ABSTRACT
Adlay (CL) has been used as food and medicinal plant, and its anti-inflammatory, anti-cancer and anti-allergic activities have been demonstrated. However, its anti-rheumatoid arthritic effect has not been evaluated. We evaluated the anti-rheumatoid arthritic activity of fermented CL (FCL) in vivo compared to fermented Achyranthes japonica Nakai (FAJN). The collagen-induced arthritis (CIA) mouse model was used, and clinical signs, histological examination, serum inflammatory and rheumatoid factors were analyzed. FCL and FAJN significantly reduced incidence of CIA, alleviated inflammation and joint destruction through inhibition of IL-1β and rheumatoid factors. In addition, they increased anti-oxidant enzyme activity such as superoxide dismutase (SOD) in the liver. Interestingly, ferulic acid was isolated from the FCL extract, which have anti-oxidant and anti-inflammatory activities. Moreover, the anti-rheumatoid arthritis (RA) effect of FCL was shown to be similar to or much better than that of FAJN. Therefore, our study suggests that FCL and FAJN are potential agents to alleviate RA.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that causes joint destruction, deformity and loss of function, which lead to pain and disability. These symptoms of chronic pain and significant disabilities reduce quality of life, and are also associated with increased morbidity and mortality of RA patients (Gonzalez et al., Citation2007; Pugner, Scott, Holmes, & Hieke, Citation2000; Wolfe & Hawley, Citation1998). Although the precise pathology and etiology of RA have not fully understood yet, the key role of TNF-α, IL-1 and Il-6 in the RA process has been reported by a number of studies (Arend & Dayer, Citation1995; Choy, Citation2012; Sarban, Kocyigit, Yazar, & Isikan, Citation2005). Likewise, because of the unclear etiology, the objective of RA treatment is to minimize the disease intensity and pain. Current drug therapy for RA includes non-steroidal anti-inflammatory drugs, disease-modifying anti-rheumatic drugs and newer biological agents that target specific pro-inflammatory cytokines, cell surface receptors or various cell types (Smolen & Steiner, Citation2003). However, their therapeutic effects are not so fully satisfactory, and prolonged consumption of them can cause serious adverse side effects. Therefore, it is necessary to develop safe and effective therapeutic agents for RA.
Natural plant products have been tried in RA treatment, and offered a promising resource for potential anti-arthritic agents (Nanjundaiah, Astry, & Moudgil, Citation2013; Rabe et al., Citation2015). Moreover, many studies have shown that fermented natural plants have enhanced biological activity and increased the original treatment efficacy (Eum et al., Citation2011; Miyake et al., Citation2005). In our previous study, fermented Achyranthes japonica Nakai (FAJN), one of the medicinal foods in Asia, showed stronger anti-inflammatory and anti-osteoarthritic effects than those of non-fermented ones (Bang et al., Citation2012; Lee et al., Citation2012). Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf: CL) is also commonly used as food and a medicinal plant in Asia. CL has shown to have anti-inflammatory, anti-cancer and anti-allergic effects (Bang et al., Citation2012; Chen, Hsu, & Chiang, Citation2012; Chung et al., Citation2010). However, its anti-rheumatoid arthritic activity has not been evaluated yet. The animal model of collagen-induced arthritis (CIA) is widely used in such studies as testing potential new therapeutics and determining the pathogenesis and etiology of RA (Hegen, Keith, Collins, & Nickerson-Nutter, Citation2008). Therefore, we investigated the anti-rheumatoid arthritic effect of fermented (F) CL compared to FAJN in the mouse CIA model.
In the present study, we found that FCL and FAJN reduce incidence of CIA and alleviate inflammation and destruction of joints by delaying disease progression into the chronic stage of arthritis.
Materials and methods
Preparation of FAJN and FCL extract
A. japonica Nakai (AJN) and Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf: CL) were purchased from an herb market in Seoul, Korea. The procedure for preparing fermented AJN (FAJN) was performed as described in our previous study (Lee et al., Citation2012). Finally, the water extract of the FAJN was lyophilized (FDU-1100, Eyela Co.) and stored at −20°C before use. The yield (w/w) obtained from the FAJN water extract was about 13.1%. The procedure for preparing fermented CL (FCL) was as follows: the CL was soaked in water, autoclaved for 25 min at 121°C and ground, and then 1.5 volumes of water was added after mixing with the same amount of boiled rice. The mixture was inoculated with 3% nu-ruk and 0.6% Saccharomyces cerevisiae and fermented at 25°C for 7 days. The FCL was produced following the same procedure mentioned above, which has an alcohol content of 14%. The FCL was filtered with cartridge paper (Whatman No 2, Maidstone, England), and then concentrated using a vacuum rotary evaporator (N-1000S-WD, Eyela Co., Tokyo, Japan). After freeze-drying, the powdered FCL was provided to the mice.
Identification and quantification of major component in the extract of FCL
In the previous study, we identified 20-hydroxyecdysone (20-HES) in FAJN extract (Lee et al., Citation2012). Through the same method using the HPLC system, we identified and quantified major component in the extract of FCL.
Animals
Male DBA/1J mice were used in the experiments and were maintained at 12 h day/dark cycles in a controlled environment (22 ± 2°C, relative humidity 50 ± 10%) and fed ad libitum with standard mouse chow. Animal experiments were performed in accordance with the Kyungpook National University guidelines for the care and use of laboratory animals (KNU2010-87)
Induction of CIA
CIA in mice was induced according to methods by Brand and colleagues (Brand, Latham, & Rosloniec, Citation2007). Briefly, one volume of bovine type II collagen (Chondrex, USA) was mixed with an equal volume of complete Freund’s adjuvant (Sigma, USA) in an electric homogenizer at 4°C to make an emulsion. Mice were immunized with the emulsion by injecting intradermally (i.d.) into the skin of the tail at approximately 2–3 cm distal to the base of the tail. Emulsified type II collagen in incomplete Freund’s adjuvant was injected once more through the same procedure after 3 weeks.
Experimental design
DBA/1J mice (male, 8-week-old) were randomized into five groups of ten animals. CIA was induced in Groups 2–5. Group 1 was the normal control and Group 2 (CIA) was CIA induction mice without therapeutic treatment. Group 3 (CIA + COX) mice were fed a diet containing 0.03% (wt/wt) Celecoxib for comparison of therapeutic effect. Groups 4 (CIA + FAJN) and 5 (CIA + FCL) were fed a diet containing 0.3% (wt/wt) of the FAJN and FCL extracts, respectively. All of the mice were fed a chow diet, and then, Groups 3–5 were fed a chow diet containing each component for 5 weeks after the second injection of type II collagen. The body weight of the mice was measured twice a week, and the weight of the livers and spleens was measured at sacrifice.
Monitoring of the arthritis incidence, clinical evaluation and measurement of paw swelling
The clinical signs of arthritis in mice were evaluated three times per week throughout the experiment. The redness and swelling of each paw were scored individually on a scale of 0–4 (Brand et al., Citation2007). The scores of all mice in the same group were summated and shown as the final score. Paw swelling was measured using a digimatic thickness gage (Mitutoyo, Japan) before sacrifice. The final score of each mouse was the mean of all paws scored.
Histological assessment of arthritis
Joint tissues were formalin-fixed, decalcified and embedded in paraffin wax for hematoxylin and eosin (H&E) and Toluidine Blue staining. The erosion and synovial inflammation of each paw were scored from 0 to 4, respectively (Plater-Zyberk et al., Citation2001). The final score of each mouse was the sum of all paws scored from 0 to 32.
Biochemical analysis
Levels of serum TNF-α, IL-1β, IgG and IgM were measured using ELISA (R&D systems, USA) according to the manufacturer’s instructions.
Statistical analysis
The results are presented as the means ± SE. For multiple comparisons, ANOVA with Duncan’s or Waller–Duncan’s multiple range test was used. All calculations were done with SPSS 12.0 K software. Statistical significance was assumed when p < .05.
Results
Analysis of major component in the extracts of FAJN and FCL
We identified 20-HES from the extracts of FAJN in our previous study (Lee et al., Citation2012), and examined that the amount of 20-HES in FAJN extract was much increased by longer period of fermentation. The amount of 20-HES in the non-fermented extract was only 15.13 ± 0.91 mg/100 g; however, this amount was increased up to over 120 mg/100 g by 5 or 7 days fermentation. In the FCL extract, we isolated and identified three components, 4-hydroxyphenethyl alcohol (4-HPA), sodium dehydroferulate (SDF) and ferulic acid (FA) (). The non-fermented CL extract contained only FA, however, HPA and SDF were isolated after fermentation. SDF is a sodium salt of FA which is water soluble and stable form of FA, and has been reported to have a broad spectrum of biological activities involving anti-inflammatory, antioxidative and antimutagenic effects (Guo et al., Citation2012; Wang & Ou-Yang, Citation2005). Therefore, we can obtain more active and stable form of FA from CL after fermentation.
Table 1. Major components in the extract of FCL (mg/100 g, dry weight).
FCL alleviates loss of bodyweight from CIA in mice
In an anti-inflammatory drug test with animals, a change in the body weight is one of the useful indexes because arthritic animals may loss their body weight due to the reduced absorption of some nutrients into the intestine (Somasundaram, Sadique, & Subramoniam, Citation1983; Winder, Lembke, & Stephens, Citation1969). The bodyweight of mice was measured twice a week (). The body weight of the control group gradually increased throughout the experiment, but the increase in body weight of the CIA group was retarded. The COX group showed a similar increase in body weight as the control group, and the body weight of the FCL group also was increased compared to the CIA group.
Table 2. Effect of FCL and FAJN on body weight (g).
FCL and FAJN reduce the incidence and severity of CIA in mice
We examined the incidence rate of CIA by observing the redness and swelling of the paws ((A)). The redness and swelling of the paws were initially examined about 30 days after the first immunization. The CIA group exhibited a faster induction of arthritis, and finally the mice in the group showed a 100% incidence rate at the end of the experiment. The COX group exhibited the slowest induction of arthritis, and 60% of the mice in the group were examined for arthritis. The induction of CIA in the FAJN and FCL groups was a bit slower than the CIA group, and consequently these groups had a 100% and 90% incidence rate, respectively, at the end of the experiment.
Figure 1. Effect of FCL and FAJN on (A) arthritis incidence, (B) clinical score and (C) swelling of the paw in collagen-induced arthritis of mice. Means ± SE. n = 10. abc means not sharing the same letter is significantly different among the groups at p < .05. Control: normal group, CIA: negative control group, COX: positive control group, FAJN: fermented Achyranthes extract, FCL: fermented Adlay extract.
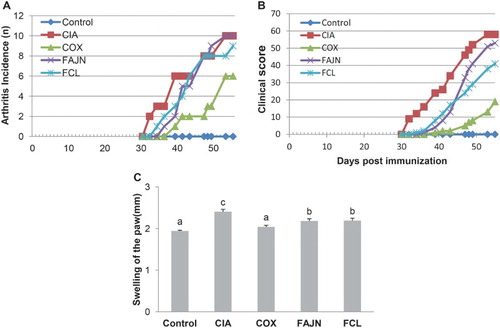
We next evaluated the clinical score of CIA ((B)). Each paw was evaluated and scored individually, and the scores of all the mice in the same group were summated and shown as a final score. The final clinical score for the CIA group was 58 while that of the COX group was 19. The final clinical scores for the FAJN and FCL groups were 53 and 41, respectively, and were lower than that of the CIA group. The swelling score of the paws from synovial inflammation was the highest in the CIA group and the lowest in the COX group. The swelling scores were significantly decreased in the FAJN and FCL groups compared to the CIA group.
FCL and FAJN alleviate the increased liver and spleen weight from CIA in mice
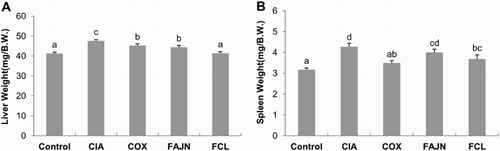
Enlarged livers and spleens have been reported in some patients with RA (Bedoya, Ceccato, & Paira, Citation2015; Tiger, Gordon, Ehrlich, & Shapiro, Citation1976). We measured the weight of the livers and spleens in all mice at sacrifice ((A) and (B)). The CIA group showed a significant increase in liver and spleen weight compared to the control group. The weight of the liver in the COX, FAJN and FCL groups was significantly lower than the CIA group. In the weight of the spleen, COX and FCL groups were significantly lower than the CIA group.
Figure 2. Effect of FCL and FAJN on (A) liver and (B) spleen weights in collagen-induced arthritis of mice. Means ± SE. n = 10. abcd means not sharing the same letter is significantly different among the groups at p < .05. Control: normal group, CIA: negative control group, COX: positive control group, FAJN: fermented Achyranthes extract, FCL: fermented Adlay extract.
FCL and FAJN alleviate inflammation and destruction of the joint in CIA
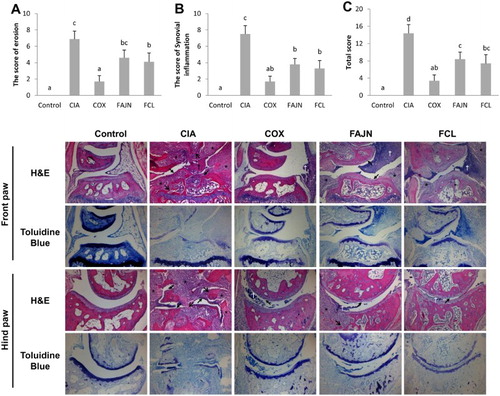
To assess the histological outcome of joints, front and hind paws were stained with H&E and Toluidine Blue for proteoglycan. The scores for joint erosion and inflammation are presented in . The control group had normal joint structures and abundant proteoglycan in the cartilages. The CIA group, however, showed severe destruction of joint cartilages with bone erosion, proteoglycan depletion and excessive infiltration of inflammatory cells in the joint capsules with hyperplasia of the surrounding tissue. The COX group showed a mild depletion of proteoglycan in the cartilages and the filtration of some inflammatory cells. The FAJN and FCL groups had mild destruction of the cartilage with moderate depletion of proteoglycan and a moderate inflammatory response. The total arthritis score of the FAJN (8.4 ± 1.6) and FCL (7.4 ± 2.03) groups was significantly decreased compared to the CIA (14.4 ± 2.01) group.
Figure 3. Histological examination of the effect of FCL and FAJN on joints in collagen-induced arthritis of mice. (A) Erosion score of the joints, (B) the score of synovial inflammation, and (C) total score of arthritis. H&E. Toluidine Blue stain for proteoglycan. Cartilage destruction (close arrow), bone erosion (open arrow), infiltration of inflammatory cells and inflammatory exudate (†), and hyperplasia of surrounding tissue (*). Control: normal group, CIA: negative control group, COX: positive control group, FAJN: fermented Achyranthes extract, FCL: fermented Adlay extract.
FCL and FAJN alleviate the increase in IgG, IgM and inflammatory cytokines in serum
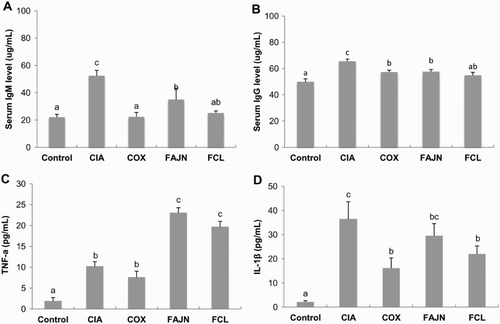
Rheumatoid factors are autoantibodies with specificity directed against the Fc region on the immunoglobulin molecules, and an increase in total IgG and IgM has been found in RA patients due to increase in IgG-RF and IgM-RF in serum. Therefore, measurement of total anti-IgG and IgM can be a useful serological tests for an evaluation of RA (Mosayyebi & Shokri, Citation2000; Renaudineau, Jamin, Saraux, & Youinou, Citation2005; Veys & Claessens, Citation1968). The CIA group showed the highest level of both serum IgM and IgG, and the COX, FAJN and FCL groups showed a significant decrease in these factors compared to the CIA group ((A) and (B)). Inflammatory cytokines play an important role in the pathogenesis of RA. Tumor necrosis factor (TNF-α) has a dominant role in activating inflammatory cells, and Interleukin (IL)-1 has a more significant impact on the disease process such as cartilage destruction through osteoclast activation (Arend & Dayer, Citation1995; Choy, Citation2012). We analyzed TNF-α and IL-1β in the serum ((C) and (D)). The COX group had a lower level of both cytokines compared to the other arthritis-induced groups. Interestingly, the CIA group had a low level of TNF-α similar to the COX group; however, it had the highest level of IL-1β among the groups. The FAJN and FCL groups had the highest level of TNF-α; on the other hand, the level of IL-1β was significantly lower than that of the CIA group. We suppose that TNF-α plays an important role in the acute stage of inflammation, but IL-1β is more involved in the chronic inflammatory process. Therefore, the COX group did not progress into the chronic stage, but just had an acute inflammatory response; however, the CIA group had already progressed into the chronic stage of arthritis. Therefore, the FCL and FAJN groups are suggested to be in the middle of the process between the acute and chronic inflammation stages.
Figure 4. Effect of FCL and FAJN on expression of immunoglobulin and inflammatory cytokines in collagen-induced arthritis of mice. (A, B) amount of serum IgM and IgG. (C, D) amount of serum TNF-α and IL-1β. Means ± SE. n = 10. abc means not sharing the same letter is significantly different among the groups at p < .05. Control: normal group, CIA: negative control group, COX: positive control group, FAJN: fermented Achyranthes extract, FCL: fermented Adlay extract.
FCL and FAJN alleviate a decrease in the antioxidant enzyme activity in CIA
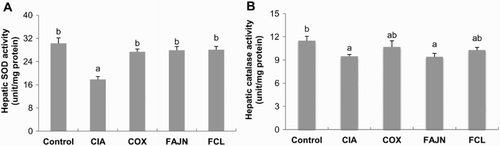
The presence of oxidative stress and decreased anti-oxidant enzymes have been reported in patients with RA (Sarban et al., Citation2005). We measured the superoxide dismutase (SOD) and catalase activities in the liver and found that CIA decreased the SOD and catalase activity; however, COX, FAJN and FCL treatments recovered the SOD activity ().
Figure 5. Effect of FCL and FAJN on the anti-oxidant enzyme activity in collagen-induced arthritis of mice. (A) hepatic SOD and (B) catalase activities. Means ± SE. n = 10. abc means not sharing the same letter is significantly different among the groups at p < .05. Control: normal group, CIA: negative control group, COX: positive control group, FAJN: fermented Achyranthes extract, FCL: fermented Adlay extract.
Discussion
Medicinal herbal plants have been used as therapeutic agents for arthritis treatment as a traditional medicine in many countries, and are considered relatively safe compared to synthetic drugs (Nanjundaiah et al., Citation2013). However, there is a lack of scientific verification for their efficacy in vivo. Therefore, we evaluated the anti-RA effect of FCL and FAJN, two of well-known traditional medicinal foods in Korea, using the CIA mouse model, and showed that they have preventive and ameliorative effects against RA.
AJN ameliorates cartilage degradation and reduces TNF-α expression in the rabbit CIA model (Lee et al., Citation2012), and recently, Bang et al., reported that AJN suppresses inflammation through the inhibition of LPS-induced nitric oxide (NO) production in macrophages related to IκB/NF-κB and mitogen-activated protein kinases signaling (Bang et al., Citation2012). Moreover, Jung et al. showed that isolated compounds from AJN have an inhibitory activity against the complement system (Jung, Lee, Lee, & Moon, Citation2012). Anti-osteoarthritis effects of FAJN were briefly evaluated using the CIA rabbit model in our previous study (Lee et al., Citation2012), and we found that FAJN contained high amount of 20-HES which has been reported to have anti-inflammatory and anti-osteoarthritic effects (Kapur, Wuttke, Jarry, & Seidlova-Wuttke, Citation2010; Zhang, Xu, Xu, & Qin, Citation2014). We further confirmed this in detail using the CIA mouse model.
CL has been believed to consist of beneficial compounds for the human body, and recent studies have reported various physiological activities of CL, such as antioxidant, anti-inflammatory, anti-allergic and antitumor effects (Chen et al., Citation2012; Chung, Hsia et al., Citation2011; Chung, Hsu et al., Citation2011; Chung et al., Citation2010; Seo et al., Citation2000; Wang, Sun, Yi, Wang, & Ju, Citation2012). Oral gavage of polyphenolic extracts of CL increases the activity of catalase and glutathione peroxidase in rat liver (Wang et al., Citation2012). The methanolic extracts of CL seeds inhibit NO and O2− production in macrophage-like RAW 264.7 cells (Seo et al., Citation2000). CL extract reduces allergic reaction through the inhibition of anti-IgE against ovalbumin antigen production and modulation of T helper 1 (Th1)/(Th2) immune reaction (Chen et al., Citation2012). The anti-proliferative activity of CL has been reported in many different types of cancer cells (Chen et al., Citation2012; Chung, Hsia et al., Citation2011; Chung, Hsu et al., Citation2011). Thus, many studies have shown that CL has inhibitory effects in the processes of various diseases; however, the efficacy of CL for the treatment of RA has not been evaluated. Moreover, we isolated three components, 4-HPA, SDA and FA. Those have been isolated from other types of plants, but have not been reported from CL to the author’s knowledge. Interestingly, FA and SDA have been reported as strong antioxidant for cell membrane and DNA damage, and ameliorated the radiation-induced inflammation through inhibition of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase-2 (iNOS-2) and tumor necrosis factor-alpha (TNF-α) (Das et al., Citation2014; Guo et al., Citation2012; Rukkumani, Aruna, Suresh Varma, & Padmanabhan Menon, Citation2004; Srinivasan et al., Citation2006; Wang & Ou-Yang, Citation2005). Therefore, we suggest that anti-inflammatory and anti-RA effects of FCL may be related with the function of FA.
Conclusion
The present study shows that FCL and FAJN delay the onset of the disease and ameliorate inflammation in the CIA mouse model, and suggests that these can be used safely for the prevention and treatment of RA. Moreover, the anti-RA effect of FCL has shown to be similar to or much better than that of FAJN.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Il-Hwa Hong works as the assistant professor in the Department of Veterinary Pathology, Gyeongsang National University, Jinju, Republic of Korea. At this department, she is in the research of pathogenesis of chronic liver diseases and aging.
Ji-Young Choi works as the post-doctoral associate in Department of Food Science and Nutrition, Kyungpook National University, Daegu, Republic of Korea. She collaborates in the research of regulation of metabolic diseases and development of functional food.
Ah-Young Kim works as the graduated student and Kyungpook National University, Daegu, Republic of Korea. At this department, she collaborates in the research of iPSCs for skeletal and osteoarthritic diseases.
Eun-Mi Lee works as the graduated student and Kyungpook National University, Daegu, Republic of Korea. At this department, she collaborates in the research of iPSCs for skeletal and osteoarthritic diseases.
Jun-Han Kim worked as the head of the Bio Health Convergence Center of Daegu Technopark, Daegu, Republic of Korea. Currently, he was retired from the position.
Jin Hong Park works in the Oriental Medicine industry Support Center of Daegu Technopark, Daegu, Republic of Korea. At this center, he provides the education and financial support for promoting bioventure business.
Sang-Won Choi works as the professor in Department of Food Science & Nutrition, Catholic University of Daegu, Republic of Korea. He is in research in bioconversion of biological compounds during fermentation and collaborates in evaluation of biological effects of the fermented plant extracts in the cell and animal model systems.
Kyu-Shik Jeong works as the professor in the Department of Veterinary Pathology, Kyungpook National University, Daegu, Republic of Korea. At this department, he collaborates in the research of iPSCs for skeletal and osteoarthritic diseases.
Additional information
Funding
References
- Arend, W. P., & Dayer, J. M. (1995). Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis and Rheumatism, 38(2), 151–160. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7848304 doi: 10.1002/art.1780380202
- Bang, S. Y., Kim, J. H., Kim, H. Y., Lee, Y. J., Park, S. Y., Lee, S. J., & Kim, Y. (2012). Achyranthes japonica exhibits anti-inflammatory effect via NF-kappaB suppression and HO-1 induction in macrophages. Journal of Ethnopharmacology, 144(1), 109–117. doi:10.1016/j.jep.2012.08.037
- Bedoya, M. E., Ceccato, F., & Paira, S. (2015). Spleen and liver enlargement in a patient with rheumatoid arthritis. Reumatología Clínica, 11(4), 227–231. doi:10.1016/j.reuma.2014.09.006
- Brand, D. D., Latham, K. A., & Rosloniec, E. F. (2007). Collagen-induced arthritis. Nature Protocols, 2(5), 1269–1275. doi:10.1038/nprot.2007.173
- Chen, H. J., Hsu, H. Y., & Chiang, W. (2012). Allergic immune-regulatory effects of adlay bran on an OVA-immunized mice allergic model. Food and Chemical Toxicology, 50(10), 3808–3813. doi:10.1016/j.fct.2012.07.011
- Choy, E. (2012). Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford), 51(Suppl. 5), v3–v11. doi:10.1093/rheumatology/kes113
- Chung, C. P., Hsia, S. M., Lee, M. Y., Chen, H. J., Cheng, F., Chan, L. C., … Chiang, W. (2011). Gastroprotective activities of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on the growth of the stomach cancer AGS cell line and indomethacin-induced gastric ulcers. Journal of Agricultural and Food Chemistry, 59(11), 6025–6033. doi:10.1021/jf2009556
- Chung, C. P., Hsu, C. Y., Lin, J. H., Kuo, Y. H., Chiang, W., & Lin, Y. L. (2011). Antiproliferative lactams and spiroenone from adlay bran in human breast cancer cell lines. Journal of Agricultural and Food Chemistry, 59(4), 1185–1194. doi:10.1021/jf104088x
- Chung, C. P., Hsu, H. Y., Huang, D. W., Hsu, H. H., Lin, J. T., Shih, C. K., & Chiang, W. (2010). Ethyl acetate fraction of adlay bran ethanolic extract inhibits oncogene expression and suppresses DMH-induced preneoplastic lesions of the colon in F344 rats through an anti-inflammatory pathway. Journal of Agricultural and Food Chemistry, 58(13), 7616–7623. doi:10.1021/jf101084e
- Das, U., Manna, K., Sinha, M., Datta, S., Das, D. K., Chakraborty, A., … Dey, S. (2014). Role of ferulic acid in the amelioration of ionizing radiation induced inflammation: A murine model. PLoS One, 9(5), e97599. doi:10.1371/journal.pone.0097599
- Eum, H. A., Lee, J. H., Yang, M. C., Shim, K. S., Lee, J. H., & Ma, J. Y. (2011). Protective effect of Ssanghwa-tang fermented by Lactobacillus fermentum against carbon tetrachloride-induced acute hepatotoxicity in rats. African Journal of Traditional Complementary, and Alternative Medicines, 8(3), 312–321. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22468011
- Gonzalez, A., Maradit Kremers, H., Crowson, C. S., Nicola, P. J., Davis, J. M.3rd, Therneau, T. M., … Gabriel, S. E. (2007). The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis and Rheumatism, 56(11), 3583–3587. doi:10.1002/art.22979
- Guo, Y., Wu, X. Q., Zhang, C., Liao, Z. X., Wu, Y., & Wang, H. (2012). Protective effect of sodium ferulate on acetaldehyde-treated precision-cut rat liver slices. Journal of Medicinal Food, 15(6), 557–562. doi:10.1089/jmf.2011.1915
- Hegen, M., Keith, J. C.Jr., Collins, M., & Nickerson-Nutter, C. L. (2008). Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Annals of the Rheumatic Diseases, 67(11), 1505–1515. doi:10.1136/ard.2007.076430
- Jung, S., Lee, J. H., Lee, Y. C., & Moon, H. I. (2012). Inhibitory effects of three oleanolic acid glycosides from Achyranthes japonica on the complement classical pathway. Immunopharmacology and Immunotoxicology, 34(2), 213–215. doi:10.3109/08923973.2011.594954
- Kapur, P., Wuttke, W., Jarry, H., & Seidlova-Wuttke, D. (2010). Beneficial effects of beta-Ecdysone on the joint, epiphyseal cartilage tissue and trabecular bone in ovariectomized rats. Phytomedicine, 17(5), 350–355. doi:10.1016/j.phymed.2010.01.005
- Lee, S. G., Lee, E. J., Park, W. D., Kim, J. B., Kim, E. O., & Choi, S. W. (2012). Anti-inflammatory and anti-osteoarthritis effects of fermented Achyranthes japonica Nakai. Journal of Ethnopharmacology, 142(3), 634–641. doi:10.1016/j.jep.2012.05.020
- Miyake, Y., Fukumoto, S., Okada, M., Sakaida, K., Nakamura, Y., & Osawa, T. (2005). Antioxidative catechol lignans converted from sesamin and sesaminol triglucoside by culturing with Aspergillus. Journal of Agricultural and Food Chemistry, 53(1), 22–27. doi:10.1021/jf048743h
- Mosayyebi, G., & Shokri, F. (2000). Comparative measurement of rheumatoid factor in serum and synovial fluid of rheumatoid arthritis patients by ELISA and latex-agglutination test. Iranian Biomedical Journal, 4(2), 63–67. Retrieved from http://ibj.pasteur.ac.ir/browse.php?a_code=A-10-1-391&slc_lang=en&sid=1
- Nanjundaiah, S. M., Astry, B., & Moudgil, K. D. (2013). Mediators of inflammation-induced bone damage in arthritis and their control by herbal products. Evidence Based Complementary and Alternative Medicine, 2013, 518094. doi:10.1155/2013/518094
- Plater-Zyberk, C., Joosten, L. A., Helsen, M. M., Sattonnet-Roche, P., Siegfried, C., Alouani, S., … Chvatchko, Y. (2001). Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. Journal of Clinical Investigation, 108(12), 1825–1832. doi:10.1172/JCI12097
- Pugner, K. M., Scott, D. I., Holmes, J. W., & Hieke, K. (2000). The costs of rheumatoid arthritis: An international long-term view. Seminars in Arthritis and Rheumatism, 29(5), 305–320. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10805355 doi: 10.1016/S0049-0172(00)80017-7
- Rabe, S. Z. T., Sahebari, M., Mahmoudi, Z., Hosseinzadeh, H., Haghmorad, D., Tabasi, N., … Mahmoudi, M. (2015). Inhibitory effect of Crocus sativaus L. ethanol extract on adjuvant-induced arthritis. Food and Agricultural Immunology, 26(2), 170–180. doi:10.1080/09540105.2013.878900
- Renaudineau, Y., Jamin, C., Saraux, A., & Youinou, P. (2005). Rheumatoid factor on a daily basis. Autoimmunity, 38(1), 11–16. doi:10.1080/08916930400022574
- Rukkumani, R., Aruna, K., Suresh Varma, P., & Padmanabhan Menon, V. (2004). Hepatoprotective role of ferulic acid: A dose-dependent study. Journal of Medicinal Food, 7(4), 456–461. doi:10.1089/jmf.2004.7.456
- Sarban, S., Kocyigit, A., Yazar, M., & Isikan, U. E. (2005). Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clinical Biochemistry, 38(11), 981–986. doi:10.1016/j.clinbiochem.2005.08.003
- Seo, W. G., Pae, H. O., Chai, K. Y., Yun, Y. G., Kwon, T. H., & Chung, H. T. (2000). Inhibitory effects of methanol extract of seeds of Job’s tears (Coix lachryma-jobi L. var. ma-yuen) on nitric oxide and superoxide production in RAW 264.7 macrophages. Immunopharmacology and Immunotoxicology, 22(3), 545–554. doi:10.3109/08923970009026011
- Smolen, J. S., & Steiner, G. (2003). Therapeutic strategies for rheumatoid arthritis. Nature Reviews Drug Discovery, 2(6), 473–488. doi:10.1038/nrd1109
- Somasundaram, S., Sadique, J., & Subramoniam, A. (1983). In vitro absorption of [14 C] leucine during inflammation and the effect of antiinflammatory drugs in the jejunum of rats. Biochemical Medicine, 29(2), 259–264. Retrieved from http://www.sciencedirect.com/science/article/pii/0006294483900467 doi: 10.1016/0006-2944(83)90046-7
- Srinivasan, M., Sudheer, A. R., Pillai, K. R., Kumar, P. R., Sudhakaran, P. R., & Menon, V. P. (2006). Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicology, 228(2–3), 249–258. doi:10.1016/j.tox.2006.09.004
- Tiger, L. H., Gordon, M. H., Ehrlich, G. E., & Shapiro, B. (1976). Liver enlargement demonstrated by scintigraphy in rheumatoid arthritis. Journal of Rheumatology, 3(1), 15–20. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/818377
- Veys, E. M., & Claessens, H. E. (1968). Serum levels of IgG, IgM, and IgA in rheumatoid arthritis. Annals of the Rheumatic Diseases, 27(5), 431–440. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4175666 doi: 10.1136/ard.27.5.431
- Wang, B. H., & Ou-Yang, J. P. (2005). Pharmacological actions of sodium ferulate in cardiovascular system. Cardiovascular Drug Reviews, 23(2), 161–172. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16007232 doi: 10.1111/j.1527-3466.2005.tb00163.x
- Wang, L., Sun, J., Yi, Q., Wang, X., & Ju, X. (2012). Protective effect of polyphenols extract of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on hypercholesterolemia-induced oxidative stress in rats. Molecules, 17(8), 8886–8897. doi:10.3390/molecules17088886
- Winder, C. V., Lembke, L. A., & Stephens, M. D. (1969). Comparative bioassay of drugs in adjuvant-induced arthritis in rats: Flufenamic acid, mefenamic acid, and phenylbutazone. Arthritis and Rheumatism, 12(5), 472–482. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/5823918 doi: 10.1002/art.1780120503
- Wolfe, F., & Hawley, D. J. (1998). The longterm outcomes of rheumatoid arthritis: Work disability: A prospective 18 year study of 823 patients. Journal of Rheumatology, 25(11), 2108–2117. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9818651
- Zhang, X., Xu, X., Xu, T., & Qin, S. (2014). Beta-ecdysterone suppresses interleukin-1beta-induced apoptosis and inflammation in rat chondrocytes via inhibition of NF-kappaB signaling pathway. Drug Development Research, 75(3), 195–201. doi:10.1002/ddr.21170
