3390/s150204291
- Gu, H., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of an immunochromatographic strip assay for ractopamine detection using an ultrasensitive monoclonal antibody. Food and Agricultural Immunology, 27, 471–483. doi: 10.1080/09540105.2015.1126808
- Khaemba, G. W., Tochi, B. N., Mukunzi, D., Joel, I., Guo, L., Suryobrobowo, S., … Xu, C. (2015). Development of monoclonal antibody and lateral test strip for sensitive detection of clenbuterol and related β2-agonists in urine samples. Food and Agricultural Immunology, 27, 111–127. doi: 10.1080/09540105.2015.1079598
- Kong, N., Song, S., Peng, J., Liu, L., Kuang, H., & Xu, C. (2015). Sensitive, fast, and specific immunoassays for methyltestosterone detection. Sensors (Basel), 15, 10059–10073. doi: 10.3390/s150510059
- Kuang, H., Xing, C., Hao, C., Liu, L., Wang, L., & Xu, C. (2013). Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors (Basel), 13, 4214–4224. doi: 10.3390/s130404214
- Li, W., Meng, M., He, F., Wan, Y., Xue, H., Liu, W., … Xi, R. (2011). Preparation of an anti-diethylstilbestrol monoclonal antibody and development of an indirect competitive ELISA to detect diethylstilbestrol in biological samples. Chinese Science Bulletin, 56, 749–754. doi: 10.1007/s11434-010-4322-x
- Liu, L., Luo, L., Suryoprabowo, S., Peng, J., Kuang, H., & Xu, C. (2014). Development of an immunochromatographic strip test for rapid detection of ciprofloxacin in milk samples. Sensors (Basel), 14, 16785–16798. doi: 10.3390/s140916785
- Liu, L., Xing, C., Yan, H., Kuang, H., & Xu, C. (2014). Development of an ELISA and immunochromatographic strip for highly sensitive detection of microcystin-LR. Sensors (Basel), 14, 14672–14685. doi: 10.3390/s140814672
- Socas-Rodríguez, B., Asensio-Ramos, M., Hernández-Borges, J., Herrera-Herrera, A. V., & Rodríguez-Delgado, M. Á. (2013). Chromatographic analysis of natural and synthetic estrogens in milk and dairy products. TrAC Trends in Analytical Chemistry, 44, 58–77. doi: 10.1016/j.trac.2012.10.013
- Song, Y., Song, S., Liu, L., Kuang, H., Guo, L., & Xu, C. (2015). Simultaneous detection of tylosin and tilmicosin in honey using a novel immunoassay and immunochromatographic strip based on an innovative hapten. Food and Agricultural Immunology, 27, 314–328. doi: 10.1080/09540105.2015.1089843
- Sun, C., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of a highly sensitive ELISA and immunochromatographic strip to detect pentachlorophenol. Food and Agricultural Immunology. doi:10.1080/09540105.2016.1148668
- Tang, J., Xiang, L., Zhao, F., Pan, F., Wang, S., & Zhan, X. (2014). Development of an up-conversion homogenous immunoassay for the determination of diethylstilbestrol in water. Analytical Letters, 48, 796–808. doi: 10.1080/00032719.2014.961605
- Tochi, B. N., Khaemba, G. W., Isanga, J., Mukunzi, D., Liu, L., Peng, J., … Xu, C. (2015). Monoclonal antibody for the development of specific immunoassays to detect Enrofloxacin in foods of animal origin. Food and Agricultural Immunology, 27, 435–448. doi: 10.1080/09540105.2015.1089844
- Tochi, B. N., Peng, J., Song, S., Liu, L., Kuang, H., & Xu, C. (2016). Determination of sarafloxacin and its analogues in milk using an enzyme-linked immunosorbent assay based on a monoclonal antibody. Analytical Methods, 8, 1626–1636. doi: 10.1039/C5AY02702K
- Wang, L., Fang Peng, C., Chen, W., & Xu, C. (2008). A direct enzyme-linked immunosorbent assay for hexoestrol residues. Food and Agricultural Immunology, 19, 61–75. doi: 10.1080/09540100801933611
- Wang, Z., Zou, S., Xing, C., Song, S., Liu, L., & Xu, C. (2016). Preparation of a monoclonal antibody against testosterone and its use in development of an immunochromatographic assay. Food and Agricultural Immunology, 27, 547–558. doi: 10.1080/09540105.2015.1137276
- Xu, C., Peng, C., Liu, L., Wang, L., Jin, Z., & Chu, X. (2006). Determination of hexoestrol residues in animal tissues based on enzyme-linked immunosorbent assay and comparison with liquid chromatography-tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 41, 1029–1036. doi: 10.1016/j.jpba.2006.01.016
- Xu, N., Li, L., Song, S., Xu, L., Kuang, H., & Xu, C. (2015). Development of a lateral flow immunoassay for the detection of total malachite green residues in fish tissues. Food and Agricultural Immunology, 26, 870–879. doi: 10.1080/09540105.2015.1039498